Abstract
Purpose
To assess clinical outcome and the role of proton-therapy (PT) for local control (LC) of osteosarcoma (OSA).
Methods
All patients who received PT or mixed photon-proton radiotherapy from 1983–2009 at the Massachusetts General Hospital were reviewed. Criteria for PT were the need for high dose in the context of highly conformal radiotherapy of unresected or partially resected OSA, positive postoperative margins, postoperative imaging studies with macroscopic disease, or incomplete resection as defined by the surgeon. The primary endpoint was local control (LC) of the site treated; secondary endpoints were disease-free survival (DFS), overall survival (OS), long term toxicity, and prognostic factors associated with clinical outcome.
Results
55 patients with a median age of 29 (2 to 76) were offered PT. The mean dose was 68.4 Gy, (standard deviation 5.4 Gy). 58.2% (11–100%) of the total dose was delivered with protons. LC after three and five years was 82 % and 72 %, respectively. The distant failure rate was 26% after three and five years. The five-year DFS was 65%, and the five-year OS was 67%. The extent of surgical resection did not correlate with outcome. Risk factors for local failure were ≥2 grade disease (p<0.0001) and total treatment length (p=0.008). Grade 3–4 late toxicity was seen in 30.1 % of patients. One patient died from treatment-associated acute lymphocytic leukemia, and one from secondary carcinoma of the maxilla.
Conclusion
PT to deliver high RT doses allows organ-preserving locally curative treatment with high doses for unresectable or incompletely resected OSA.
Keywords: osteosarcoma, proton radiotherapy
INTRODUCTION
Osteosarcoma (OSA) is a disease typically of young patients, with a peak incidence in the second decade; when arising in adults, the highest incidence appears after the age of 55. In the young, OSA generally presents as an osteoid-forming tumor around the epiphyses of the long bones. Besides local destruction, metastatic dissemination to the lungs or other bones can also occur. As chemotherapy is highly active and the risk for metastatic disease is high, treatment generally starts with induction chemotherapy as soon as pathologic diagnosis has been confirmed. Anthracyclines and cisplatin, as well as methotrexate or ifosfamide form the backbone of systemic treatments 1. After chemotherapy induction, complete surgical resection generally yields high rates of local control in patients with extremity lesions. Chemo-responsiveness can be assessed after resection and the degree of chemotherapy-induced necrosis carries important prognostic information 2. Postoperative chemotherapy usually continues for nearly a year.
Local tumor control, an important component of successful treatment, may be improved in selected patients with external beam radiation, although the potential of radiotherapy as an integral therapeutic component for local curative treatment has been debated 3, 4. Depending on the clinical presentation, function-sparing surgery might be difficult to achieve in certain anatomical sites, such as the head, the spine, or the pelvis. Furthermore, OSA has been traditionally viewed as a neoplasm that responds poorly to radiation 5, and current knowledge about the role of high dose radiotherapy (RT) in the setting of non-resectable or marginally resectable OSA has remained limited. Overall, successful local control rates can be expected in ~70% of cases with modern surgery 6. The Cooperative OsteoSarcoma Study (COSS) group used photon-based RT in a minority of their trial patients: in case of unresectable disease they employed doses of 30 to 56 Gy for disease of the spine, and 56 to 68 Gy for OSA of the pelvis, resulting in LC rates of less than 20% 7, 8. On the other hand, Machak et al. reported local control rates of 60% with photon RT for non-resected OSA in 31 patients with doses of 60 Gy(given in 2.5–3 Gy daily or 1.25.–1.5 Gy b.i.d. fractions), and patients who responded to chemotherapy had even higher rates of response 9. A previous report from the Massachusetts General hospital on 41 patients reported benefits of high dose RT resulting in local control rates between 50 and 80%, depending of the extent of surgical resection 10. Thus, the current use of RT for OSA follows the concept that high doses of ionizing radiation may add to local control as has been the case in the treatment of sarcomas in general 10, 11.
The current experience with proton therapy (PT) remains limited for curative treatment of OSA. Most of the studies reporting on particle therapy include a variety histological entities, of which OSA mostly represents the minority, limiting interpretation 11, 12. Thus, it has remained unclear in what context PT should be incorporated a priori in the curative treatment of OSA not amendable to complete surgical resection. Currently, the best data have been reported from Chiba in Japan, using heavy ion-based particle treatment. They reported local control rate after five years of 65% with an overall survival rate of 29% in 58 patients with OSA of the trunk treated with carbon ions from 1994 until 2007 at the National Institute of Radiological Sciences, 13.
A reassessment of the role of particle treatment in the multimodality approach to OSA is of growing clinical importance, as the access to particle treatment facilities is increasing worldwide. While the clinical benefits of this costly and complex technology are still being assessed and debated, the reduction in integral radiation dose is likely to be of most importance to the young patient with a large tumor such as the patients with pelvic disease 14, 15. Here we review the entire series of OSA patients at the Massachusetts General Hospital who were treated with proton therapy for limited or disseminated OSA.
PATIENTS AND METHODS
During the period from 1983 to 2009, there were 55 patients in the Sarcoma Database of the Department of Radiation Oncology at the Massachusetts General Hospital who were treated for OSA with PT. Approval from the Investigational Review Board was obtained to analyze long-term outcome in these patients. All available department and hospital charts and records were reviewed to assess local control as the primary endpoint, as well as secondary endpoints of disease-free survival, metastasis-free survival, overall survival, and long-term toxicity.
Patient Characteristics
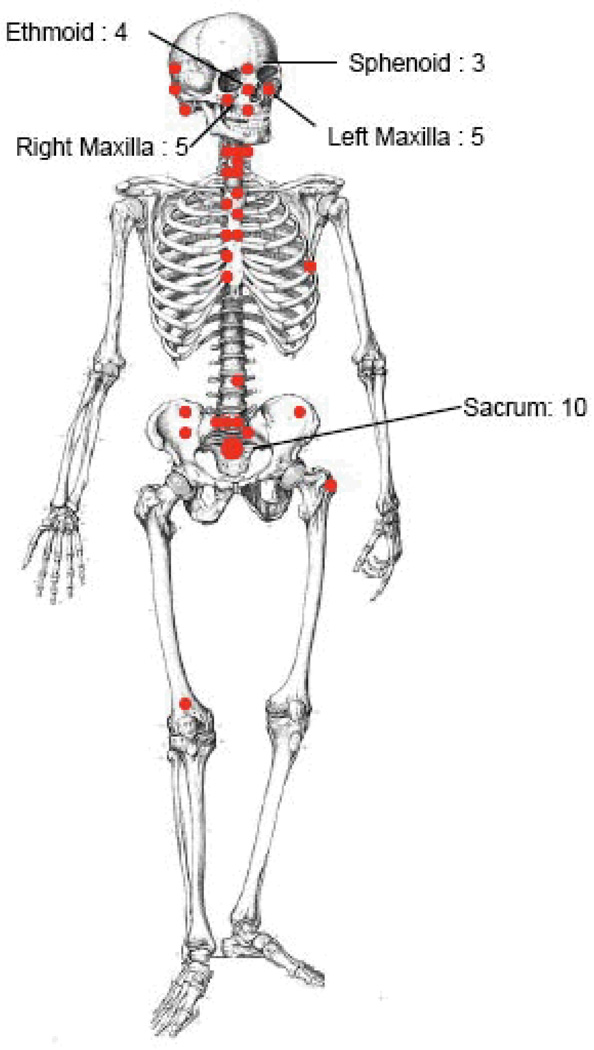
Table 1 summarizes the characteristics of the cohort. The median age was 29 (range 2 to 76), while the mean age was 32 year (SD 18). The male to female ratio was 5:6. The median follow-up was 27 months (range 0 to 196). The distribution of disease manifestations is shown in Figure 1. Histology was available for all patients and histological subtype was osteoblastic in 29 patients (52.7%), chondroblastic in 21 patients (38.2%), OSA with giant cells in 2 (3.6%), fibroblastic in 2 (3.6%), and myxoid in one patient (1.8%). Grade 1 disease was seen in 12 patients (22%), grade 2 in 23 patients (41.2%), and grade 3 disease, in 20 patients (36%) each. This grade distribution may be more favorable than in some series, perhaps because the patient population is slightly older, likely reflective of the anatomic distribution with more craniofacial lesions, rather than the more common population of adolescents with high grade, extremity lesions. AJCC stage IA disease was noted in 3 (5.5%) patients, IB in 9 (16.4%) cases, 12 patients (21.8%), stage IIA disease in 24 patients (43.6%), stage IIB disease in 14 patients (25.5%), and stage IV disease in five patients (7.3%). In 23 patients (41.8%), surgery consisted of “biopsy only” or minor resection with residual gross tumor. Positive surgical margins were present in 27 patients (49.1%), and surgeon’s notes indicating residual disease in 5 patients (9.1%).
Table 1. Patients’ characteristics.
Characteristics of 55 patients treated with proton therapy. Each red solid circle indicates one case, or as specified. All patients were offered cytotoxic PT with the aim to achieve long-term local control of OSA. Therefore, the four cases treated to or treated with metastatic disease at the time of treatment were retained in the analysis.
| Patients' characteristics | n = 55 | (%) |
|---|---|---|
| Age (median) | 26.9 (2–76) | |
| Male:Female ratio | 5:6 | |
| Disease Location | ||
| Head (cranium) | 22 | 40 |
| Cervical spine | 5 | 9 |
| Thoracic spine | 8 | 15 |
| Lumbar spine | 4 | 7 |
| Pelvis or sacrum | 13 | 24 |
| Femur | 1 | 2 |
| Hip | 1 | 2 |
| Rib/chest wall | 1 | 2 |
| Stage | ||
| I / II / IV | 12 / 38 / 5 | 22 / 69 / 9 |
| Histology | ||
| Osteoblastic/Chondroblastic | 29 / 21 | 53 / 38 |
| Grade | ||
| 1, 2, 3 | 12 / 23 / 20 | 22 / 42 / 36 |
| Extent of Surgery | ||
| No surgery | 12 | 22 |
| Partially resected/debulking | 19 | 35 |
| Grossly resected with positive margins | 24 | 43 |
| Chemotherapy | ||
| No systemic treatment | 5 | 9 |
| Some chemotherapy | 31 | 56 |
| Intensive chemotherapy | 19 | 35 |
| Primary presentation | 35 | 64 |
| Relapse after curative surgery | 17 | 31 |
| Metastatic site | 3 | 5 |
Figure 1.
Distribution of anatomical sites with OSA treated with PT.
Radiotherapy
The initial fields were designed to cover the preoperative clinical tumor. All cases were treated after CT-based 3D planning and PT was delivered with 160 MeV protons via a fixed beam line prior to 2001 and with 230 MeV protons via a rotational gantry after 2001. Radiation therapy consisted of daily external beam using either protons or a combination of protons and photons, except for three cases, where hyperfractionation was used (one in 1994, one in 1998, and one in 2001). The total dose was selected based on the patient’s condition, anatomic localization of the disease, the extent of prior surgery and degree of resection, grading characteristics, and normal critical structures in close proximity to the target volumes. Doses of <60 Gy were given to five patients (9.1%) only. One patient had a resected OSA of the third lumbar vertebral body at the age of 37 years and received an adjuvant dose of 50.4 Gy with protons only, one patient at the age of 14 received 57.6 Gy for incompletely resected disease at the lumbo-sacral junction, one patient at the age of 19 was treated with 59.4 Gy for positive margins for disease of the distal femur, one patient at the age of 51 years received 54.6 Gy for resected disease of the fourth-and fifth thoracic vertebral bodies, and one patient received 58 Gy after microscopically incomplete resection of OSA of the maxilla. 22 patients (40%) received a dose between >60Gy and <70Gy, and 28 patients (50.1%) received a total dose of ≥70Gy.
The total dose was applied with protons only in eleven patients (20%), and 7 of these patients (63.6%) presented with OSA of the bones of the head. ≥50% of the total dose was delivered with protons in 31% of the patients. <30% of the total dose was applied with protons in 9 patients (16.4%). These nine patients had either disease of the spine, sacrum, pelvis, or femur.
Preoperative radiation therapy was used in a total of seven (13%) patients with a dose of 19.8 Gy, except in one case, a dose of 50.4 Gy was used for disease of the spine. The rationale for delivering a portion of the dose prior to surgery is to minimize the risk of intraoperative tumor cell seeding. Post-operative radiation was started as soon as patients recovered from surgery to minimize overall treatment time. Unplanned treatment breaks were given at the discretion of the treating physician for servere acute mucositis, dermatitis, febrile neutropenia, or other acute radiation-related toxicity. Intraoperative treatment with electrons (6MV) was used in two patients with doses of 7.5 to 15 Gy, while in one patient, radioactive 90Y plaques were used to deliver dose to the dura adjacent to disease invading the spinal canal.
The median volume treated to the maximal dose (boost dose) covering the GTV (gross tumor volume) or the CTV I (highest risk area for relapse in the tumor bed in the patients without residual macroscopic tumor) as visualized on the treatment planning CT was 82.0 mL (range 8 to 1090 mL, mean 194.2 mL) and for the volumes covering the initial clinical target volume which also included areas at risk for subclinical disease beyond the original gross tumor was 213.0 mL (range 14–1624 mL, mean 380 mL), respectively. Four patients had only one tumor target volume after microscopically incomplete resection: one case of OSA of the maxilla, one in the mastoid bone, one in the second cervical vertebra, and one in the sacrum; all four were treated with doses of 67–68 Gy.
Chemotherapy
Chemotherapy was delivered neoadjuvantly in 48 patients (87%) and consisted of anthracyclines in combination with cisplatin, and / or high dose methotrexate. In case of undesired side effects or failure to respond, treatment was switched to ifosfamide with etoposide. Ifosfamide was administered during radiotherapy in 38 of the patients (69%), in combination with etoposide (65%) including some recent patients with unresected OSA or methotrexate (4% of patients). After radiotherapy, adjuvant chemotherapy was given in 75% of cases, while unknown chemotherapy status after surgery was scored in 6 patients (11%). Chemotherapy was scored as full intensive standard chemotherapy of intensity defined by the number of cycles and the drugs and drug combinations used. All others were scored as “some chemotherapy”.
Statistics
Statistical analysis was performed using Stata (StataCorp. 2009. Release 11.0. College Station, TX). Statistical significance of various risk factors for primary and secondary end-points was assessed using competing risks regression methodology. Death was considered a competing event to local and distant failure. P values of less than 0.05 were considered statistically significant.
RESULTS
Local control
12 patients suffered from local treatment failure (Figure 2a). Disease progression within 2 months after RT was observed in 4 patients, and 3 patients suffered from local relapse more that 3 years after RT. At 3 and 5 years, the LC rate was 82% (95%CI: 68 – 90%) and 72% (95%CI: 52 – 84%). No local relapse was seen in grade 1 disease (p < 0.0001). Prolonged treatment time was a risk factor for local failure with a hazard ratio of 1.02 (CI 1.01 – 1.03; p = 0.008). An indication of increased risk for local failure with a hazard ratio of 2.6 (95%CI, 0.8 – 9.0; p = 0.1) was observed for patients with disease of the skull, as compared to other anatomical sites. Figure 3 illustrates the difference of local control over time in patients with disease in the cranium versus other sites (p=0.09). Primary presentation or presentation at relapse did not discriminate for successful local control, as 16 patients (29.1%) presented with local recurrence after prior resection, and another two were treated to metastatic sites after successful prior therapy to the primary site.
Figure 2.
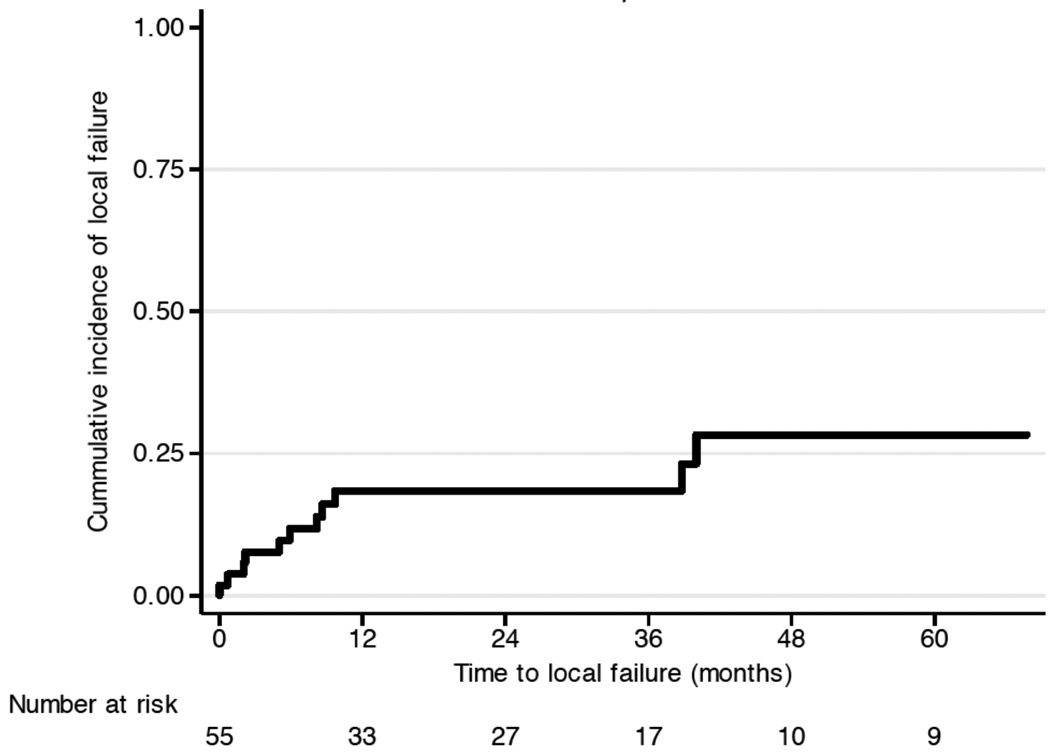
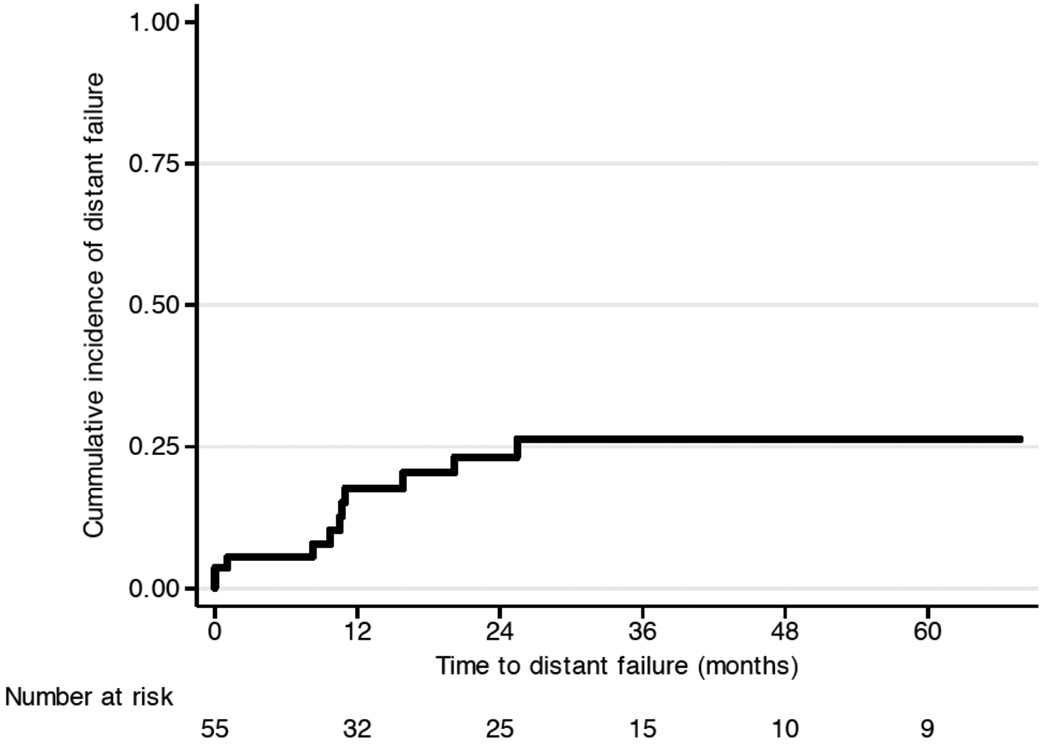
Clinical outcome: (a) local failure and (b) metastatic failure.
Figure 3.
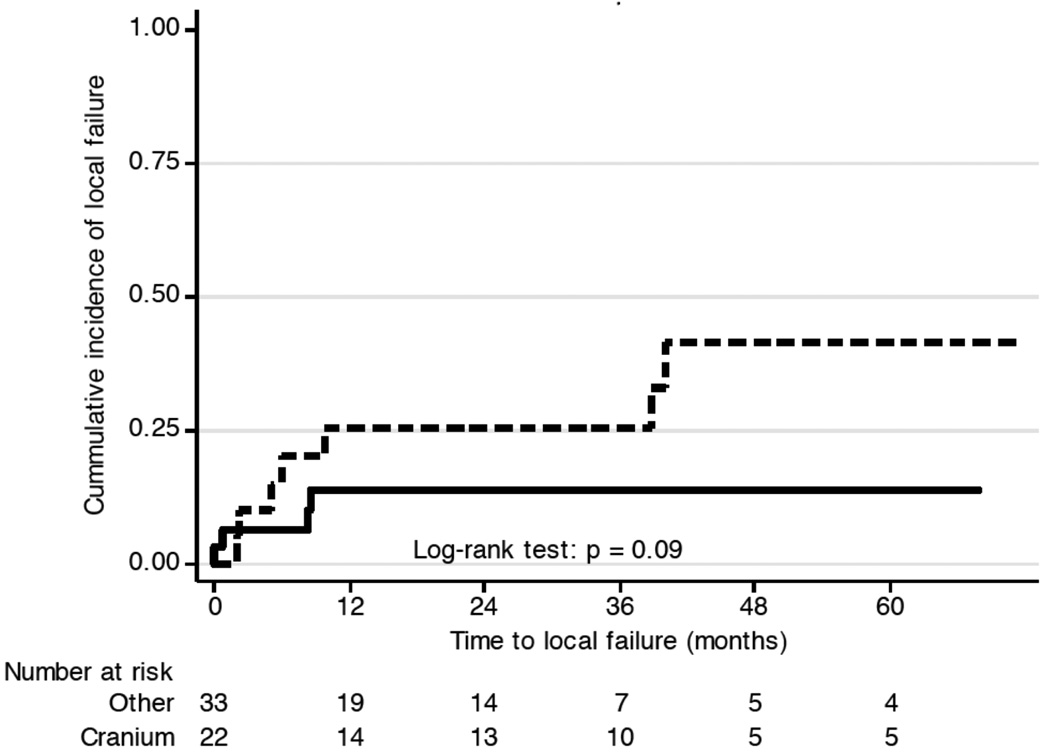
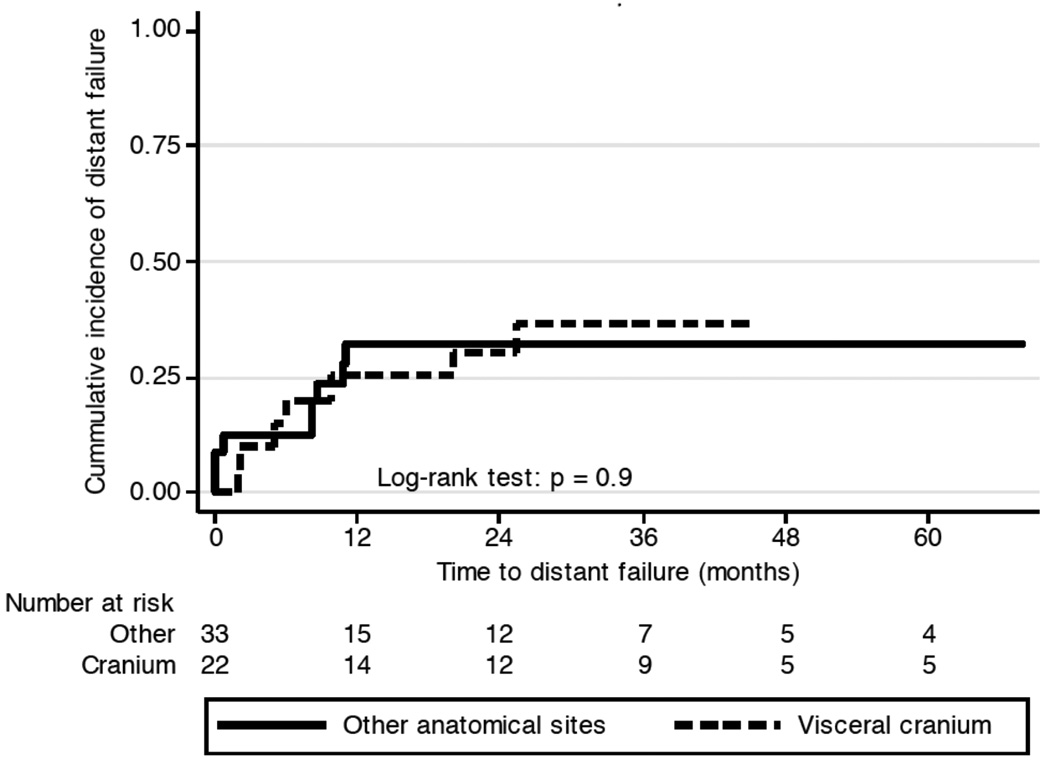
Local failure of OSA of the cranium compared with OSA of other anatomical sites (p=0.1).
With regard to the pattern of failure, 10 patients failed within the radiation treatment field. Of these, 8 patients had OSA of the bones of the skull. The other two cases were one patient with extensive disease from the distal cervical spine to the mid-thoracic spine and another patient with extensive sacral disease spanning S1 to S4. Marginal misses were likely in two cases. One patient with disease of the thoracic spine showed disease progression in the vertebral body growing from the irradiated high-dose volume into the contralateral part of the vertebral body and beyond it. Another patient with pelvic disease involving the ileum showed distal disease progression, and retrospectively, a caudal marginal miss can not be excluded. Treatment volumes or the absence of surgery did not correlate with local treatment failure (p = 0.5). The fraction of dose delivered with protons or photons was not associated with local disease control or disease progression.
The extent of surgery, however, did impact on local control probability when conducting a meta-analysis of published data based on Table 2, illustrated in Figure 5. Patients who received lower doses seemed to achieve higher local control when combined with surgery but interestingly the impact on surgery is no longer evident when high dose radiation is applied.
Table 2.
Local control rates as a function of the dose and cohorts analyzed based on reports with more than five patients. ns = not stated.
| Reference | LC rate | Dose (Gy) | Surgery | Site | Patient # |
|---|---|---|---|---|---|
| Ozaki et al. | ≤ 27.4% | 56 | 42% | Pelvis | 11 |
| Ozaki et al. | ≤ 64% | 48 | 100% | Pelvis | 4 |
| Ozaki et al. | 6% | 61 | 0% | Pelvis | 7 |
| Kassir et al. | 50% | ns | 100% | Head-and-neck | 46 |
| Jasnau et al. | 30% | ns | 1% | Cranio-facial | 6 |
| Ozaki et al. | 32% | 50 | 14% | Spine | 7 |
| DeLaney et al. | 68% | 66 | 88% | Variable | 41 |
| DeLaney et al. | 78% | 66 | 100% | Variable | 36 |
| DeLaney et al. | 40% | 66 | 0% | Variable | 9 |
| Machak et al. | 56% | 60 | 0% | Extremities | 31 |
| Kamada et al. | 73% | 70.4 | 0% | Variable/ns | 15 |
| Kamada et al. | 76% | 70.4 | 0% | Variable/ns | 58 |
| present series | 85% | 68.4 | 58% | Variable | 55 |
| Caceres et al. | 80% | 60 | 100% | Extremities | 15 |
Figure 5.
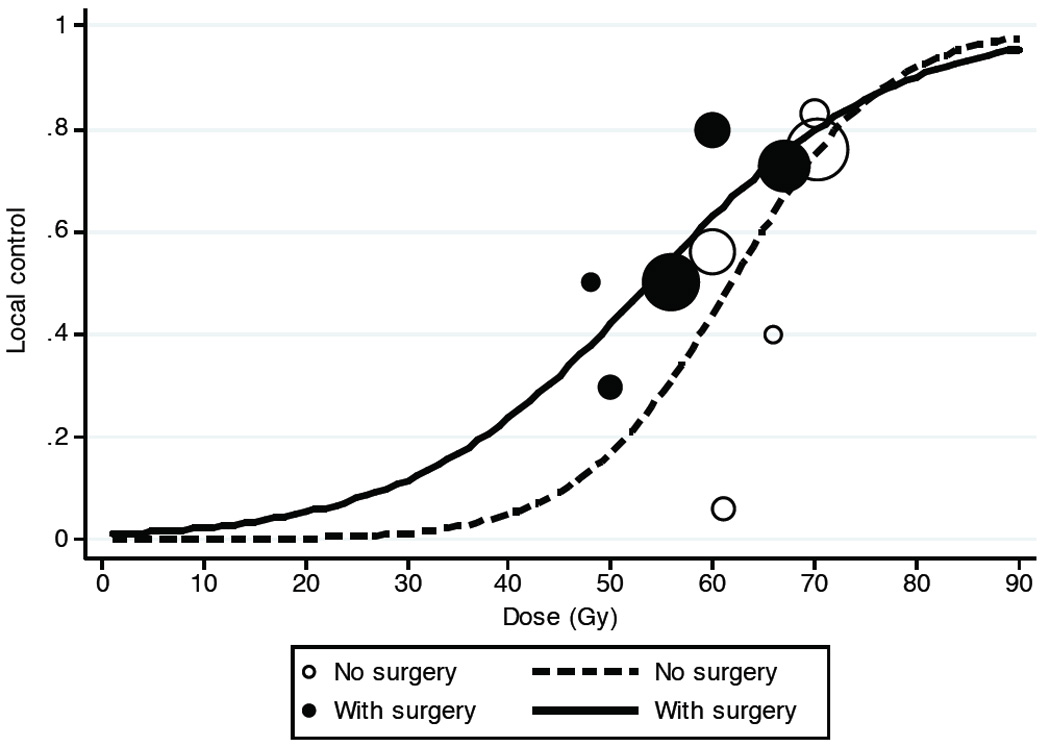
Metanalysis of local control rates reported data in the literature from table 2 as a function of surgical resection prior to radiotherapy. Resection prior to radiotherapy appears to only enhance local control when lower RT doses < ~70 Gy are delivered.
Distant metastasis
A total of 11 patients suffered from distant failure (Figure 2b). Four of the 12 patients with local failure suffered from distant failure 0 to 7 months after radiotherapy, as well. Ten of the 11 patients with distant failure had Grade 2 or higher of the disease but the difference was not statistically significant (p=0.2).
The role of chemotherapy
Chemotherapy was used in most patients. However, the quality of chemotherapy was reviewed, and intensive chemotherapy as defined by the drugs used (anthracyclines and cisplatin alternating with methotrexate) and documentation of the number of cycles given revealed highly intensive state-of-the art treatment was delivered to only 19 patients. Of these 19, two patients (10.5%) suffered local failure, and 5 (26.3%) failed distantly. Of the 31 patients receiving non-intensive chemotherapy, 10 patients (32.3%) failed locally and 6 patients (19.4%) showed metastatic disease progression. The differences with respect to chemotherapy intensity were not significant, although adherence to standard high intensive chemotherapy did weakly associate with successful local treatment control in univariate analysis (hazard ratio 5.9, CI 0.75–46.5; p = 0.09), but not in multivariate analysis. None of the five patients treated without chemotherapy, three with grade 1 disease and two with grade 2 disease, showed either local or distant failure.
Disease-free survival and overall survival
DFS was 68% (95%CI: 53 – 80%) at 2 years and 65% (95%CI: 49 – 77%) at 5 five years (Figure 4a). The overall survival rate at 2 years was 84% (95%CI: 69 – 92%), and 67% (95%CI: 47 – 80%) at five years (Figure 4b). Four patients died without disease, two of therapy-related mortality, one at the age of 16 (1.5 years after completion of treatment) due to acute lymphocytic leukemia, and another patient 15 years after completion of treatment of OSA from squamous carcinoma of the maxilla. Two patients died to non-cancer-related disease 65 and 96 months after the end of radiotherapy.
Figure 4.
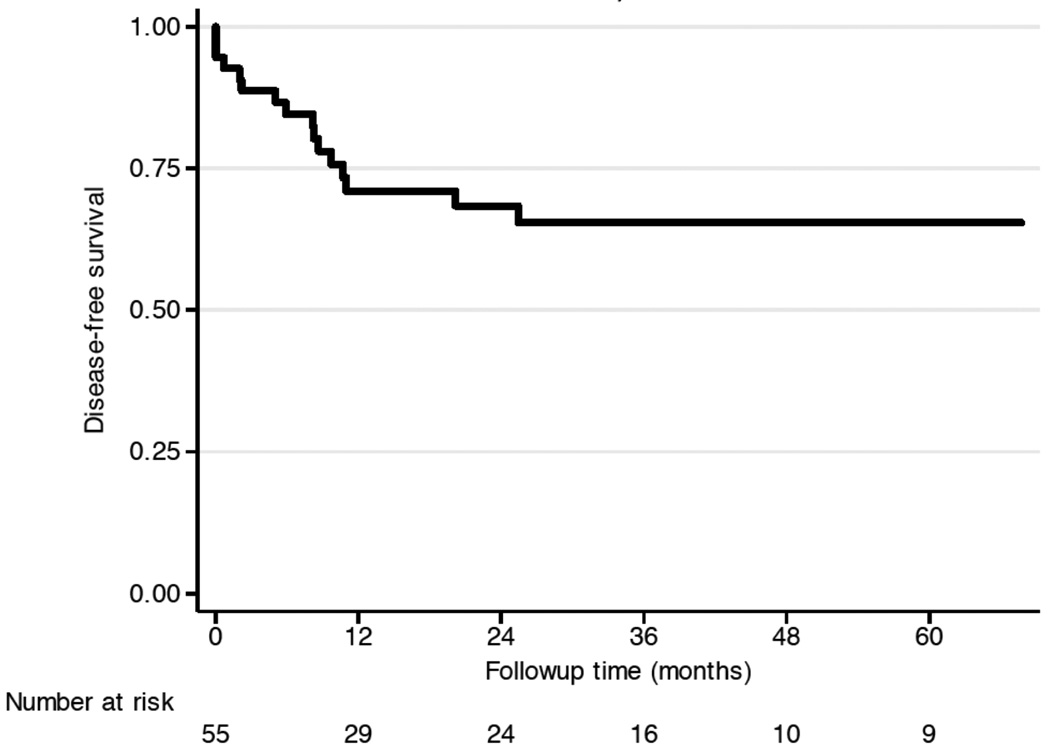
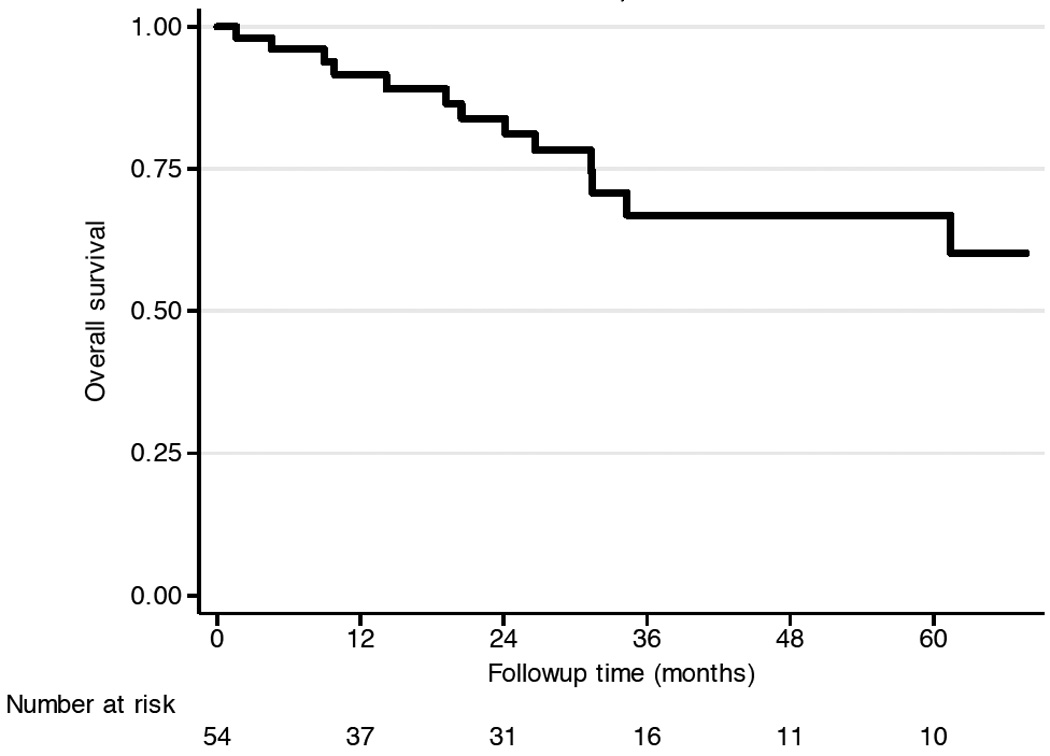
Overall clinical outcome: (a) DFS, and (b) OS.
Treatment-associated toxicity
Nine patients did not have any significant late term toxicity, and were treated to the mastoid (1), maxillary bone (1), cervical spine (3), thoracic spine (2), lumbo-sacral bones (1), and the hip (1). Grade 1 toxicity was reported in 12 patients, treated to the cranium (4), cervical spine (1), chest wall (1), thoracic spine (2), lumbo-sacral sites (3), and distal femur (1). Grade 2 toxicity, as defined by pain controlled with non-opioid medication, and minor complaints interfering with the activities of daily living, were reported in 12 patients, consisting of pain, paresthesia, trophical changes (atrophy) (one patient with cervical spine OSA), ineffective gait and foot drop, radiation myelopathy, and distal neuropathy. Complaints were possibly caused by radiation alone in three patients, while most cases of neuronal dysfunction were either preexisting or possibly related to surgery. Grade 3 and 4 toxicity was recorded in 17 patients. Grade 3 toxicity consisted of severe pain requiring morphine-based medication (3), cranial nerve damage with diplopia (1), immobility of limb (2), severe bowel dysfunction with distal functional obstruction due to denervation (1), and severe headaches (1). Grade 4 toxicity was defined by loss of organ or complete loss of organ function and was reported in 9 patients. In four patients, the eye had to be removed after RT, one patient suffered from ipsilateral loss of vision and orbital pain, and one patient suffered from ipsilateral hearing loss and blindness. Two patients were severely handicapped due to immobility or impairment of gait. One patient suffered from extensive structural alteration of the maxillary bone needing repeated adaptation of the prosthesis. Two patients died from treatment-related illnesses: one patient developed acute lymphocytic leukemia one year and a half after successful treatment of OSA and the other patient died from a secondary squamous cell carcinoma of the maxilla almost 16 years after successful treatment of the osteosarcoma with protons and chemotherapy.
DISCUSSION
The present series reviews the experience with proton therapy for local control of incompletely or unresected OSA at the Massachusetts General Hospital since 1983. In a prior series from the MGH spanning 1980 until 2002, the role of radiotherapy in 41 cases of OSA was analyzed. Only 56% of the patients received PT as a part of their treatment. Additional differences from the prior cohort exist. (1) The median dose in the present series was 68.4 Gy compared to 66 Gy in the earlier cohort, (2) no patient received doses less than 50.4 Gy compared to 10 % patients in the former cohort who received ≤30Gy and some received < 50 Gy, (3) and chemotherapy was given to 91% of patients compared to 85% in the previous cohort. Despite the fact that 22 patients (40%) from the previous analysis were retained in the present series, the results differ significantly. The differences regarding treatment modalities, the doses applied and the time frame analyzed allow us to more fully explore the role of PT for OSA.
An important observation is that differences in treatment dose did not emerge as a risk factor for local control in the present series, and the lack of radical resection did not seem to be detrimental. The reason for this finding may rely in the overall higher mean dose applied in the current series. PT generally allows more conformal dose application than 3D conformal photons and lower integral doses than either 3D conformal photons or IMRT. Treating in high dose ranges may reduce the importance of the extent of surgery, as illustrated with data derived from reports with photons or particle treatment in Figure 5.
High control rates with particle treatment without prior surgical resection have been reported by the Kamada et al., based on their analysis of unresected OSA patients treated with carbon ions at the National Institute for Radiological Sciences in Chiba, Japan 13. Table 2 summarizes the current literature in respect with regard to local control and complete surgical resection status. It is noteworthy, that the results from particle-based radiotherapies yield high control rates exceeding 70%, which are seemingly superior to the results obtained with conventional radiotherapy. Indeed, such findings are not surprising, as charged particle treatment is generally associated with the feasibility to deliver higher doses. The lack of association of the extent of surgery with local control in the present series, besides being possibly the result of lack of statistical power, is particularly interesting in reference to the data of local control obtained with carbon ions 13. Furthermore, conventional fraction schedules as used for PT in the vast majority of cases in the present series compare favorably with the hypofractionated schedules using 16 fractions over 4 weeks to deliver 64 to 70.4 Gy with carbon ions 16. Figure 5 illustrates the relationship between dose and local tumor control rates as reported in the literature in Table 2. In fact, the optimal combined approach of surgical resection with radiotherapy when proton or carbon ion therapy is available must consider the relative long-term toxicities of the different approaches, especially for disease of the axial skeleton or pelvis.
Another important finding in the present series is the association of impaired local control in patients with disease of the skull. This finding has not been anticipated due to the prior experience at the MGH 10. However, the data from the literature have been reporting on control rates below 50% in small series 17, 18, and some hypothesis can be evoked why PT might have reduced efficacy in the head and neck region. First, the use of induction chemotherapy in patients with OSA in the head has not been uniformly applied. Indeed, some patients may require immediate surgery because disease close to critical structures may mandate immediate surgical decompression 19. Resection, however, is rarely complete due to invasion and spatial relationship to critical structures. Another consideration, especially in the maxillary bone and bones close to the ethmoid cavities, is that air-tissue in-homogeneities might impair the accuracy of dose delivery, as noted for other situations when treating areas with air cavities with PT such as in the chest 20. Dose constraints placed on critical structures in close proximity to the tumor may also be a contributing factor. The present finding that head-and-neck region may still be a risk factor for local relapse may be of clinical importance. …(removed)
Chemotherapy in the present series was marginally associated with better local control. However, in the present series considerable uncertainties about chemotherapy delivered after radiotherapy persist, because documentation of treatment after radiotherapy and during follow-up was incomplete in some cases. The importance of optimal chemotherapy has been clearly defined in the pediatric population with osteosarcomas, and the possible association with improved local control is supported by the present series. During radiotherapy, chemotherapy with ifosfamide is being routinely used at the Massachusetts General Hospital. Whether ifosfamide acts as a radiation sensitizer, as well, and not merely as a cytotoxic, systemically active agent against OSA has not been conclusively shown.
The present cohort analysis highlights an important general consideration with regard to many rare diseases entities such as incompletely resected OSA. The difficulty of conducting randomized controlled trials presents challenges in the prospective assessment of novel medical and technical innovations once they are introduced into the clinic 6, 15. Controlled registries or data bases collecting data from multiple institutions could facilitate the assessment and implementation of novel technologies and agents in these rare medical conditions. These approaches should be considered as more particle treatment facilities come on line and permit the collaborative evaluation of this technology when employed for uncommon diseases such as unresectable or incompletely resected osteosarcoma.
In summary, particle-based radiotherapy can be effective for local control of OSA in areas where radical resection is problematic due to the impossibility of complete resection or potential morbidity of surgery. PT especially is especially suitable for young patients with OSA in whom reductions in the integral dose to non-target tissue is important to minimize the risk for normal tissue development as well as secondary malignancies 21. Even with the use of conformal 3D proton therapy, some patients in this series experienced significant radiation related toxicity. There may be the possibility for some reduction in this toxicity as well as further improvements with the future implementation of intensity modulated proton therapy, which will better conform the prescribed dose to the target than 3D conformal protons, as well as reduce normal tissue dose that is in the beam trajectory proximal to the tumor target22, 23. Optimal coordination of surgery, chemotherapy, new targeted agents and particle-based radiotherapy is likely to result in the best local control rates while minimizing toxicity; the best sequencing and integration of multimodality approaches, however, remain to be further defined 24.
Acknowledgment
Supported in part by grant PO1CA021239 from the National Cancer Institute and in part by the Zurich Cancer League, Switzerland.
References
- 1.Ta HT, Dass CR, Choong PF, et al. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev. 2009;28:247–263. doi: 10.1007/s10555-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 2.Ritter J, Biellack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–vii325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 3.Hayden JB, Hoang BH. Osteosarcoma: basic science and clinical implications. Orthop Clin North Am. 2006;37:1–7. doi: 10.1016/j.ocl.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Wang VY, Potts M, Chou D. Sarcoma and the spinal column. Neurosurg Clin N Am. 2008;19:71–80. doi: 10.1016/j.nec.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa Y, Takahashi T, Kobayashi T, et al. Mechanism of apoptotic resistance of human osteosarcoma cell line, HS-Os-1, against irradiation. Int J Mol Med. 2003;12:453–458. [PubMed] [Google Scholar]
- 6.Nagarajan R, Clohisy D, Weigel B. New paradigms for therapy for osteosarcoma. Curr Oncol Rep. 2005;7:410–414. doi: 10.1007/s11912-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 7.Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069–1077. [PubMed] [Google Scholar]
- 8.Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21:334–341. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 9.Machak GN, Tkachev SI, Solovyev YN, et al. Neoadjuvant chemotherapy and local radiotherapy for high-grade osteosarcoma of the extremities. Mayo Clin Proc. 2003;78:147–155. doi: 10.4065/78.2.147. [DOI] [PubMed] [Google Scholar]
- 10.DeLaney TF, Park L, Goldberg SI, et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys. 2005;61:492–498. doi: 10.1016/j.ijrobp.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 11.DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732–739. doi: 10.1016/j.ijrobp.2008.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada T, Tsujii H, Tsuji H, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Kamada T, Imai R, Serizawa I, et al. Carbon ion radiotherapy in bone and soft tissue sarcoma; Proceedings of NRS-MD Anderson Symposium on Clinical Issues for Particle Therapy; 2008. pp. 24–29. ISBN 978-4-938987-50-3. [Google Scholar]
- 14.Brower V. European boost for particle therapy. Nature. 2009;457:139. doi: 10.1038/457139a. [DOI] [PubMed] [Google Scholar]
- 15.Terasawa T, Dvorak T, Ip S, et al. Systematic Review: Charged-Particle Radiation Therapy for Cancer. Ann Intern Med. 2009 doi: 10.7326/0003-4819-151-8-200910200-00145. [DOI] [PubMed] [Google Scholar]
- 16.Imai R, Kamada T, Tsuji H, et al. Cervical spine osteosarcoma treated with carbon-ion radiotherapy. Lancet Oncol. 2006;7:1034–1035. doi: 10.1016/S1470-2045(06)70978-0. [DOI] [PubMed] [Google Scholar]
- 17.Kassir RR, Rassekh CH, Kinsella JB, et al. Osteosarcoma of the head and neck: meta-analysis of nonrandomized studies. Laryngoscope. 1997;107:56–61. doi: 10.1097/00005537-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Jasnau S, Meyer U, Potratz J, et al. Craniofacial osteosarcoma Experience of the cooperative German-Austrian-Swiss osteosarcoma study group. Oral Oncol. 2008;44:286–294. doi: 10.1016/j.oraloncology.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Guadagnolo BA, Zagars GK, Raymond AK, et al. Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer. 2009;115:3262–3270. doi: 10.1002/cncr.24297. [DOI] [PubMed] [Google Scholar]
- 20.Krayenbuhl J, Hartmann M, Lomax AJ, et al. Proton therapy for malignant pleural mesothelioma after extraleural pleuropneumectomy. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.11.006. (revision submitted) [DOI] [PubMed] [Google Scholar]
- 21.Chung CS, Keating N, Yock T, et al. Comparative analysis of second malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int J Radiat Oncol Biol Phys. 2008;72(Suppl 1) abst17. [Google Scholar]
- 22.Weber DC, Trofimov AV, Delaney TF, et al. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58:1596–1606. doi: 10.1016/j.ijrobp.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner TD, Kobayashi W, Dean S, et al. Combination short-course preoperative irradiation, surgical resection, and reduced-field high-dose postoperative irradiation in the treatment of tumors involving the bone. Int J Radiat Oncol Biol Phys. 2009;73:259–266. doi: 10.1016/j.ijrobp.2008.03.074. [DOI] [PubMed] [Google Scholar]