Abstract
Major features of the transcellular signaling mechanism responsible for endothelium-dependent regulation of vascular smooth muscle tone are unresolved. We identified local calcium (Ca2+) signals (“sparklets”) in the vascular endothelium of resistance arteries that represent Ca2+ influx through single TRPV4 cation channels. Gating of individual TRPV4 channels within a four-channel cluster was cooperative, with activation of as few as three channels per cell causing maximal dilation through activation of endothelial cell intermediate (IK)- and small (SK)-conductance, Ca2+-sensitive potassium (K+) channels. Endothelial-dependent muscarinic receptor signaling also acted largely through TRPV4 sparklet-mediated stimulation of IK and SK channels to promote vasodilation. These results support the concept that Ca2+ influx through single TRPV4 channels is leveraged by the amplifier effect of cooperative channel gating and the high Ca2+ sensitivity of IK and SK channels to cause vasodilation.
Endothelial cells (ECs) line all blood vessels and regulate the smooth muscle contractile state (tone). The concentration of intracellular free calcium ([Ca2+]i) in ECs is increased by influx and by release from intra-cellular stores through inositol trisphosphate receptors (IP3Rs) in the membrane of the endoplasmic reticulum. Although Ca2+-influx pathways are incompletely characterized, members of the transient receptor potential (TRP) family of nonselective cation channels have been implicated in this function. In particular, results from gene-knockout studies suggest that the vanilloid (TRPV) family member TRPV4 is involved in endothelium-dependent vascular dilation in response to flow and acetylcholine (ACh) (1–5).
Increases in endothelial [Ca2+]i activate EC pathways that terminate in the release of soluble factors or initiation of processes that hyperpolarize the membrane of adjacent vascular smooth muscle cells, and thus promote dilation. These Ca2+-dependent vasodilatory influences fall into three broad categories: (i) nitric oxide (NO), a tissue-permeable gas generated as a by-product of the oxidation of arginine to citrulline catalyzed by endothelial nitric oxide synthase (eNOS) (6); (ii) prostaglandins, produced through phospholipase A2–dependent activation of cyclooxygenase (COX) (7); and (iii) endothelial-derived hyper-polarizing factor (EDHF), characterized by its strict dependence on the activity of EC intermediate-conductance (IK; KCa3.1) and small-conductance (SK; KCa2.3), Ca2+-sensitive potassium (K+) channels (8). Although a number of factors have been suggested as EDHF, accumulating evidence points to the importance of electrotonic spread of EC IK and/or SK channel–mediated hyperpolarizing current to smooth muscle cells through gap junctions (8, 9).
Studies of Ca2+ signaling in ECs using conventional Ca2+-binding fluorescent dyes (e.g., Fluo-4) are limited by interference from the vigorous Ca2+-signaling activity of adjacent smooth muscle cells, which also readily take up such dyes. A recently developed alternative is a transgenic mouse that expresses a genetically encoded Ca2+ biosensor (GCaMP2) exclusively in the endothelium of the vascular wall (10, 11). GCaMP2 is a fusion protein of the Ca2+-binding protein calmodulin and a circularly permutated enhanced green fluorescent protein (EGFP) that fluoresces when Ca2+ binds to calmodulin. The GCaMP2 protein is homogeneously expressed throughout the EC (10) and allows long, stable recordings of intracellular Ca2+ in ECs in the intact blood vessel wall, without contamination of signals from smooth muscle. Using this model, we previously identified local, IP3R-mediated Ca2+ events in ECs, termed Ca2+ pulsars (10), that had previously gone undetected with conventional imaging protocols.
To identify Ca2+-influx pathways in the ECs of resistance arteries (i.e., arteries important in regulating peripheral resistance and blood pressure), we imaged Ca2+ fluorescence in isolated, small (100 μm diameter) mesenteric arteries from GCaMP2 mice using confocal microscopy (12). Isolated arteries were surgically opened and pinned down with the EC surface facing up (en face preparation) to improve optical resolution (10). In a single field of view, local Ca2+ signals in ~14 individual ECs could be recorded simultaneously with high spatial (0.3 μm) and temporal (15 ms) resolution. Events were analyzed offline by measuring the fluorescence intensity over time within defined 1.7-μm2 regions of interest on images corresponding to active sites.
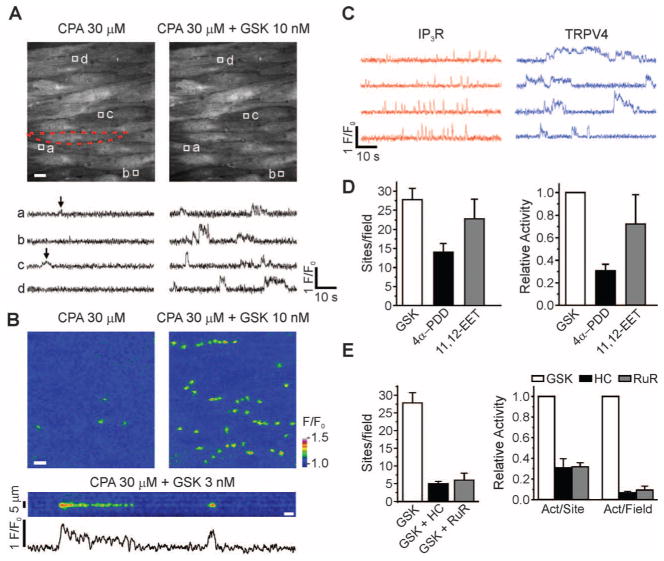
With IP3R-mediated signaling eliminated by pretreatment with the sarcoplasmic reticulum/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitor, cyclopiazonic acid (CPA), or the phospholipase C (PLC) blocker, U73122 (10), local Ca2+ signals distinct from pulsars could be detected [Fig. 1, A (left) and B (upper left); fig. S2A (left); and movie S1], albeit at a very low frequency [2.8 ± 0.8 (SEM) event sites per field per 2 min; n = 5], possibly reflecting extracellular Ca2+ influx. Exposure of the endothelial surface to the potent, selective TRPV4 channel agonist GSK1016790A (GSK; 10 nM) (13, 14) induced a marked increase [30.4 ± 2.5 (SEM)–fold, n = 5] in the activity of these Ca2+ signals [Fig. 1, A (right) and B (upper right); fig. S2, A and B; and movie S2], without increasing global [Ca2+]i (Fig. 1B and movies S1 to S4).
Fig. 1.
GSK-induced Ca2+ signals represent Ca2+ influx through plasmalemmal TRPV4 channels. (A) Ca2+ imaging of an en face preparation of third-order mesenteric arteries from a GCaMP2-expressing mouse, showing changes in the activity of local, IP3R-independent Ca2+ signals recorded over time (traces, below) from regions of interest (1.7 μm2), denoted by boxes in images (above). An EC in the field is outlined by red dashes (above, left). Scale bar: 10 μm. Bottom left: Localized events (arrows) detected in the absence of IP3R-mediated signaling; bottom right: localized events after addition of GSK (10 nM). (B) Top panels: Pseudocolor overlay images showing all IP3R-independent Ca2+ signaling events detected in the absence or presence of GSK (10 nM) in a single field over a 94-s interval. Scale bar: 10 μm. Bottom panel and trace: Pseudo–line-scan image and associated trace recorded from a single site over the same interval. Scale bar: 2 s. Calibration bar at right indicates intensity of signals (F/F0; fluorescence F divided by baseline fluorescence F0). (C) Representative traces illustrating differences in the kinetic properties of IP3R-mediated Ca2+ pulsars (left) and TRPV4-mediated Ca2+ events (right) from the same field of view in the presence of 10 nM GSK (without CPA). (D) Increased numbers of sites per 2 min per field and activity per field (right; n = 4 to 6 arteries) in the presence of GSK (10 nM), 4α-PDD (5 μM), or 11,12-EET (1 μM). (E) Inhibition of GSK-induced increases in activity per site and activity per field by HC (1 μM; n = 5) and RuR (5 μM; n = 3). Error bars (C and D), SEMs.
Unlike Ca2+ pulsars, which are brief (<300 ms), spikelike signals, GSK-induced Ca2+ events exhibited a plateau phase with discrete amplitudes (Fig. 1C). Their spatial spread within an EC was 11.2 ± 0.4 (SEM) μm2 (50 sites, n = 5 arteries), or about 0.6% of the total cell surface area, and they occurred repetitively at the same sites (Fig. 1, A and B). Also in contrast to Ca2+ pulsars, which predominantly occur at “holes” in the internal elastic lamina (IEL) corresponding to the locations of endothelial projections to smooth muscle (10), TRPV4-mediated Ca2+ signals were approximately evenly distributed between such holes (39%) and the ends of cells (31%; fig. S2B).
4α-Phorbol 12,13-didecanoate (4α-PDD), a pharmacological activator of TRPV4 channels, and 11,12-epoxyeicosatrienoic acid (11,12-EET), an endogenous activator of TRPV4 channels, induced IP3R-independent Ca2+ signals comparable to GSK-induced events (Fig. 1D and fig. S3C). The selective TRPV4 channel antagonist HC-067047 (HC; 1 μM) (13, 15), and the non-selective TRPV pore blocker ruthenium red (RuR; 5 μM), inhibited GSK (10 nM)–induced Ca2+ signals by about 93%, reducing both the number of sites and the activity per site (Fig. 1E and figs. S2D and S3B). Moreover, GSK had no effect on arteries from TRPV4−/− mice [P = 0.88, n = 5; paired two-sample t test (12)]. Notably, rapid removal of external Ca2+ eliminated GSK-induced Ca2+ events (n = 4; fig. S2C). Thus, these IP3R-independent optical signals reflect Ca2+ influx through plasmalemmal TRPV4 channels.
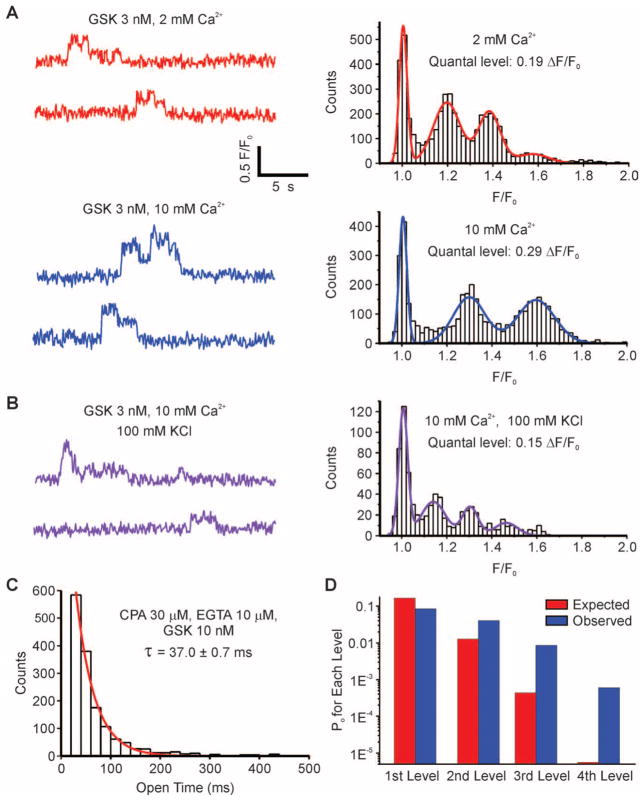
TRPV4-mediated Ca2+ signals exhibited characteristics of single channels, including square, discrete amplitudes (Fig. 1, A to C; fig. S2, A, C, and D; and fig. S3, A to C). Such fluorescence signals can be inferred to represent Ca2+ influx through single channels if they meet the following criteria (16): (1) Recording volume is very small (<1 fL); (2) events are quantal (i.e., exhibit fixed amplitude steps); (3) amplitude steps depend on the Ca2+ electrochemical gradient; (4) amplitude steps do not depend on the concentration or nature of the agonist or antagonist; (5) durations of events are exponentially distributed; and (6) channels have high Ca2+ permeability and single-channel conductance. The first criterion may exist naturally for native ECs, which are about 0.5 μm thick (yielding an approximate recording volume of 0.8 fL), and satisfaction of the last criterion has been demonstrated for TRPV4 channels (17). A fit of multiple Gaussians to an all-points histogram of fluorescence signals (Fig. 2A) (12) revealed quantal amplitudes (criterion 2), with evenly spaced F/F0 increments (F/F0: change in fluorescence relative to baseline fluorescence) of 0.19 with 2 mM extracellular Ca2+. Increasing extracellular Ca2+ from 2 to 10 mM increased the amplitude of these events (Fig. 2A), and membrane depolarization with 100 mM K+ decreased their amplitude (Fig. 2B), indicating a dependence on the Ca2+ electrochemical gradient (criterion 3). Moreover, these events were independent of the concentration and nature of the agonist or antagonist used (criterion 4): Increasing GSK concentration from 3 to 10 nM increased activity (fluorescence integral) 3.7 ± 0.8 (SEM)–fold (n = 3 arteries; fig. S3A, left), but did not increase amplitude (fig. S3A, right); the specific TRPV4 inhibitor HC reduced activity, but not amplitude (fig. S3B); and other TRPV4 channel activators (4α-PDD and 11,12-EET) did not differentially affect amplitudes (fig. S3C). Finally, the durations of GSK (3 nM)–induced events were exponentially distributed (criterion 5), with a time constant of 37.0 ± 0.7 ms (Fig. 2C) (12). Thus, each quantal level appears to represent Ca2+ influx through a single TRPV4 channel in the plasma membrane of a vascular EC. By analogy to the L-type Ca2+ channel–mediated events first described in cardiac myocytes (18), we use the term TRPV4 sparklet to refer to the optically detected influx of extracellular Ca2+ through a single TRPV4 channel.
Fig. 2.
TRPV4 Ca2+ sparklets as unitary events that exhibit cooperative activation. (A) A fit of multiple Gaussians to all-points histograms showing evenly spaced ΔF/F0 levels of 0.19 at 2 mM extracellular Ca2+ and 0.29 at 10 mM Ca2+. (B) Effect of decreasing Ca2+ electrochemical gradient by membrane depolarization with 100 mM K+ on Ca2+ signals. (C) Exponential distribution of the durations of GSK (10 nM)–induced sparklets with Fluo-4 and 10 μM EGTA-AM. Sampling rate, 50 to 60 images/s. τ, time constant. (D) Cooperative channel gating demonstrated by deviation of the frequencies of second, third, and fourth levels (measured as open probabilities, PO) from a binomial distribution predictive of independent events. Same conditions as in (C).
Each sparklet site exhibited up to four quantal levels interspersed with long quiescent periods. If these discrete amplitude steps represent independently functioning channels, their probabilities should follow a binomial distribution. The observed distribution deviated significantly from a binomial distribution (P < 0.01; χ2 test), with an excess of double, triple, and quadruple events (Fig. 2D) (12), indicating that TRPV4 channels open cooperatively, likely reflecting potentiation of nearby TRPV4 channels by intracellular Ca2+ (19). The degree of spread and termination of sparklet activity may reflect inhibition by higher concentrations of intracellular Ca2+ (17). A fifth level, which should have been resolvable under the imaging conditions used, was not reliably detected, suggesting that TRPV4 channels form a four-channel metastructure.
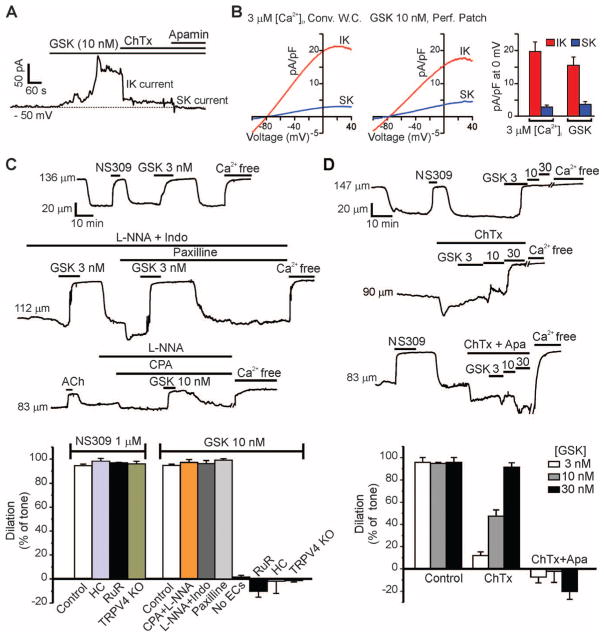
Ca2+-sensitive IK and SK channels are potentially important targets of Ca2+ influx through TRPV4 channels (20). Measurement of membrane currents in freshly isolated ECs using the perforated-patch configuration of the patch-clamp technique (12) showed that, at physiological salt concentrations and voltages (−50 mV), GSK (10 nM) induced an outward current that was substantially reduced by the IK blocker charybdotoxin (ChTx) and further reduced by subsequent addition of the SK blocker apamin (Fig. 3A). Using a voltage-ramp protocol (−100 to +40 mV, 200 ms), we found that activation of TRPV4 channels with GSK (10 nM) increased IK and SK channel current densities to 15.6 ± 2.5 and 3.6 ± 0.9 pA/pF (±SEM; n = 5 cells), respectively, at 0 mV, values similar to maximal IK and SK current densities obtained by dialyzing cells with 3 μM Ca2+ (Fig. 3B).
Fig. 3.
Activation of IK and SK channels and induction of EDHF-dependent vasodilation by Ca2+ influx through TRPV4 channels. (A) Inhibition of GSK (10 nM)–induced outward current by 300 nM ChTx (n = 5 cells) or 300 nM apamin (n = 5 cells). (B) Densities of IK and SK channel currents in cells treated with GSK (10 nM) or in cells dialyzed with 3 μM Ca2+ (n = 8 cells). Conv. W.C., conventional whole-cell configuration; Perf. Patch, perforated-patch configuration. (C) Top trace: GSK (3 nM)–induced vasodilation of pressurized (80 mmHg) mesenteric arteries; middle trace: effects of 100 μM L-NNA + 10 μM indomethacin (Indo; n = 5), or 1 μM paxilline (n = 3) on GSK-induced dilations; bottom trace: effects of L-NNA and 30 μM CPA (n = 5) on GSK (10 nM)–induced dilations; bar graph (left): absence of effects of the TRPV4 antagonists HC (1 μM; n = 4) and RuR (5 μM; n = 4), and TRPV4 knockout (n = 3) on dilations induced by the IK and SK agonist NS309 (1 μM); bar graph (right): quantification of effects of CPA + L-NNA (n = 5), L-NNA + Indo (n = 5), paxilline (n = 3), endothelium removal (n = 5), RuR (n = 5), HC (n = 5), and TRPV4 knockout (n = 4) on GSK (10 nM)–induced dilations. (D) Vasodilation in response to GSK (3, 10, 30 nM) in the presence or absence of 200 nM ChTx (n = 7), or ChTx + 300 nM apamin (Apa). Effects on dilation [bar graphs in (C) and (D)] determined relative to initial tone [26 ± 1% (SEM), n = 10], defined as the percentage decrease in arterial diameter to pressure (80 mmHg) relative to the diameter in external Ca2+-free solution. Error bars (B to D), SEMs.
Our imaging data indicated that GSK (≤10 nM) activates a small number of channels per cell [average activity, 2.8 ± 0.4 (SEM) sparklets per cell with 10 nM GSK; n = 15 cells from five fields]. To estimate the number of TRPV4 channels per cell, we measured whole-cell K+ currents through TRPV4 channels at +100 mV under conditions that minimize Ca2+ entry through TRPV4 (12). Under these conditions, TRPV4 current was 63 ± 27 pAwith 10 nM GSK and 1228 ± 242 pA with 100 nM GSK (±SEM; n = 5 cells each; fig. S4), currents that correspond to the activation of 8 and 152 channels per cell, respectively.
Although TRPV4 activation by 3 to 10 nM GSK opened only a few TRPV4 channels in an EC (fig. S5 and movie S5), it hyperpolarized the smooth muscle membrane potential by about 10 mV (fig. S6) and led to a maximal, endothelium-dependent dilation of pressurized arteries (Fig. 3C) (12). GSK-induced dilations were blocked by RuR and HC and were absent in arteries from TRPV4−/− mice (Fig. 3C), conditions that did not affect dilations in response to the direct IK and SK channel agonist NS309 (Fig. 3C); they were also eliminated by removing the endothelium and unaffected by inhibition of smooth muscle Ca2+-sensitive K+ (BK) channels with paxilline (Fig. 3C). Notably, TRPV4-mediated dilations induced by 10 nM GSK were unaffected by inhibition of NOS and COX with nitro-L-arginine (L-NNA) and indomethacin, respectively (Fig. 3C). Block of IK channels with ChTx eliminated the dilation in response to 3 nM GSK and greatly reduced the dilation in response to 10 nM GSK (Fig. 3D). Treatment with apamin in the presence of ChTx completely prevented dilations in response to 10 and 30 nM GSK (Fig. 3D), indicating that the greater activation of TRPV4 channels at these higher GSK concentrations resulted in increased signaling through SK channels. Thus, local Ca2+ influx through TRPV4 channels acts through IK and SK channels and not through eNOS or COX, demonstrating a specific link between TRPV4 activation and the EDHF pathway of endothelial-mediated vascular dilation.
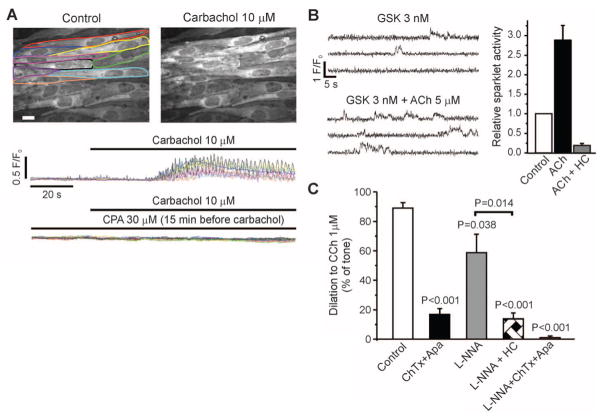
Muscarinic receptor activation by ACh or carbachol (CCh) induces vasodilation through both NO and EDHF mechanisms (1–6, 8, 9). The NO-dependent component of ACh-induced dilation is modestly reduced in larger mesenteric arteries from TRPV4−/− mice, whereas the EDHF-mediated dilation is more considerably reduced (3–5). Thus, NO-mediated dilation in response to muscarinic receptor activation may be driven primarily by IP3R-mediated increases in global Ca2+ and Ca2+ pulsars, whereas the EDHF component may reflect the action of local increases in Ca2+ influx through a small number of TRPV4 channels. Global Ca2+ and Ca2+ pulsars were increased by muscarinic receptor stimulation and were eliminated by blocking PLC or SERCA (Fig. 4A) (10). ACh (5 μM) alone increased basal sparklet activity in the absence of IP3R-mediated Ca2+ release and TRPV4 agonist [2.3 ± 0.3 (SEM)–fold, n = 4 arteries], indicating that the muscarinic signaling pathway can increase Ca2+ influx through TRPV4 channels. In the presence of a submaximal concentration of GSK (3 nM), ACh (5 μM) increased TRPV4 sparklet activity by 2.9 ± 0.4 (SEM)–fold (n = 5), an effect that was inhibited by the TRPV4 antagonist HC (Fig. 4B). Endothelial-dependent dilation by CCh of arteries constricted by intravascular pressure (80 mmHg) was dependent on NO (34%) and EDHF (66%) (Fig. 4C). Our demonstration that muscarinic receptor stimulation induces TRPV4 sparklets suggests that this pathway should cause vasodilation. Indeed, TRPV4 inhibition by HC reduced muscarinic receptor–induced EDHF dilations by 76% (Fig. 4C), indicating that the EDHF component of these dilations largely reflects TRPV4 sparklet–mediated activation of IK and/or SK channels.
Fig. 4.
Effects of muscarinic receptor activation on global Ca2+ and TRPV4 sparklets. (A) Global Ca2+, shown as fractional fluorescence from outlined whole cells treated with CCh (10 μM) with and without CPA (30 μM). (B) GSK (3 nM)–induced sparklets in the presence or absence of 5 μM ACh (left) and 1 μM HC (right; n = 5). (C) Effects of 200 nM ChTx + 300 nM apamin (Apa; n = 5), 100 μM L-NNA (n = 6), L-NNA + HC (n = 5), and ChTx + Apa + L-NNA (n = 4) on dilations in response to the muscarinic agonist CCh (1 μM). Error bars (B and C), SEMs. Except where indicated by a bracket (Student’s t test), P-values are for comparison to control (one-way analysis of variance).
Our results demonstrate that a small number of active TRPV4 channels (about three to eight per cell) mediate local Ca2+ signals that activate IK and SK channels (primarily IK) to cause maximal dilation of resistance arteries. We propose that cooperative activation of TRPV4 channels in a cluster leverages the large Ca2+ influx through a single TRPV4 channel to produce a more substantial Ca2+ signal (fig. S1), which is further boosted by the high Ca2+ sensitivity of IK and SK channels, conferred by calmodulin (20). The current caused by activation of IK and SK channels is likely spread to the surrounding smooth muscle through myoendothelial gap junctions, resulting in hyperpolarization of smooth muscle cells and vasodilation (8, 9). IK channels are localized to endothelial projections where a portion (39%) of TRPV4 sparklets occur and myoendothelial gap junctions are concentrated (10, 21, 22), an arrangement that may facilitate activation of IK channels by a local Ca2+ signal.
Whereas low-level activation of TRPV4 channels with synthetic agonists (e.g., 3 to 10 nM GSK) or via muscarinic receptor stimulation caused significant vasodilation (P < 0.0001; paired two-sample t test), higher-level activation (100 nM GSK) led to rapid global Ca2+ overload in ECs and oscillations of blood-vessel diameter (fig. S7 and movie S6). Notable in this context, systemic activation of TRPV4 channels by GSK causes a reduction in blood pressure and generalized circulatory failure (14). Collectively, these observations indicate that small numbers of EC TRPV4 channels regulate vascular physiology and suggest that pathologies characterized by blood-pressure reduction and vascular permeability increases (e.g., septic shock) may involve excessive activation of EC TRPV4 channels.
Supplementary Material
Acknowledgments
We thank HydraBiosciences for HC-067047, Neurosearch A/S for NS309, and J. E. Brayden, K. Freeman, M. Koide, L. W. Nausch, and G. C. Wellman for comments on the manuscript. This work was supported by a grant from the COBRE Program of the National Center for Research Resources funded by the NIH (2-P20-RR-016435-06), an award from the American Heart Association Founders Affiliate and the Pulmonary Hypertension Association (10POST3690006) to S.K.S, and by grants NIH GM086736 to M.I.K.; FRSQ (Junior 1)/NIA (HSFC) to J.L.; and NIH HL044455, 1P01HL095488, R37DK053832, R01HL098243, and the Totman Medical Research Trust to M.T.N.
Footnotes
The authors have no conflicts of interest.
References and Notes
- 1.Hartmannsgruber V, et al. PLoS ONE. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendoza SA, et al. Am J Physiol Heart Circ Physiol. 2010;298:H466. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saliez J, et al. Circulation. 2008;117:1065. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 4.Zhang DX, et al. Hypertension. 2009;53:532. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earley S, et al. Am J Physiol Heart Circ Physiol. 2009;297:H1096. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming I, Busse R. J Mol Cell Cardiol. 1999;31:5. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- 7.Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD. Microvasc Res. 2005;69:107. doi: 10.1016/j.mvr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Edwards G, Félétou M, Weston AH. Pflugers Arch. 2010;459:863. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 9.Coleman HA, Tare M, Parkington HC. Clin Exp Pharmacol Physiol. 2004;31:641. doi: 10.1111/j.1440-1681.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 10.Ledoux J, et al. Proc Natl Acad Sci USA. 2008;105:9627. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tallini YN, et al. Circ Res. 2007;101:1300. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 12.Materials and methods are available as supplementary materials on Science Online.
- 13.GlaxoSmithKline compound GSK1016790A: (N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl] amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide; HydraBiosciences compound HC067047: (2-methyl-1-[3-{4-morpholinyl}propyl]-5-phenyl-N-[3-{trifluoro-methyl}phenyl]-1H-pyrrole-3-carboxamide.
- 14.Willette RN, et al. J Pharmacol Exp Ther. 2008;326:443. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- 15.Everaerts W, et al. Proc Natl Acad Sci USA. 2010;107:19084. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker I, Smith IF. J Gen Physiol. 2010;136:119. doi: 10.1085/jgp.200910390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everaerts W, Nilius B, Owsianik G. Prog Biophys Mol Biol. 2010;103:2. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang SQ, Song LS, Lakatta EG, Cheng H. Nature. 2001;410:592. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 19.Strotmann R, Semtner M, Kepura F, Plant TD, Schöneberg T. PLoS ONE. 2010;5:e10580. doi: 10.1371/journal.pone.0010580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledoux J, Werner ME, Brayden JE, Nelson MT. Physiology (Bethesda) 2006;21:69. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 21.Dora KA, et al. J Vasc Res. 2003;40:480. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 22.Sandow SL, Neylon CB, Chen MX, Garland CJ. J Anat. 2006;209:689. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.