Abstract
Stroke is a leading cause of death and long-term disability. Non-invasive magnetic resonance imaging (MRI) has been widely used for the early detection of ischemic stroke and the longitudinal monitoring of novel treatment strategies. Recent advances in MRI techniques have enabled improved sensitivity and specificity to detecting ischemic brain injury and monitoring functional recovery. This review describes recent progresses in the development and application of multimodal MRI and image analysis techniques to study experimental stroke in rats and non-human primates.
Keywords: Magnetic resonance imaging, Perfusion–diffusion mismatch, Apparent diffusion coefficient, Cerebral blood flow, Diffusion-weighted imaging, Perfusion-weighted imaging, Experimental stroke model, Rodents, Oxygen challenge, Predictive mode, Rats, Hyperperfusion, Functional magnetic resonance imaging
Introduction
Stroke is a leading cause of death and a cause of long-term disability.1 Ischemic stroke, the most prevalent form of stroke, is caused by a loss of blood flow to the brain, resulting in loss of brain functions. The prevalence of stroke and the financial burden of stroke care are steadily rising because the conditions that put people at risk for stroke (such as heart disease, diabetes, and obesity) are steadily on the rise. The ability to minimize neurological deficit in stroke patients remains extremely limited. The ability to reliably distinguish salvageable versus non-salvageable tissue and longitudinally monitor novel treatment strategies remain high priority for the treatment and management of acute stroke. This paper reviews recent progresses in the development and application of magnetic resonance imaging (MRI) and image analysis techniques to study ischemic tissue at risk in experimental stroke models using rodents and non-human primates (NHPs).
Conventional T1 and T2 MRI
Conventional T1 and T2 MRI are part of standard imaging protocols in stroke imaging2 and, along with normal non-contrast computed tomography (CT), have been used in acute stroke primarily to rule out hemorrhage.3 While both T1WI and T2WI identify vasogenic edema at later times during stroke,4 90% of infarctions are visible on T2WI at 24 hours, relative to only 50% on T1WI, and thus T2WI is the `gold standard' in clinical settings for imaging cerebral infarction.5 However, both normal non-contrast CT and conventional MRI have shown sensitivities of <50% in imaging ischemic stroke within six hours of onset,6,7 despite the fact that T2WI has signal changes as early as 30 minutes post-stroke in cats and primates.8 In addition, T1WI and T2WI have shown high false negative rates during the first day after stroke onset.5
Perfusion and Diffusion MRI
Diffusion-weighted imaging (DWI)9 in which contrast is based on water apparent diffusion coefficient (ADC) is known for its ability to detect stroke within minutes after onset, whereas CT and other imaging modalities fail to detect stroke injury for at least a few hours. Tissue ADC decline has been correlated with energy failure and breakdown of membrane potential in animal models.10–12 The reduction in cerebral blood flow (CBF) in the brain can be imaged using an exogenous intravascular contrast agent or by arterial spin labeling of the endogenous water in blood.13,14 The `perfusion–diffusion mismatch' – defined as the difference in area between perfusion abnormality and diffusion abnormality (the region of interest outlined in Fig. 1) – approximates the `ischemic penumbra.' The mismatch concept is being used in preclinical investigation and clinical decision-making in the management of acute stroke.
Figure 1.
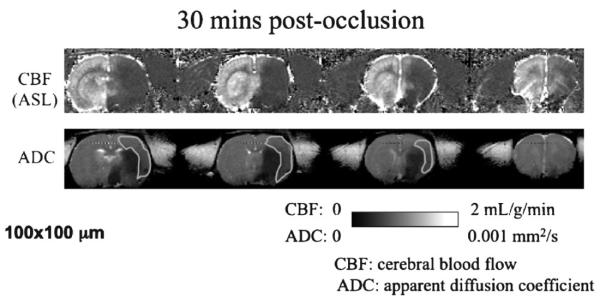
Cerebral blood flow (CBF) and apparent diffusion coefficient (ADC) of a stroke rat obtained at 30 minutes after middle cerebral artery occlusion. The green regions of interest indicate the perfusion–diffusion mismatch.
Perfusion and diffusion MRI has been applied to evaluate the spatiotemporal progression of middle cerebral artery occlusion in rats during the acute phase.15,16 Areas with ADC reduction grow with time after permanent occlusion, eventually reaching the CBF-defined lesion volume (Fig. 2, left). Reperfusion salvages substantial amount of mismatch tissue (Fig. 2, right). The ADC and CBF viability threshold in this rat stroke model was determined to be 0.53±0.02 × 10−3 mm2/s (30%±2% reduction) and 0.30±0.09 ml/gram/minute (57%±11% reduction), respectively, in rats.15,16 Although these thresholds are model dependent, they are potentially useful and easy to use. Reperfusion at 60 minutes post-occlusion showed that some `perfusion–diffusion' mismatch defined by these thresholds was salvaged, with ADC lesion volume at 180 minutes reaching ~50% of the permanent occlusion group.16,17 The degree of salvaged mismatch tissue is dependent on occlusion durations as expected.18 Using this approach, a few drugs have been shown to be effective in reducing infarct volume in rats by salvaging the perfusion–diffusion mismatch defined by the viability thresholds.19,20
Figure 2.
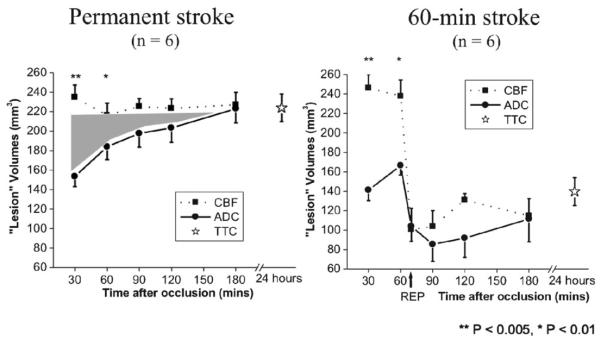
Temporal evolution of lesion volumes defined by cerebral blood flow (CBF) and apparent diffusion coefficient (ADC) thresholds for permanent and 60-minute middle cerebral arterial occlusion.
Alternative to the threshold method, automated cluster analysis based on ISODATA (self-organizing data analysis algorithm) technique21 has been used to characterize the spatiotemporal dynamic evolution of ischemic brain injury based on high-resolution, quantitative perfusion, and diffusion measurements. Multiple clusters were automatically resolved after stroke, with pixels corresponding to the `normal', `at risk' (`perfusion–diffusion' mismatch), and `ischemic core' tissue. The unique advantage of the ISODATA is that the number clusters in the data set can be statistically determined. Tissue volumes, ADC, and CBF of each ISODATA cluster were quantified. Pixels of different ISODATA clusters can be color-coded and mapped onto the image and ADC-CBF spaces. In permanent stroke, the ADC regions decreased precipitously as ischemia progressed. In transient stroke, reperfusion salvaged the majority of the mismatch pixels and some core pixels. This approach allows objective classification of different tissue types, which can be mapped back onto the image spaces, providing a powerful and objective means for pixel-by-pixel visualization of different tissue fate.
Prediction of Ischemic Tissue Fate
The ultimate goal of acute stroke imaging is to predict tissue fate based on acute MRI data. Sophisticated algorithms have been developed to predict ischemic tissue fate on a pixel-by-pixel basis. They included predictive models based on generalized linear model,22,23 probability-of-infarct,24,25 and artificial neural network (ANN)26 and support vector machines (SVM).27 These predictive models provide statistical or probabilistic maps of infarct likelihood on a pixel-by-pixel basis utilizing only the acute MRI data. Performance analysis showed accurate prediction when compared with endpoint T2 MRI and/or histology. In addition, the effects of neighboring pixels and infarct incidence on prediction accuracy were also evaluated. Other potential a priori information can be incorporated in these predictive models.24,25 Prediction accuracy can be quantified using receiver-operating characteristic (ROC) analysis.
BOLD fMRI of Perfusion–Diffusion Mismatch
In addition to anatomical MRI techniques based on tissue perfusion and diffusion, functional MRI of stroke animals can also be performed to evaluate the functional status of the `perfusion–diffusion mismatch.' fMRI is a non-invasive imaging modality and has been widely exploited for mapping brain processes, ranging from perceptions to cognitive functions.28fMRI applications to neurological diseases in animal models are emerging. We and others have previously demonstrated that bilateral forepaw somatosensory stimulation activated the somatosensory cortices of both hemispheres in a normal rat using isoflurane as the anesthetics29,30 instead of the more common α-chloralose (which is not compatible with survival study).31,32 Recent development29 also allows the addition of oxygen-consumption imaging to map oxidative metabolism and neural-vascular coupling in stroke rats. In the stroke rat 30 minutes after occlusion, we demonstrated that activations in the somatosensory cortices were not detected in the ischemic hemisphere.18 Functional MRI in stroke should be useful in determining whether risky therapeutic intervention should be performed if the `perfusion–diffusion' mismatch is already non-functional. More recently, T2*-weighted MRI of transient oxygen challenge33,34 has been proposed to improve delineation of reversible from irreversibly damaged ischemic brain tissue in ischemic stroke. The at-risk regions surrounding the infarct core showed an exaggerated increase in OC T2*-weighted signal intensity. OC brings in oxygenated blood displacing the high deoxyhemoglobin concentration in the at-risk region where CBF is partially compromised but its metabolic activity remains significant. Thus, tissue with exaggerated increase in T2*-weighted signal intensity during OC is potentially salvageable.
Non-human primate stroke
Although rodent stroke models are necessary to develop imaging protocols and test novel treatment strategies, large NHPs are also important animal models for studying stroke because one can take advantage of brain organization and vascular circuitry that is more homologous with humans than the widely used rodents for stroke modeling. Non-human primates allow for the use of well-established behavioral test batteries that assess a wide range of cognitive functions in much the same way we do for clinical patients. Imaging technologies developed on the clinical scanners can be seamlessly translated to humans and vice versa. As such a few laboratories have investigated clinically relevant NHP stroke models paired with innovative high-resolution MRI. NHP stroke imaging studies are, however, challenging and sparse because of (i) high cost, (ii) NHP resources and trained staff are not widely available, and (iii) acute stroke imaging studies necessitate an expensive imaging facility to be located in very close proximity.
Liu et al., measured ADC and fractional anisotropy (FA) changes in permanent and transient MCAO in macaques, and compared with T2 and histology to determine endpoint lesion size.35 This study established the temporal profile of diffusion changes in NHP stroke. They found that reperfusion accelerated the changes of diffusion parameters when compared with permanent stroke. Decreases in ADC within lesion territory were apparent in the acute phase, and it pseudo-normalized ~10 days in macaques.35 Wey et al. implemented perfusion MRI using arterial spin labeling technique, which is ideally suited for serial MRI within one imaging session, and demonstrated the spatial-temporal characteristics of perfusion–diffusion mismatch in baboon stroke.36,37 An advantage of using baboon is that it offers a larger brain compared to macaques making them more amenable to MRI studies. Reperfusion salvaged substantial amount of the perfusion–diffusion mismatch tissue.37 The mismatch volume progressively decreased for up to six hours post-occlusion, in contrast to mismatch volumes in rodent models that lasted up to three hours post-occlusion.15,16,17,38 In humans, the perfusion–diffusion mismatch is often detected up to 6–12 hours after stroke onset, and the frequency of detection decreased with time.39 Consistent with previous serial diffusion MRI studies in NHP stroke which reported temporal profiles of diffusion parameters concord more with humans than rodents, the spatial-temporal dynamics of perfusion–diffusion mismatch appear to be more similar to humans than rodents as well.
Perfusion–diffusion mismatch in human stroke
Perfusion–diffusion mismatch in stroke patients has also been widely observed.40–42 Clinical trials have demonstrated the benefits of thrombolytic therapy using recombinant tissue plasminogen activator (t-PA) within three hours after the onset of ischemia and the clinical benefit was associated with smaller late infarcts on CT.43 More recently, t-PA treatment window has been extended to 4.5 hours after the onset of stroke with the help of perfusion and diffusion imaging.44 Diffusion-weighted imaging and PWI are now widely used to help identify potential candidate patients for intravenous thrombolysis, intra-arterial thrombolysis or mechanical clot retrieval,45 thus enhancing patient selection that could lead to a greater chance for therapeutic benefit. Stroke clinical trials have now routinely included perfusion and diffusion MRI.
While there is apparent clinical utility of perfusion–diffusion mismatch, there are also studies that suggest that diffusion–perfusion mismatch does not optimally define the penumbra, early diffusion lesions are in part reversible and often include both irreversibly infarcted tissue and penumbra.46 The visible zone of perfusion abnormality could also overestimate the penumbra by including regions of benign oligemia. Despite these shortcomings, perfusion and diffusion MRI remains to be a very practical method for stroke imaging and some perfusion–diffusion mismatch is salvageable in humans.
Conclusions
Magnetic resonance imaging provides powerful tools to improve characterization of cerebral ischemia, to longitudinally monitor ischemic progression, statistically predict ischemic tissue fate, as well as the potential to evaluate of drug efficacy. Animal stroke models in which the `perfusion–diffusion mismatch' can be reproducibly studied under controlled conditions are highly valuable for establishing novel MRI modalities and to characterize ischemic tissue fate. Non-invasive MRI can be applied to study stroke in high-order animals, such as NHPs, as well as in humans.
Acknowledgements
This work was supported by the NIH (R01-NS45879) and the American Heart Association (EIA 0940104N and SDG 0830293N).
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs MA, Mitsias P, Soltaniaan-Zadeh H, Santhakumar S, Ghanei A, Hammound R, et al. Multiparametric MRI tissue characterization in clinical stroke with correlation to clinical outcome: part 2. Stroke. 2001;32:950–7. doi: 10.1161/01.str.32.4.950. [DOI] [PubMed] [Google Scholar]
- 3.Mullins ME, Schaefer PW, Sorensen AG, Halpern EF, Ay H, He J, et al. CT and conventional and diffusion-weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology. 2002;224:353–60. doi: 10.1148/radiol.2242010873. [DOI] [PubMed] [Google Scholar]
- 4.Quast MJ, Huang NC, Hillman GR, Kent TA. The evolution of acute stroke recorded by multimodal magnetic resonance imaging. Magn Reson Imaging. 1993;11:465–71. doi: 10.1016/0730-725x(93)90465-p. [DOI] [PubMed] [Google Scholar]
- 5.Yuh WTC, Crain MR, Loes DJ, Greene GM, Ryals TJ, Sato Y. MR imaging of cerebral ischemia: findings in the first 24 hours. Am J Neuroradiol. 1991;12:621–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez RG, Schaefer PW, Buonanno FS, Schwamm LH, Budzik RF, Rordorf G, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999;210:155–62. doi: 10.1148/radiology.210.1.r99ja02155. [DOI] [PubMed] [Google Scholar]
- 7.Mohr JP, Biller J, Hilal SK, Yuh WT, Tatemichi TK, Hedges S, et al. Magnetic resonance versus computed tomographic imaging in acute stroke. Stroke. 1995;26:807–12. doi: 10.1161/01.str.26.5.807. [DOI] [PubMed] [Google Scholar]
- 8.Brant-Zawadzki M, Pereira B, Weinstein P, Moore S, Kucharczyk W, Berry I, et al. MR imaging of acute experimental ischemia in cats. AJNR Am J Neuroradiol. 1986;7:7–11. [PMC free article] [PubMed] [Google Scholar]
- 9.Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and t2-weighted MRI and spectroscopy. Magn Reson Med. 1990;14:330–46. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- 10.Hoehn-Berlage M, Norris DG, Kohno K, Mies G, Leibfritz D, Hossmann KA. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab. 1995;15:1002–11. doi: 10.1038/jcbfm.1995.126. [DOI] [PubMed] [Google Scholar]
- 11.Kohno K, Hoehn-Berlage M, Mies G, Back T, Hossmann KA. Relationship between diffusion-weighted MR images, cerebral blood flow, and energy state in experimental brain infarction. Magn Reson Imaging. 1995;13:73–80. doi: 10.1016/0730-725x(94)00080-m. [DOI] [PubMed] [Google Scholar]
- 12.Back T, Hoehn-Berlage M, Kohno K, Hossmann KA. Diffusion nuclear magnetic resonance imaging in experimental stroke correlation with cerebral metabolites. Stroke. 1994;25:494–500. doi: 10.1161/01.str.25.2.494. [DOI] [PubMed] [Google Scholar]
- 13.Barbier EL, Lamalle L, Decorps M. Methodology of brain perfusion imaging. J Magn Reson Imaging. 2001;13:496–520. doi: 10.1002/jmri.1073. [DOI] [PubMed] [Google Scholar]
- 14.Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19:701–35. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Shen Q, Meng X, Fisher M, Sotak CH, Duong TQ. Pixel-by-pixel spatiotemporal progression of focal ischemia derived using quantitative perfusion and diffusion imaging. J Cereb Blood Flow Metab. 2003;23:1479–88. doi: 10.1097/01.WCB.0000100064.36077.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol. 2004;55:207–12. doi: 10.1002/ana.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Q, Fisher M, Sotak CH, Duong TQ. Effects of reperfusion on ADC and CBF pixel-by-pixel dynamics in stroke: characterizing tissue fates using quantitative diffusion and perfusion imaging. J Cereb Blood Flow Metab. 2004;24:280–90. doi: 10.1097/01.WCB.0000110048.43905.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1265–79. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tagaris GA, Richter W, Kim SG, Pellizzer G, Andersen P, Ugurbil K, et al. Functional magnetic resonance imaging of mental rotation and memory scanning: a multidimensional scaling analysis of brain activation patterns. Brain Res Brain Res Rev. 1998;26:106–12. doi: 10.1016/s0165-0173(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 20.Bardutsky J, Meng X, Bouley J, Duong TQ, Ratan R, Fisher M. Effects of iv dimethyl sulfoxide on ischemia evolution in permanently occluded rats. J Cereb Blood Flow Metab. 2005;25:968–77. doi: 10.1038/sj.jcbfm.9600095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Q, Ren H, Bouley J, Fisher M, Duong TQ. Dynamic tracking of acute ischemic tissue fates using improved unsupervised isodata analysis of high-resolution quantitative perfusion and diffusion data. J Cereb Blood Flow Metab. 2004;24:887–97. doi: 10.1097/01.WCB.0000124321.60992.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu O, Sumii T, Asahi M, Sasamata M, Ostergaard L, Rosen BR, et al. Infarct prediction and treatment assessment with MRI-based algorithms in experimental stroke models. J Cereb Blood Flow Metab. 2007;27:196–204. doi: 10.1038/sj.jcbfm.9600328. [DOI] [PubMed] [Google Scholar]
- 23.Wu O, Koroshetz WJ, Ostergard L, Buonanno FS, Copen W, Gonzales G, et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke. 2001;32:933–42. doi: 10.1161/01.str.32.4.933. [DOI] [PubMed] [Google Scholar]
- 24.Shen Q, Ren H, Fisher M, Duong TQ. Statistical prediction of tissue fates in acute ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:1336–45. doi: 10.1038/sj.jcbfm.9600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Q, Duong TQ. Quantitative prediction of ischemic stroke tissue fate. NMR Biomed. 2008;21:839–48. doi: 10.1002/nbm.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Shen Q, Duong TQ. Artificial neural-network prediction of ischemic tissue fate in acute stroke imaging. J Cereb Blood Flow Metab. 2010;30:1661–70. doi: 10.1038/jcbfm.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Shen Q, Duong TQ. Quantitative prediction of acute ischemic tissue fate using support vector machine. Brain Res. 2011;1405:77–84. doi: 10.1016/j.brainres.2011.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S-G, Merkle H, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–85. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia and hypercapnia on baseline and stimulus-evoked BOLD, CBF and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–8. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, et al. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23:472–81. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements Magn Reson Med. 2000;43:383–92. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Santosh C, Brennan D, McCabe C, Macrae IM, Holmes WM, Graham DI, et al. Potential use of oxygen as a metabolic biosensor in combination with T2*-weighted MRI to define the ischemic penumbra. J Cereb Blood Flow Metab. 2008;28:1742–53. doi: 10.1038/jcbfm.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Q, Huang S, Du F, Duong TQ. Probing ischemic tissue fate with BOLD fMRI of brief oxygen challenge Brain Res. 2011;1425:132–141. doi: 10.1016/j.brainres.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, D'arceuil HE, Westmoreland S, He J, Duggan M, Gonzalez RG, et al. Serial diffusion tensor MRI after transient and permanent cerebral ischemia in nonhuman primates. Stroke. 2007;38:138–45. doi: 10.1161/01.STR.0000252127.07428.9c. [DOI] [PubMed] [Google Scholar]
- 36.Wey HY, Wang DJ, Duong TQ. Baseline CBF, and BOLD, CBF, and CMRO2 fMRI of visual and vibrotactile stimulations in baboons. J Cereb Blood Flow Metab. 2011;31:715–24. doi: 10.1038/jcbfm.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wey HY, Kroma GM, Li J, Leland MM, Jones L, Duong TQ. MRI of perfusion-diffusion mismatch in non-human primate (baboon) stroke: a preliminary report. Open Neuroimag J. 2011;5:6. doi: 10.2174/1874440001105010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai V, Shen Q, Duong TQ. Incorporating ADC temporal profiles to predict ischemic tissue fate in acute stroke. Brain Res. 2012;1458:86–92. doi: 10.1016/j.brainres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darby DG, Barber PA, Gerraty RP, Desmond PM, Yang Q, Parsons M, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–52. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 40.Warach S, Dashe J, Edelman R. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging. J Cereb Blood Flow Metab. 1996;16:53–9. doi: 10.1097/00004647-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Albers GW. Expanding the window for thrombolytic therapy in acute stroke: the potential role of acute MRI for patient selection. Stroke. 1999;30:2230–7. doi: 10.1161/01.str.30.10.2230. [DOI] [PubMed] [Google Scholar]
- 42.Davis D, Ulatowski J, Eleff S, Loes DJ, Greene GM, Ryals TJ, et al. Rapid monitoring of changes in water diffusion coefficients during reversible ischemia in cat and rat brain. Magn Reson Med. 1994;31:454–60. doi: 10.1002/mrm.1910310416. [DOI] [PubMed] [Google Scholar]
- 43.NINDS (The national institute of neurological disorder, and stroke rt-PA stroke study group) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 44.Davis SM, Donnan GA. 4.5 hours: the new time window for tissue plasminogen activator in stroke. Stroke. 2009;40:2266–7. doi: 10.1161/STROKEAHA.108.544171. [DOI] [PubMed] [Google Scholar]
- 45.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. MRI profile and response to endovascular reperfusion after stroke (defuse 2): a prospective cohort study. Lancet Neurol. 2012;11:860–7. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–35. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]


