Abstract
Vocalizations are a dominant means of communication for numerous species, including nonhuman primates. These acoustic signals are encoded with a rich array of information available to signal receivers that can be used to guide species-typical behaviors. Here we examined the communicative content of common marmoset phee calls, the species-typical long distance contact call, during antiphonal calling. This call type has a relatively stereotyped acoustic structure, consisting of a series of long tonal pulses. Analyses revealed that calls could be reliably classified based on the individual identity and social group of the caller. Our analyses did not, however, correctly classify phee calls recorded under different social contexts, though differences were evident along individual acoustic parameters. Further tests of antiphonal calling interactions showed that spontaneously produced phee calls differ from antiphonal phee calls in their peak and end frequency, which may be functionally significant. Overall, this study shows that the marmoset phee call has a rich communicative content encoded in its acoustic structure available to conspecifics during antiphonal calling exchanges.
Keywords: Common Marmoset, Phee Calls, Antiphonal Calling, Dialects, Information Content
Introduction
Vocalizations are a central means of communicating information to conspecifics for most, if not all, vertebrate species. The significance of these signals in the evolutionary history of a species is reflected both in the complex array of information encoded within vocalizations and their functional role in mediating conspecific interactions. The common marmoset (Callithrix jacchus) produces a rich diversity of vocalizations [Bezera and Souto 2008; Epple 1968]. The most thoroughly studied of these vocal signals is the phee call, which has been the subject of several studies of acoustics, behavior and neurobiology [Chen et al. 2009; Eliades and Wang 2008; Jones et al. 1993; Miller et al. 2009a; Miller et al. 2009b; Miller and Wang 2006; Norcross and Newman 1993; Norcross and Newman 1997; Norcross et al. 1994; Pistorio et al. 2006]. Detailed acoustic analyses of the marmoset phee call in adults revealed acoustic cues for the caller's individual identity [Jones et al. 1993] and sex [Norcross and Newman 1993]. As these calls are primarily used for communicating with conspecifics occluded by vegetation or distance, other acoustic information may be encoded within the structure of this vocalization related to either the caller's identity or the behavioral context of the vocalization.
A critical vocal behavior exhibited by several species of nonhuman primates when visually occluded from conspecifics is antiphonal calling, a behavior involving the reciprocal exchange of species-specific contact calls between conspecifics [Biben 1993; Miller et al. 2001a]. This vocal behavior in marmosets involves their species-typical phee call [Chen et al. 2009; Miller and Wang 2006]. Our initial study showed that the timing of antiphonal calling exchanges changed due to the social relationship of the two animals engaged in the vocal interaction, suggesting that subjects recognize the caller's identity and relative relatedness [Miller and Wang 2006]. Subsequent interactive playback experiments showed that the timing of the antiphonal call response is critical to maintaining the behavior [Miller et al. 2009a], suggesting that, as in squirrel monkeys [Biben 1993], social rules govern the temporal pattern of the antiphonal call sequences. In order for such interactions to occur, however, marmosets must be able to extract specific categorical information about other callers from the acoustic structure of the phee alone.
Building on earlier work, here we sought to quantify the acoustic structure and communicative content of common marmoset phee calls during antiphonal calling. Identifying the various sources of acoustic variation in the call could provide insight into the types of information available to marmosets during antiphonal calling. We performed an acoustic analysis on a large corpus of phee calls to determine the various sources of communicative information available to conspecific signal receivers. Our analysis looks at three levels of information. First, we analyze the overall structure of the phee call in order to characterize its core spectro-temporal structure. Second, each vocalization communicates multiple levels of categorical information about the caller [Gerhardt 1992; Miller and Cohen 2010]. To examine the additional sources of acoustic information in the marmoset phee call, we used discriminant function analysis to test whether calls could be reliably classified based on the caller's individual identity, sex and group membership. And third, we tested whether changes in behavioral context affect the structure of phee calls. As the phees in this study were recorded during antiphonal calling exchanges between animals that varied in their social relationship, we analyzed whether consistent acoustic differences were evident in the call structure in these social scenarios.
Methods
Subjects
We recorded 1313 phee calls produced by 8 adult common marmosets (4 male, 4 female) housed at Johns Hopkins University (Baltimore, MD). The common marmoset is a small-bodied (∼400g), New World primate endemic to the rainforests of northeastern Brazil [Bezera and Souto 2008; Rylands 1993]. Subjects comprised the pair-bonded breeding pairs of 4 different social groups. These social groups consisted of their pair-bonded breeding pair and up to two generations of offspring. These groups had all been together for a minimum of one year prior to testing. Animals are given ad libitum access to water and are fed a diet consisting primarily of monkey chow and supplemented with other items such as fruit, nuts and yogurt. All experimental protocols were approved by the Johns Hopkins University Animal Use and Care Committee and complied with the American Society of Primatologists' Principles for the Ethical Treatment of Non Human Primates.
Acoustic Recording Procedure
We transported subjects from the colony to the testing room in transport cages. During transport, we prevented any visual recognition of the other individual in the experiment by ensuring that subjects were visually occluded from each other at all times. The testing room was 7m × 4m in size and had the walls covered completely in acoustic attenuating foam and a carpet floor. This testing room is situated a far distance from the colony room. Animals in the testing room could not hear any vocalizations produced by animals in the colony room. Once inside the room, we placed subjects in wire mesh cages – each animal in an individual cage - separated by 2m with an opaque cloth occluder equidistant between the two cages. Animals could interact vocally, but could not obtain visual cues from each other during the length of the experiment. We aimed a directional microphone (Sennnheiser ME-66: Frequency Response 50-20000Hz) at each cage and recorded (44.1kHz sampling rate) all vocalizations produced by subjects directly to the hard drive on either an Apple G4 powerbook or G5 Desktop computer using a Digidesign Mbox I/O device and Raven Bioacoustics Software (Cornell, Lab of Ornithology). Each test session lasted for 15 minutes. Following an experiment, we returned subjects to their home cage and cleaned the cages in the test room.
Behavioral Contexts
The vocalizations of all subjects were recorded in four different behavioral contexts. Three of these conditions consisted of pairing animals with individuals of different social relationships: Cagemate (CM), Non-Cagemate of the Same Sex (NCM-SS) and Non-Cagemate of the Opposite Sex (NCM-OS). For the fourth condition, the vocalizations produced by an isolated single animal in the test cage were recorded (ALONE). Subjects participated in each condition 3 times in randomized order. In the CM condition, subjects were always paired with their mate. For all behavioral conditions, we distinguished between phee calls produced as antiphonal and spontaneous calls. Following our earlier work [Miller et al. 2009a; Miller and Wang 2006], we considered a vocalization an antiphonal call if the marmoset produced a phee call within 10s of the other subject producing a phee call. All other phees were classified as spontaneous calls.
Acoustic Analysis
Phee calls were digitized as individual files for analysis. Using custom Matlab (Mathworks, Inc) code written by CTM, we analyzed the following spectro-temporal features for each phee call: call duration(s), inter-pulse interval(s), pulse duration(s), duration from phee onset to peak frequency(s), duration from peak frequency to phee offset(s), pulse start frequency(Hz), pulse end frequency(Hz), pulse mean frequency(Hz), pulse minimum frequency(Hz), pulse peak frequency(Hz), pulse delta frequency(Hz), Slope 1: slope from phee onset to peak frequency(Hz/s), Slope 2: slope from peak frequency to phee offset (Hz/s). The Matlab code used for this analysis was semi-automated. For each call, a spectrogram was generated and the onset and offsets of each pulse marked manually. The F0 contour was then extracted automatically from between these time events.
Statistical Analyses
All statistical analyses were performed using SPSS (v16.0). The data presented for the ‘Core Acoustic Structure’ section are descriptive and, as such, have no statistical tests. Analyses of the ‘Information Content’ and differences in phee calls between ‘Social Contexts’ primarily utilized discriminant function analyses (DFA). This test uses a multi-dimensional space of independent variables for predicting group membership to a specific categorical dependent variable. We utilized discriminant functions to test whether a model could be generated to correctly classify the ‘Information Content’ and ‘Social Context’ of phee calls based on the set of acoustic features. For cross-validation, half of the dataset for a particular test was chosen at random and used to build the function and then the second half of the data set was then run through the original function to test how accurately these new data were classified. As the same dataset was used in each of the three DFA tests, we used a Bonferroni corrected alpha level of p<0.01. We followed this analysis up with a nested permutation test in which the identity of the caller was nested in the analysis for the main effect of the sex and group identity of the caller. This analysis also determines the extent to which a category can be classified, but is considered a more conservative estimate as it accounts for variability that is specific to individual differences. In order to examine whether individual acoustic features were distinguishable along these experimental categories, we used multivariate multiple regression analysis. As this latter analysis involved 24 different variables that were repeatedly tested, a Bonferroni corrected significance level was used: p<0.002 (two-tailed).
Results
Core Acoustic Structure
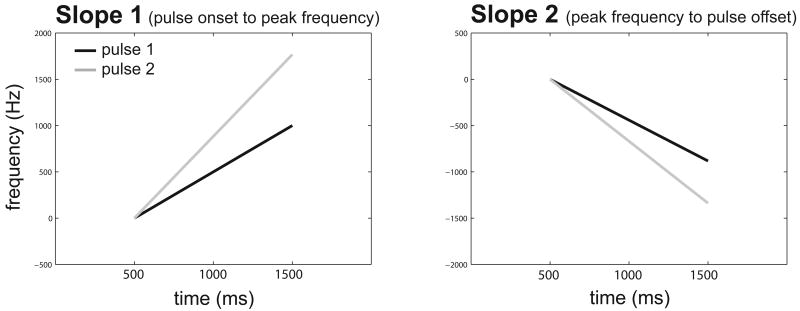
We recorded 1313 phee calls from 8 adult common marmosets. The number of calls recorded from each individual were as follows: Female-1: 84; Female-2: 214; Female-3: 275; Female-4: 90; Male-1: 81; Male-2: 271; Male-3: 140; Male-4: 158. The majority of these calls (n=865) consisted of 2 pulses (Figure 1A). The number of 2 pulse phee calls recorded from each individual were as follows: Female 1: 46; Female 2: 173; Female 3: 212; Female 4: 82; Male 1: 40; Male 2: 129; Male 3: 78; Male 4: 105. As the most typical phee calls consists of two pulses, our analyses focused on calls with this structure. We observed no difference in the number of pulses produced between any of the measures tested here (i.e. information content or social context). Figure 1 plots the mean (s.d.) for the temporal (Figure 1B) and spectral (Figure 1C) features measured in our analysis. Overall, the phee call is a tonal vocalization consisting of a series of relatively long duration, gradually frequency modulating pulses. Each pulse increases in frequency over its length followed by a rapid drop in frequency within ∼200-300ms of pulse cessation (Figure 1A). Both pulses have similar durations, though the first pulse (‘p1’) generally exhibits a smaller change in frequency (mean=1558.9Hz, s.d=27.04) than the second pulse (‘p2’) (mean=2426.7Hz, s.d=42.1). The second pulse in the phee call also typically has a higher mean and peak frequency, as well as a greater frequency bandwidth (Figure 1C). The differences in duration and frequency modulation are also reflected in the slopes of the two pulses. Data show that the second pulse of the marmoset phee call has a sharper onset and offset slope relative to the first pulse (Figure 2).
Figure 1.
Spectro-temporal structure of marmoset phee calls. A. A spectrogram of a phee call. B. Temporal features measured for all phee calls. Features measured in both the first and second pulses of the phee are noted by ‘p1’ (pulse 1) and ‘p2’ (pulse 2). The mean of each feature is noted with a ‘o’, error bars mark standard deviation. C. Spectral features measured for all phee calls. Features measured in both the first and second pulses of the phee are noted by ‘p1’ (pulse 1) and ‘p2’ (pulse 2). The mean of each feature is noted with a ‘*’, error bars mark standard deviation.
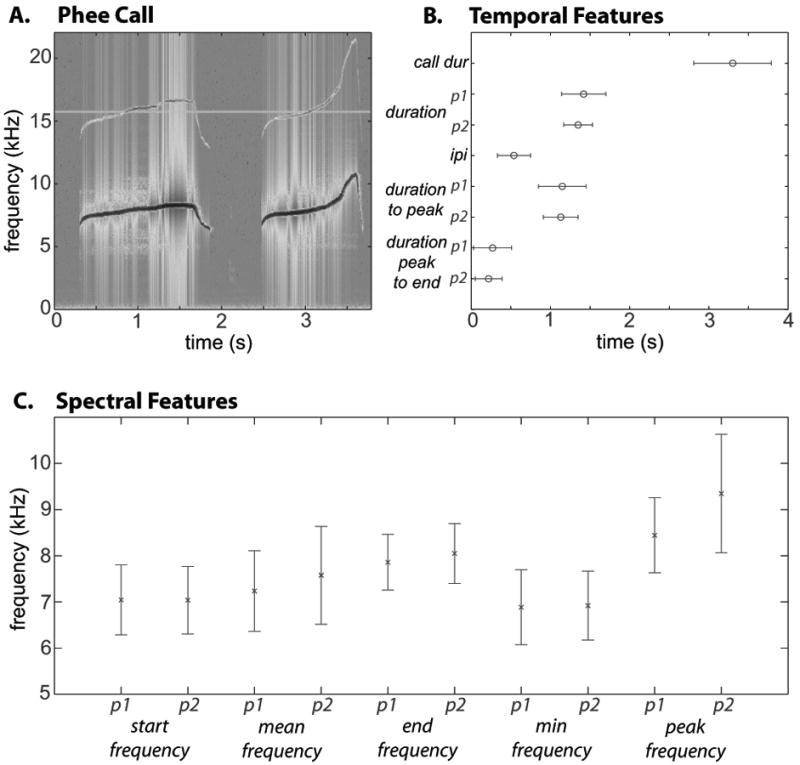
Figure 2.
Slopes for phee calls. Slope 1, shown to the left, plots the rising slope (Hz/s) in phee calls that occurs from the pulse onset to the peak frequency. Slope 2, shown to the right, plots the descending slope (Hz/s) from the peak frequency to the pulse onset. Pulse 1 is shown in the black line, while pulse 2 is shown by the grey line.
Information Content
We performed a series of discriminant function analyses to test whether phee calls could be correctly classified into distinct categories based on the acoustic structure. The first analysis tested the individual identity of the caller. The discriminant function performed here was able to correctly classify the individual caller 92.0% of the time, while a cross validation test correctly classified the caller 90.5% (Figure 3). The first two functions were able to account for 82% of the variance (F1: eigenvalue=10.25, wilks' lambda p <0.0001; F2: eigenvalue=8.68, wilks' lambda p<0.0001) suggesting that acoustic structure of the marmoset phee call during antiphonal calling is idiosyncratic for each caller.
Figure 3.
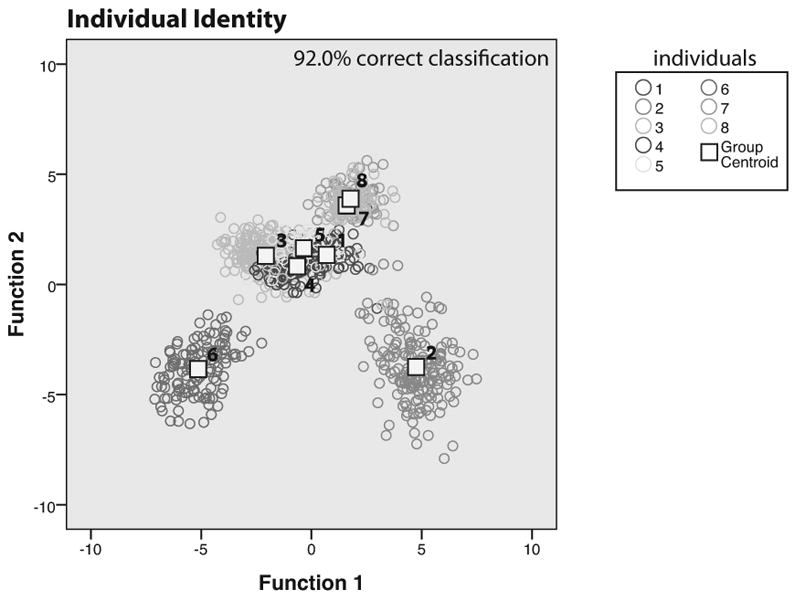
Discriminant functions for caller identity information. Plots the first and second functions from the discriminant function analysis for ‘Individual Identity’. Squares mark the group centroids for each of the 8 individuals whose calls were analyzed in the study, while colored open circles depict individual vocalizations produced by each individual.
Overall, the following acoustic features were significantly different between male and female phee calls: call duration, pulse duration (p1&2), duration to peak frequency (p1&2), duration from peak frequency to pulse end ((p1&2), start frequency (p2) end frequency (p1&2), mean frequency (p2), minimum frequency (p2), peak frequency (p2), delta frequency (p2), slope 1 (p1&2), slope 2 (p1&2). Discriminant functions performed here were able to correctly classify the call as being produced by either a male or female 92.5% of the time, while the cross validation test had a 91.9% correct classification. The function was able to account for 99% of the variance (eigenvalue: 1.863, wilks' lambda: p<0.0001). The more conservative nested permutation test, however, was not able to significantly classify the call as being produced by either a male or female. This analysis correctly classified the sex of the caller only 22% of the time, with a cross validation test of 31% correct classification.
To test whether common marmoset phee calls showed evidence of group signatures in the acoustic structure of phee calls, we performed a discriminant function using the original social group of subjects as the classifier. The 8 animals used in this analysis were the pair bonded adult animals in 4 different social groups. The analysis was able to correctly classify 87.1% of the phee calls to the appropriate social group, while the cross validation test classified 85.4% of the calls correctly. The first function alone was able to account for 84% of the variation (eigenvalue: 5.49, wilks' lambda: p<0.0001). The more conservative nested permutation test, however, was able to classify calls as being produced by a particular social group 60% of the time. The cross validation test was able to correctly classify calls in this analysis 53% of the time. Both are notably higher than the 25% correct classification that would be expected by chance.
Equal N Analysis
Given the variability in the number of phee calls contributed by each individual, we performed the same analysis with an equal sample of vocalizations for each marmoset (n=40). Overall the results were comparable to the above analyses using all the recorded phee calls. A DFA performed to test for individual identity in phee call structure was able to correctly classify the calls to the individual caller 97.5% of the time, while the cross-validation test correctly classified 93.1% of phees to the caller. The DFA performed to test for sex differences in phee calls correctly classified phees as either male or female 91.6% of the time and 90.3% in the cross-validation test. The final DFA tested for group signatures in the phee calls. This analysis correctly classified phees as belonging to one of the four groups for 91.3% of the vocalizations. The cross-validation test also performed well, correctly classifying 88.1% of the calls.
Social Context
During recording sessions, subjects were placed in the testing room either alone (ALO) or with a second conspecific in a visually occluded separate test cage. These paired recordings occurred with conspecifics that varied in social context. Specifically, the pair of subjects were either cagemates (CM), non-cagemates of the same sex (NCM-SS) or non-cagemates of the opposite sex (NCM-OS). A discriminant function, however, was only able to classify 42.8% of the phees to the correct social context. While this degree of classification is slightly above chance (25%), it does suggest considerable overlap in the acoustic structure of the phee call across these four social contexts. Thirteen individual acoustic features were significantly different across the contexts, though no consistent pattern was evident.
With the exception of the ALO context, subjects produced both spontaneous and antiphonal calls during these recording sessions. A discriminant function was able to correctly classify the calls as antiphonal or spontaneous only at chance (59.0%) suggesting that the global acoustic differences may not be consistent enough to determine their context. Two acoustic features, however, were significantly different between antiphonal and spontaneous calls. Both the end frequency (p < 0.0001) and peak frequency (p = 0.002) were significantly higher in the second pulse of spontaneously produced phee calls. Although the general structure of the phee call in these two contexts may be quite similar, particular features may signal whether the call was produced either spontaneously or as an antiphonal response.
Discussion
Vocalizations convey an assemblage of information. The aim here was to build on earlier work [Miller et al. 2009a; Miller and Wang 2006] and quantify the relationship between the acoustic structure of the marmoset phee call and the communicative content of the signal during antiphonal calling by correlating the changes in its spectro-temporal features with behaviorally meaningful levels of information. Clearly more detailed perceptual studies are needed to determine the extent to which the animals themselves attend to the different sources of communicative content in the signal [Fischer et al. 2001; Gerhardt 1991; Ghazanfar et al. 2002; Miller and Hauser 2004; Miller et al. 2005; Nelson and Marler 1989; Nowicki et al. 2001], but a detailed quantitative analyses of signal structure and any contextual changes that occur are necessary to guide these studies.
The phee call has a relatively stable, stereotyped acoustic structure (Figure 1) and is encoded with a rich array of categorical acoustic information available to conspecific signal receivers during antiphonal calling exchanges. Consistent with earlier work (Jones et al., 1993; Norcross & Newman, 1993), discriminant function analyses showed that phee calls produced during antiphonal calling exchanges contain acoustic signatures for the individual identity (Figure 3) of the caller. Like an earlier study of the cotton-top tamarin (Saguinus oedipus) [Weiss et al. 2001], a closely related Callitrichid species, the same analysis showed evidence of sex-specific signatures in the marmoset phee call. A more conservative permutation test, however, did not find the same type of classification suggesting that individual differences may underlie these other acoustic categories. This is somewhat surprising given that several studies of primates [Rendall et al. 2004], including cotton-top tamarins [Miller et al. 2004], found that individuals readily discriminated between the calls of males and females. More work is needed to resolve this issue and determine the relationship between the acoustic features of marmoset calls and how reliably the sex of a caller can be recognized by the conspecifics. Following in the tradition of earlier work in tamarins [Miller et al. 2001b; Miller et al. 2004; Weiss et al. 2001], future studies of the marmoset phee call will aim to perceptually test the functional salience and significance of the categorical information encoded in the acoustic structure of this vocalization.
The presence of consistent acoustic differences between individuals of different social groups indicates the presence of cage signatures in this colony. For such acoustic signatures to develop, animals must possess the necessary mechanisms for sensory-feedback and vocal control to modify their vocalizations by matching the acoustic properties of animals within the social group. Previous studies of other Callitrichid species showed similar evidence [Snowdon and Elowsen 1999; Weiss et al. 2001]. These cage signatures are particularly interesting because all animals within the colony are able to hear the vocalizations of all the other animals. Common marmosets' ability to develop signatures under captive conditions may be related to the regional dialects reported in wild populations of pygmy marmosets (Cebuella pygmaea) [De la Torre and Snowdon 2009]. One possible explanation for the extensive evidence of group signatures and dialects in Calltrichid species may relate to their strong territoriality [Garber et al. 1993; Lazaro-Perea 2001]. In addition to physical territorial markers, vocalizations may provide a further means of making an in-group/out-group distinction. Although historically, many believed nonhuman primates possessed little or no control over their vocalizations [Egnor and Hauser 2004], recent evidence suggests a more sophisticated system of vocal control in this taxonomic group [Egnor et al. 2006; Egnor et al. 2007; Miller et al. 2009b; Miller et al. 2003; Suguira 1998]. Callitrichids in particular appear to possess one of the most extensive systems of vocal control in primates.
The common marmoset phee call is rich with communicative information. Despite its stereotyped structure, subtle changes in spectro-temporal features yield at least three stable sources about the caller: individual identity, sex, and social group. In all, this study shows that common marmosets are provided with a diverse array of information when hearing a phee call during antiphonal calling. The extent to which this information is perceived and used by receivers, however, is not known. Future studies will build on this result to experimentally test the perceptual and social significance of the acoustic information available in the phee call during antiphonal calling at both the behavioral and neural levels.
Acknowledgments
We thank Yi Zhou for her helpful comments on this manuscript and Roger Mundry for his generous help performing the permutation test analyses. This work was supported by grants from the NIH to CTM (F32 DC007022, K99 DC009007) and XW (R01 DC005808). All experimental protocols were approved by the Johns Hopkins University Animal Use and Care Committee and complied with the American Society of Primatologists' Principles for the Ethical Treatment of Non Human Primates (www.asp.org/society/resolutions/EthicalTreatmentofNonHumanPrimates.html).
Literature Cited
- Bezera BM, Souto A. Structure and usage of the vocal repertoire of Callithrix jacchus. International Journal of Primatology. 2008;29:671–701. [Google Scholar]
- Biben M. Recognition of order effects in squirrel monkey antiphonal call sequences. American Journal of Primatology. 1993;29:109–124. doi: 10.1002/ajp.1350290204. [DOI] [PubMed] [Google Scholar]
- Chen HC, Kaplan G, Rogers LJ. Contact calls of common marmosets (Callithrix jacchus): influence of age of caller on antiphonal calling and other vocal responses. American Journal of Primatology. 2009;71:165–170. doi: 10.1002/ajp.20636. [DOI] [PubMed] [Google Scholar]
- De la Torre S, Snowdon CT. Dialiects in pygmy marmosets? population variation in call structure. American Journal of Primatology. 2009;71:1–10. doi: 10.1002/ajp.20657. [DOI] [PubMed] [Google Scholar]
- Egnor SER, Hauser MD. A paradox in the evolution of primate vocal learning. Trends in Neurosciences. 2004;27:649–654. doi: 10.1016/j.tins.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Egnor SER, Iguina C, Hauser MD. Perturbation of auditory feedback causes systematic pertubation in vocal structure in adult cotton-top tamarins. Journal of Experimental Biology. 2006;209:3652–3663. doi: 10.1242/jeb.02420. [DOI] [PubMed] [Google Scholar]
- Egnor SER, Wickelgren JG, Hauser MD. Tracking silence: adjusting vocal production to avoid acoustic interference. Journal of Comparative Physiology A. 2007;193:477–483. doi: 10.1007/s00359-006-0205-7. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Epple G. Comparative studies on vocalizations in marmoset monkeys. Folia primatologica. 1968;8:1–40. doi: 10.1159/000155129. [DOI] [PubMed] [Google Scholar]
- Fischer J, Metz M, Cheney DL, Seyfarth RM. Baboon responses to graded bark variants. Animal Behaviour. 2001;61:925–931. [Google Scholar]
- Garber PA, Pruetz JD, Isaacson J. Patterns of range use, range defense and intergroup spacing in moustached tamarin monkeys (Saguinus mystax) Primates. 1993;34:11–25. [Google Scholar]
- Gerhardt HC. Female mate choice in treefrogs: static and dynamic acoustic criteria. Animal Behaviour. 1991;42:615–636. [Google Scholar]
- Gerhardt HC. Multiple messages in acoustic signals. Seminars in the Neurosciences. 1992;4:391–400. [Google Scholar]
- Ghazanfar AA, Smith-Rohrberg D, Pollen A, Hauser MD. Temporal cues in the antiphonal calling behaviour of cotton-top tamarins. Animal Behaviour. 2002;64:427–438. [Google Scholar]
- Jones BA, Harris DHR, Catchpole CK. The stability of the vocal signature in phee calls of the common marmoset, Callithrix jacchus. American Journal of Primatology. 1993;31:67–75. doi: 10.1002/ajp.1350310107. [DOI] [PubMed] [Google Scholar]
- Lazaro-Perea C. Integroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Animal Behaviour. 2001;62:11–21. [Google Scholar]
- Miller CT, Beck K, Meade B, Wang X. Antiphonal call timing in marmosets is behaviorally significant: Interactive playback experiments. Journal of Comparative Physiology A. 2009a;195:783–789. doi: 10.1007/s00359-009-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Cohen YE. Vocalizations as auditory objects: behavior and neurophysiology. In: Platt M, Ghazanfar AA, editors. Primate Neuroethology. New York, NY: Oxford University Press; 2010. pp. 237–255. [Google Scholar]
- Miller CT, Dibble E, Hauser MD. Amodal completion of acoustic signals by a nonhuman primate. Nature Neuroscience. 2001a;4:783–784. doi: 10.1038/90481. [DOI] [PubMed] [Google Scholar]
- Miller CT, Eliades SJ, Wang X. Motor-planning for vocal production in common marmosets. Animal Behaviour. 2009b;78:1195–1203. doi: 10.1016/j.anbehav.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Flusberg S, Hauser MD. Interruptibility of cotton-top tamarin long calls: implications for vocal control. Journal of Experimental Biology. 2003;206:2629–2639. doi: 10.1242/jeb.00458. [DOI] [PubMed] [Google Scholar]
- Miller CT, Hauser MD. Multiple acoustic features underlie vocal signal recognition in tamarins: antiphonal calling experiments. Journal of Comparative Physiology A. 2004;190:7–19. doi: 10.1007/s00359-003-0468-1. [DOI] [PubMed] [Google Scholar]
- Miller CT, Iguina C, Hauser MD. Processing vocal signals for recognition during antiphonal calling. Animal Behaviour. 2005;69:1387–1398. [Google Scholar]
- Miller CT, Miller J, Costa RGD, Hauser MD. Selective phontaxis by cotton-top tamarins (Saguinus oeidpus) Behaviour. 2001b;138:811–826. [Google Scholar]
- Miller CT, Scarl JS, Hauser MD. Sensory biases underlie sex differences in tamarin long call structure. Animal Behaviour. 2004;68:713–720. [Google Scholar]
- Miller CT, Wang X. Sensory-motor interactions modulate a primate vocal behavior: antiphonal calling in common marmosets. Journal of Comparative Physiology A. 2006;192:27–38. doi: 10.1007/s00359-005-0043-z. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Marler P. Categorical perception of a natural stimulus continuum: Birdsong. Science. 1989;244:976–978. doi: 10.1126/science.2727689. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Context and gender specific differences in the acoustic structure of common marmoset (Callithrix jacchus) phee calls. American Journal of Primatology. 1993;30:37–54. doi: 10.1002/ajp.1350300104. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Social context affects phee call production by nonreproductive common marmosets (Callithrix jacchus) American Journal of Primatology. 1997;43:135–146. doi: 10.1002/(SICI)1098-2345(1997)43:2<135::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD, Fitch WT. Responses to natural and synthetic phee calls by common marmosets. American Journal of Primatology. 1994;33:15–29. doi: 10.1002/ajp.1350330103. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Searcy WA, Hughes M, Podos J. The evolution of bird song: male and female response to song innovation in swamp sparrows. Animal Behaviour. 2001;135:615–628. [Google Scholar]
- Pistorio A, Vintch B, Wang X. Acoustic analyses of vocal development in a New World primate, the common marmoset (Callithrix jacchus) Journal of the Acoustical Society of America. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Rendall D, Owren MJ, Weerts E, Hienz RD. Sex differences in the acoustic structure of vowel-like vocalizations in baboons and their perceptual discrimination by baboon listeners. Journal of the Acoustical Society of America. 2004;115:411–421. doi: 10.1121/1.1635838. [DOI] [PubMed] [Google Scholar]
- Rylands AB. Marmosets and Tamarins: Systematics, Behaviour, and Ecology. Oxford, UK: Oxford University Press; 1993. [Google Scholar]
- Snowdon CT, Elowsen AM. Pygmy marmosets modify call structure when paired. Ethology. 1999;105:893–908. [Google Scholar]
- Suguira H. Matching of acoustic features during the vocal exchange of coo calls by Japanese macaques. Animal Behaviour. 1998;55:673–687. doi: 10.1006/anbe.1997.0602. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Garibaldi BT, Hauser MD. The production and perception of long calls by cotton-top tamarins (Saguinus oedipus): Acoustic analyses and playback experiments. Journal of Comparative Psychology. 2001;11:258–271. doi: 10.1037/0735-7036.115.3.258. [DOI] [PubMed] [Google Scholar]