Abstract
Background:
Postoperative wound infection is a preventable risk that can lead to significant adverse outcomes and increased cost of care. Minimally invasive surgeries (MIS) have been found to have lower rates of postoperative infection compared with the traditional approach. To assess if the reported difference is related to intraoperative contamination or to other factors, we assessed the surgical field for sterility.
Methods:
We compared 10 MIS versus 10 traditional microdiscectomies. Swabs of the operating field were obtained before and after the procedure from multiple sites in the operating room. Positive and negative controls were taken of the skin immediately before and after preparation of the incision site. All swabs were plated out on Columbia blood agar plates and grown for 48 hours. Colony counting was performed to determine growth.
Results:
There was no statistically significant difference in the colony counts of swab sites in traditional microdiscectomies compared with MIS microdiscectomies. There was no significant contamination of the operating field using either approach.
Conclusions:
In this prospective study, we found that there was no significant difference in bacterial counts in swabs of operative sites in either traditional or MIS microdiscectomies, suggesting that the decreased rate of postoperative infection in the reported literature for MIS cases may be related to other factors, such as patient selection and/or postoperative care.
Keywords: Infection, lumbar surgery, microdiscectomy, sterility
INTRODUCTION
Lumbar microdiscectomies are one of the most common spinal surgeries performed for back and leg pain in the United States.[17,18,19] Traditional microdiscectomies are performed with significant amounts of paraspinous muscle dissection. Exposure is maintained by the usage of retractors that laterally displace the muscles off the spinal lamina and spinous process.[12] Recently, minimally invasive surgeries (MIS) microdiscectomy has been performed with increasing frequency. These approaches, which minimize the amount of muscular dissection performed have been associated with faster recovery times,[7,10] smaller amounts of intraoperative blood loss,[8] and less tissue trauma.[13] MIS procedures have also gained popularity with the public as patients often perceive MIS surgeries as a newer approach.[8]
One of the possible complications following microdiscectomies is postoperative wound infection. Patients undergoing spinal procedures may suffer from postoperative infections due to the long operative times, instrumentation, and the amount of tissue trauma associated with lumbar spinal surgeries.[13] Interestingly, it has been reported that contamination of the surgical field does not necessarily correlate with rates of postoperative infection.[11] Some authors have shown a >10% contamination of many instruments in the operative field.[3,14,15] In particular, 14-57% of gloves used by the operating room staff, including surgeons, cosurgeons, and scrub nurses, in orthopedic cases have been reported as contaminated depending on the type of surgery performed.[4,9]
It has been suggested that there may be less postoperative infections following MIS surgeries. In a retrospective analysis of 1275 patients who underwent an open lumbar spine operation compared with 791 who underwent MIS, the authors found a decreased incidence of surgical site infection in MIS surgeries (odds ratio = 0.580).[16] Given the possibility that MIS cases may offer a lower rate of postoperative infection, we compared the bacterial counts of operating room equipment between MIS and traditionally performed microdiscectomies to assess whether differences in intraoperative contamination may contribute to the reported differences in rates of postoperative infection.
MATERIAL AND METHODS
Ten MIS microdiscectomies were compared with 10 traditional microdiscectomies performed by three spine surgeons at the UCLA-Santa Monica Orthopedic Hospital. Sites for bacterial swabs (BD Liquid Amies Elution Swab (ESwab); Becton, Dickinson and Company, Sparks, MD) were predetermined and are listed in Table 1. A standard protocol was followed for all swabs and the scrub nurse in each case acquired all swab samples. Samples from gloves were collected from the palmar surface of both hands. Swabs of the microscope were taken from the portion of the drape closest to the operating field. The inside diameter of the METRx™ (Medtronic; Minneapolis, MN) tube was sampled for MIS cases while the retractor heads used to maintain operative exposure was sampled in the traditional cases. The patient's skin was prepped in each case using the ChloraPrep 26 mL applicator (CareFusion, Leawood, KS) and a negative control was taken of the skin right after prepping. A positive control was taken from the patient's skin prior to the preparation. All preoperative samples were taken before incision and all postoperative samples were taken after the wound was closed but before the surgery team touched anything outside the sterile field. Personnel and operating room characteristics are shown in Table 2.
Table 1.
Sites of swabbing
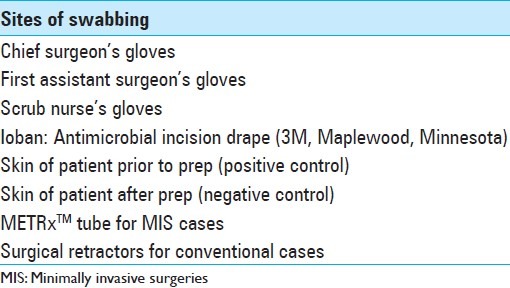
Table 2.
Surgical environment

Cultures were grown on BD Columbia Blood Agar (VWR, West Chester, PA) for 48 hours at 37°C before colony counts were performed. One research technician performed counts uniformly. Columbia blood agar is a nonselective media that can culture both aerobic and anaerobic microorganisms.[5] Colony counting is a verified method for determining contamination rate.[6] To measure the sensitivity of our tests, swabs outside the surgical field were taken and cultured for growth. Pre- and postoperative colony counts and postoperative counts from MIS and traditional discectomies were compared using the paired Student t-test.
RESULTS
Average total mean colony counts are shown in Table 3. Mean colony count is reported per 90 mm2, the surface area of the plates each swab was cultured on, and after 48 hours growth. A dose response curve comparing colony count versus surface area swabbed was obtained and this verified that the surface area swabbed during the study was of sufficient size to detect organism growth. In all surfaces sampled, the average colony count was within range of the standard deviation and there was no statistically significant difference [Table 3] between the pre- and postoperative samples. Representative plate cultures are shown in Figure 1 showing the difference between a sampling site in the surgical field (e.g., surgeon's glove) and the positive control taken from the patient's skin prior to sterilization comparing MIS versus open surgeries. There was a statistically significant difference between bacterial counts from the positive control and every surgical sample (<0.05 in all cases). A representative P value of the positive control compared against the wound site is reported in Table 3.
Table 3.
Mean TMC from sterile field
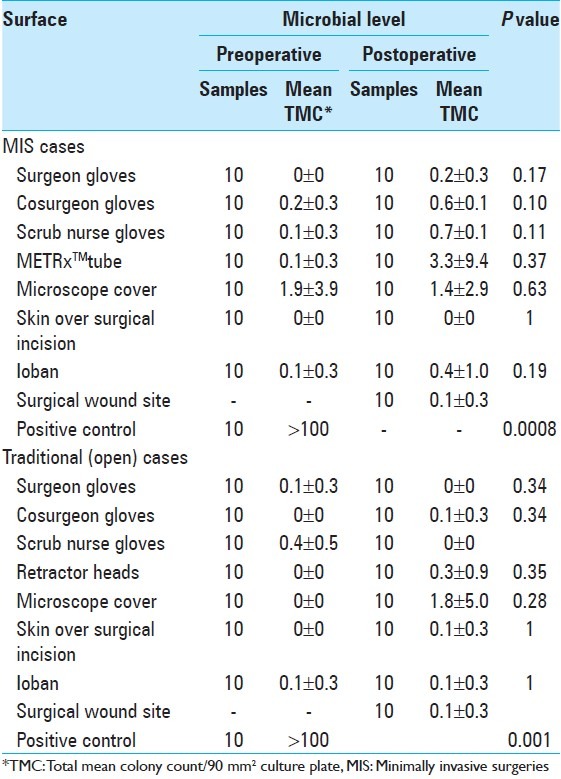
Figure 1.
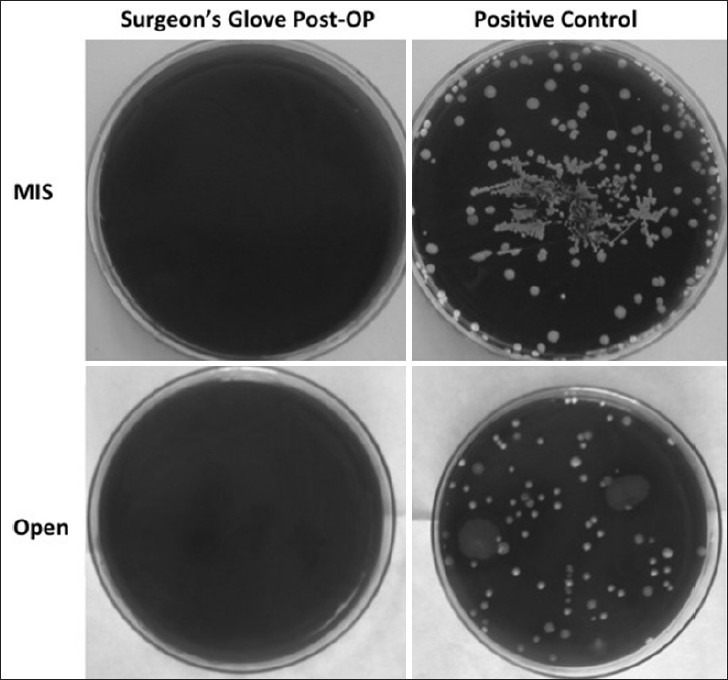
Representative positive and negative cultures
DISCUSSION
Our results indicate that there is very little bacterial contamination within the surgical field for either MIS or traditionally performed microdiscectomies. Additionally, there was no statistically significant difference in contamination between the two types of surgeries. Sampling within the surgical field revealed very little baseline growth and the averages shown in Table 3 were usually the result of one colony growth on one plate in a trial of 10. As evident from our numbers, the few colonies that grow out are most likely not due to contamination in the operating field. Baseline contamination of the plates from the environment during plating was likely to have contributed to the isolated colonies (usually just 1 per plate) that were cultured on some of the samples.
Studies have suggested that in longer surgeries, equipment that is further away from the sterile field is associated with an increased risk of contamination.[1,2] Specifically, studies of the operating microscope[1] have shown that the most likely area of contamination was on the overhead shaft of the microscope (44%) and similarly, on the C-arm fluoroscope[2] the most contaminated areas were the top (56%) and upper front of the receiver (28%). In our study, we did find an increase in contamination when swabbing the microscope cover, although the difference was not statistically significant. In all cases, these areas were near the border between the sterile field and nonsterile environment, and as such, are at the greatest risk of contamination. Our study utilized a sampling protocol similar to the ones from these prior reports but we found significantly lower rates of contamination. Additionally, regression analysis of the time of surgery compared with total colony counts found that there was no significant correlation between the length of surgery and colony growths (R2 = 0.19). Therefore, other factors could be responsible for rates of infection postsurgery. The difference between blood loss for MIS versus open surgeries was minimal and it is unlikely that this small difference would have contributed to a difference in the degree of infection. More tissue destruction and more exposure of tissue to outside environment could theoretically lead to more exposure to infection. However, the tissue exposure even in open surgeries typically involves small incisions and the duration of the surgery may not be long enough to cause significant infection.
CONCLUSIONS
In our case-controlled study of traditional compared with minimally invasive microdiscectomies, we found no difference in contamination inside the surgical field and in both approaches there was minimal contamination.
ACKNOWLEDGMENT
The authors thank resident and fellows, Drs. Ausaf Bari, Michael Dorsi, Jason Hauptman, Brandon Rebholz, and Jonathan Pribaz for allowing them to collect samples during their surgeries. The authors also thank Dr. Romney Humphreys for her help with microbiological sampling. Funding for this research was made possible by the Yang Family Foundation.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/3/295/111434
Contributor Information
Charles H. Li, Email: chli@mednet.ucla.edu.
Andrew Y. Yew, Email: andrewyew@mednet.ucla.edu.
Jon A. Kimball, Email: JKimball@mednet.ucla.edu.
Duncan Q. McBride, Email: DMcBride@mednet.ucla.edu.
Jeff C. Wang, Email: JWang@mednet.ucla.edu.
Daniel C. Lu, Email: dclu@mednet.ucla.edu.
REFERENCES
- 1.Bible JE, O’Neill KR, Crosby CG, Schoenecker JG, McGirt MJ, Devin CJ. Microscope sterility during spine surgery. Spine. 2012;37:623–7. doi: 10.1097/BRS.0b013e3182286129. [DOI] [PubMed] [Google Scholar]
- 2.Biswas D, Bible JE, Whang PG, Simpson AK, Grauer JN. Sterility of C-arm fluoroscopy during spinal surgery. Spine. 2008;33:1913–7. doi: 10.1097/BRS.0b013e31817bb130. [DOI] [PubMed] [Google Scholar]
- 3.Davis N, Curry A, Gambhir AK, Panigrahi H, Walker CR, Wilkins EG, et al. Intraoperative bacterial contamination in operations for joint replacement. J Bone Joint Surg Br. 1999;81:886–9. doi: 10.1302/0301-620x.81b5.9545. [DOI] [PubMed] [Google Scholar]
- 4.Eckersley JR, Williamson DM. Glove punctures in an orthopaedic trauma unit. Injury. 1990;21:177–8. doi: 10.1016/0020-1383(90)90090-h. [DOI] [PubMed] [Google Scholar]
- 5.Ellner PD, Stoessel CJ, Drakeford E, Vasi F. A new culture medium for medical bacteriology. Am J Clin Pathol. 1966;45:502–4. doi: 10.1093/ajcp/45.4_ts.502. [DOI] [PubMed] [Google Scholar]
- 6.Frabetti A, Vandini A, Balboni P, Triolo F, Mazzacane S. Experimental evaluation of the efficacy of sanitation procedures in operating rooms. Am J Infect Control. 2009;37:658–64. doi: 10.1016/j.ajic.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Harrington JF, French P. Open versus minimally invasive lumbar microdiscectomy: Comparison of operative times, length of hospital stay, narcotic use and complications. Minim Invasive Neurosurg. 2008;51:30–5. doi: 10.1055/s-2007-1004543. [DOI] [PubMed] [Google Scholar]
- 8.Lau D, Han SJ, Lee JG, Lu DC, Chou D. Minimally invasive compared to open microdiscectomy for lumbar disc herniation. J Clin Neurosci. 2011;18:81–4. doi: 10.1016/j.jocn.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Maffulli N, Capasso G, Testa V. Glove perforation in pediatric orthopaedic surgery. J Pediatr Orthop. 1991;11:25–7. doi: 10.1097/01241398-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Mayer HM, Brock M. Percutaneous endoscopic discectomy: Surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78:216–25. doi: 10.3171/jns.1993.78.2.0216. [DOI] [PubMed] [Google Scholar]
- 11.Moussa FW, Anglen JO, Gehrke JC, Christensen G, Simpson WA. The significance of positive cultures from orthopedic fixation devices in the absence of clinical infection. Am J Orthop (Belle Mead, NJ) 1997;26:617–20. [PubMed] [Google Scholar]
- 12.Osterman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation: A randomized controlled trial with 2 years of follow-up. Spine. 2006;31:2409–14. doi: 10.1097/01.brs.0000239178.08796.52. [DOI] [PubMed] [Google Scholar]
- 13.Righesso O, Falavigna A, Avanzi O. Comparison of open discectomy with microendoscopic discectomy in lumbar disc herniations: Results of a randomized controlled trial. Neurosurgery. 2007;61:545–9. doi: 10.1227/01.NEU.0000290901.00320.F5. [DOI] [PubMed] [Google Scholar]
- 14.Robinson AH, Drew S, Anderson J, Bentley G, Ridgway GL. Suction tip contamination in the ultraclean-air operating theatre. Ann R Coll Surg Engl. 1993;75:254–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Sankar B, Ray P, Rai J. Suction drain tip culture in orthopaedic surgery: A prospective study of 214 clean operations. Int Orthop. 2004;28:311–4. doi: 10.1007/s00264-004-0561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddaraju VM, Yap VB, Ong B, Guo CM, Chen J, Tow B, et al., editors. Society for Minimally Invasive Spinal Surgery 2011 Annual Conference. Las Vegas, NV: 2011. Oct 21-23, Comparison of surgical site infections and risk factors for minimally invasive versus open spinal surgery [abstract] [Google Scholar]
- 17.Smith JS, Fu KM, Polly DW, Jr, Sansur CA, Berven SH, Broadstone PA, et al. Complication rates of three common spine procedures and rates of thromboembolism following spine surgery based on 108,419 procedures: A report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2010;35:2140–9. doi: 10.1097/BRS.0b013e3181cbc8e7. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451–9. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, et al. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA. 2006;296:2441–50. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]


