Abstract
Suppression of dendritic cell function in HIV-1 infection is thought to contribute to inhibition of immune responses and disease progression, but the mechanism of this suppression remains undetermined. Using the rhesus macaque model, we show B7-H1 (PD-L1) is expressed on lymphoid and mucosal dendritic cells (both myeloid DC and plasmacytoid DC) and its expression significantly increases after SIV infection. Meanwhile, its receptor, PD-1 is upregulated on T cells in both peripheral and mucosal tissues, and maintained at high levels on SIV-specific CD8+ T cell clones in chronic infection. However, both B7-H1 and PD-1 expression in SIV controllers was similar to controls. Expression of B7-H1 on both peripheral mDCs and pDCs positively correlated with levels of PD-1 on circulating CD4+ and CD8+ T cells, viremia and declining peripheral CD4+ T cells levels in SIV-infected macaques. Importantly, blocking DCs B7-H1 interaction with PD-1+ T cells could restore SIV-specific CD4+ and CD8+ T cell function as evidenced by increased cytokine secretion and proliferative capacity. Combined, the results indicate interaction of B7-H1/PD-1 between APCs and T-cell correlates with impairment of CD4+T-helper cells and CTL responses in vivo, and all are associated with disease progression in SIV infection. Blockade of this pathway may have therapeutic implications for HIV-infected patients.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that play a key role in bridging the innate and adaptive immune responses. Upon antigen exposure, DCs undergo phenotypic and functional maturation and migrate to secondary lymphoid tissues where they initiate adaptive T-cell responses (1–3). Distinct subsets of circulating DCs can be identified in blood, including myeloid dendritic cells (mDCs, Lin-HLA-DR+CD11c+) and plasmacytoid dendritic cells (pDCs, Lin-HLA-DR+CD123+), which show different phenotypic and functional properties (4–5). Functionally impaired DCs have been proposed to be responsible for the failure of T cell immunity and antiviral immune responses (6). During SIV or HIV infection, both mDCs and pDCs have been shown to decrease in frequency and/or function (7). HIV-infected DCs also facilitate transfer of virus to CD4+ T cells in vitro, which may play a role in HIV-1 transmission and CD4+ T cell loss (8). Thus, DCs play a key role in regulation of immune responses and disease progression in HIV-1-infected individuals.
B7-H1 (also called PD-L1) is a member of the B7 family of costimulatory molecules, which are emerging as important mediators of various host immune responses. B7-H1 is differentially expressed on various cell subsets, and to different extents on human and murine cells. Human B7-H1 is constitutively expressed at low levels on dendritic cells, activated T cells (compared with high-expression on activated murine T cells), and is also highly expressed on monocytes and tumor cells (9). Both B7-H1 and B7-DC (PD-L2) are ligands for the PD-1 receptor, which is a negative regulatory receptor on activated T and B cells. For example, B7-H1 expression has been correlated with increased IL-10 production (a TH-2 or T cell “dampening” cytokine), suppression of CTL responses, induction of apoptosis of tumor-specific T cells, FoxP3+Treg cell production, as well as various states of unresponsiveness including T-cell anergy and exhaustion (10–14). Blocking PD-L1, but not PD-L2 significantly restored Ag-specific T cell responses, and promotes CD8 T-cell differentiation into functional cytolytic T lymphocytes (CTLs) (15,16), also suggesting a role for B7-H1 in the inhibition of T-cell responses.
Several groups have shown that PD-1 expression is elevated on HIV-specific (17–19), HBV-specific (20), and HCV specific T cells (21). B7-H1 is also reported to be upregulated on peripheral lymphocytes and associated with HIV-1 disease progression (22). Here, we show that up-regulation of B7-H1 on both mDCs and pDCs in peripheral and mucosal tissues from SIV-infected macaques correlates with PD-1 up-regulation on T-cell subsets, and with declining peripheral CD4+ T cell counts. Furthermore, blockade of B7-H1 on monocyte-derived DCs improved SIV-specific T cell function and proliferation. These findings suggest that up-regulation of B7-H1 on DCs may contribute to suppression of SIV-specific immune responses and disease progression. These studies were designed to address the significance and impact of this phenomenon in regulation of SIV infection and disease progression.
Materials and Methods
Animals and virus
A total of twenty-one adult rhesus macaques (Macaca mulatta) of Indian origin, which were initially negative for HIV-2, SIV, type D retrovirus, and STLV-1 infection were examined in this study. All animals were housed at the Tulane National Primate Research Center in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. Of these, five were uninfected controls, and the rest infected with SIVmac251 and euthanized in either acute infection (8–10 days; n=4) chronic asymptomatic stage, in which no overt signs of disease were detected (n=4), symptomatic AIDS stage (n=4), or animals previously infected with high viremia, but subsequently controlled viral replication to undetectable levels (<125 copies per ml) in plasma (elite controllers; n=4). Macaques with AIDS all had either opportunistic infections including Pneumocystis carinii pneumonia, disseminated Mycobacterium avium infection, or SIV encephalitis). All tissue samples were collected at necropsy except for those from elite controllers, which were collected by biopsy, as they remain clinically healthy and are considered important for further study.
Phenotyping blood and tissue mononuclear cells
Peripheral blood, lymph node, and intestinal tissues were isolated and processed using previously described techniques (41). Briefly, tissues were collected from the jejunum and mesenteric lymph nodes within minutes of euthanasia and processed immediately to prepare cell suspensions. Multi-color flow cytometry was used to enumerate percentages of various cell types and to phenotypically characterize macaque pDCs and mDCs in EDTA-treated whole blood, or single-cell tissue suspensions of mesenteric lymph node and jejunum using previously described criteria (23). Briefly, CD123+ pDCs or CD11c+ mDCs were identified within HLA-DR+ (clone L243) and lineage negative (Lin-) populations using: a FITC-conjugated antibody cocktail to Lin markers CD3ε/(SP34-2); CD14/(M5E2); CD16 (3G8); and CD20/(L27), and then APC-conjugated anti-CD11c and PerCP-Cy5.5-conjugated anti-CD123 (7G3) to define pDCs and mDCs respectively. B7-H1 expression was detected using either Biotin-anti-B7-H1 (R & D Systems) and indirect staining using streptavidin–Alexa 700, or PE-conjugated anti-B7-H1 (29E.2A3, BioLegend). B7-H1 up-regulation on DCs after SIV infection was consistent using both indirect and direct detection methods for B7-H1 (data not shown). Expression of PD-1 was evaluated on T cell subsets using the following antibodies: FITC-conjugated-anti-CD4 (L-200), PE-TxR-conjugated-anti-CD8 (3B5, Caltag), APC-conjugated-anti-PD-1 (EH12.2H7, BioLegend) and Alexa 700-conjugated-anti-CD3 (SP34-2). Soluble fluorochrome-labeled pMamu-A*01 tetramers (Gag CM9 and Tat SL8) were kindly provided by Dr. Marcelo Kuroda (TNPRC). Isotype-matched controls for each fluorochrome were included in all experiments. All antibodies and reagents were purchased from BD Biosciences Pharmingen (San Diego, CA), unless otherwise noted, Stained samples were resuspended in BD Stabilizing Fixative (BD Biosciences) and stored in the dark at 4°C overnight and acquired on a BD LSRII flow cytometer (Becton Dickinson) the next day. Data was analyzed with Flowjo software (Tree star, Ashland, OR).
Phenotyping DC in tissues by multilabel confocal microscopy
Three color immunofluorescent staining for CD4, CD3 (T cells) and PD-1 or/and B7-H1 was performed on mesenteric lymph nodes of SIV-infected animals to visualize the distribution of PD-1+ and B7-H1+ subsets by confocal microscopy. Tissues were stained using unconjugated primary antibodies and then with secondary antibodies conjugated to Alexa 488 (green), Alexa 568 (red) or Alexa 633 (blue)(Molecular Probes, Eugene, OR). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Individual optical slices representing 0.2 um and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.62) and Adobe Photoshop (version 7.0) were used to assign colors to the channels collected: HNPP/Fast Red, which fluoresces when exposed to a 568-nm wavelength laser, appears red; Alexa 488 (Molecular Probes) appears green; Alexa 633 (Molecular Probes) appears blue; and the differential interference contrast (DIC) image is gray scale. The four channels were collected simultaneously. In some tissues and to differentiate between individual cells, To-pro3 (nuclear marker, Molecular Probes) was used at 1 μg/ml, incubated for 5 minutes, and tissues were then washed in PBS. Co-localization of antigens is demonstrated by the addition of colors as indicated in the figure legends.
Preparation of monocyte-derived DCs from blood for in vitro assays
Monocyte derived dendritic cells (MoDCs) from chronically SIV-infected Rhesus macaques (n=3) were generated from heparinized peripheral blood using previously established protocols, with minor modifications (27). In brief, CD14+ monocytes were positively selected using magnetic beads (Miltenyi Biotec), as assessed by a minimum 95% purity for CD14 expression by flow cytometry, and cultured at 1.5–2×106 cells/3 ml in RPMI 1640 medium, supplemented with 2 mM L-glutamine, 50 μM 2-ME, 10 mM HEPES, penicillin (100 U/ml), streptomycin (100 μg/ml) (all Invitrogen), human rGM-CSF (50ng/ml, R&D Systems), human rIL-4 (25ng/ml, R&D Systems), and 5% human AB serum (PAN Biotech). On day 6, an equivalent 20μg/ml SIV lysate (Zymetrix, NY) was added for an additional 24 h incubation. Then, MoDCs were matured by incubation with IL-1β, TNF-a, IL-6 (all at 10ng/ml, R&D Systems), and 10 μM PGE2 (Sigma-Aldrich) for another 24 h at 5 × 105/well in 24-well plates. Numbers of viable large DCs were determined by trypan blue exclusion and mature MoDCs were used for phenotypic analysis or other experiments. The phenotype of DCs was characterized by flow cytometry using FITC-, PE- or PerCP-labeled anti-human mAbs with known cross-reactivity to rhesus macaque CD80 (L307.4), CD83 (HB15e), CD86 (Fun-1) and HLA-DR or the appropriate isotype controls (all from BD Pharmingen), and PE-B7-H1 (29E.2A3) from BioLegend. Cells were analyzed on a FACSCalibur cytometer with FlowJo software (BD Biosciences).
Cytospin preparation of SIV Ag-loaded DC
For examining antigen ingestions and presentation of DC, viable SIV antigen-loaded and unloaded DCs (5–10×103 cells/slide) were spun onto glass slides, dried overnight, and examined for p28 antigen by immunofluorescence. Images were acquired at 100X magnification by confocal microscopy.
Enrichment of T cells
To enrich for rhesus macaque resting T cells, 1×108/ml PBMCs were labeled with PE-conjugated anti-human HLA-DR mAb (BD Biosciences), followed by anti-PE-mAb-conjugated magnetic beads (Miltenyi Biotec). HLA-DR-negative cells were then used as responder cells in T cell response assays below. Purity of T cells as monitored by staining with FITC-conjugated mAb to CD3 was ≥90% in all experiments.
Antigen presentation assay
Purified T cells (5×105 T cells) were cocultured with autologous SIV Ag-loaded and unloaded MoDCs at DC to T cell ratios of 1:20, 1:60, and 1:180 in the presence of 10μg/ml anti-B7-H1 or control Ig. All samples were incubated for 1 h at 37°, followed by the addition of Brefeldin A (5μg/ml, BD Biosciences) and incubated for an additional 5 h. 5×105 T cells in media with and without SEB (1μg/ml) were used as positive and negative controls, respectively. Cells were then stained with Pacific Blue-anti-CD3, APC-anti-CD4 and PE-TxR-anti-CD8, followed by fixation and permeabilization for subsequent intracellular staining with PE-Cy7-anti-IFN-γ. Samples were acquired on a FACSAria (Becton Dickensen) and data analyzed using FlowJo software (Tree Star, Inc.)
T cell proliferation with antigen-loaded DC co-cultures
Proliferation assays were based on carboxyflorescein diacetate succinimidyl ester (CFSE) dilution assays. Responder T cells were prepared as above and labeled with 5μM CFSE (Molecular Probes, Invitrogen), and adjusted to 1×106/ml. SIV lysate Ag-loaded and unloaded MoDCs were added at DC to T cell ratios of 1:20, 1:60, 1:180 to 105 autologous CFSE-T cells in the presence of 10μg/ml anti-B7-H1 or control Ig., Controls consisted of 105 T cells with and without SEB as above. On day 6, the cells were stained using APC-conjugated CD3 Ab. T cell proliferation was evaluated via dilution of CFSE on a FACS Calibur flow cytometer. Percentages of cells with diluted CFSE (CFSE+low) were determined in gated populations of total CD3+ T cells. Cytokines in supernatants were detected using a BD FACSAarray Bioanalyzer according to manufacture’s instruction.
Statistics
Graphical presentation and statistical analysis of the data were performed using GraphPad Prism 4.0 (GraphPad Software, SanDiego, CA). Comparisons among groups were analyzed by a one-way ANOVA and Mann-Whitney T-test. P values <0.05 were considered statistically significant. Correlations between samples were calculated and expressed using the Spearman’s coefficient of correlation.
Results
Distribution, phenotype, and B7-H1 expression on mDCs and pDCs in SIV-infected rhesus macaques
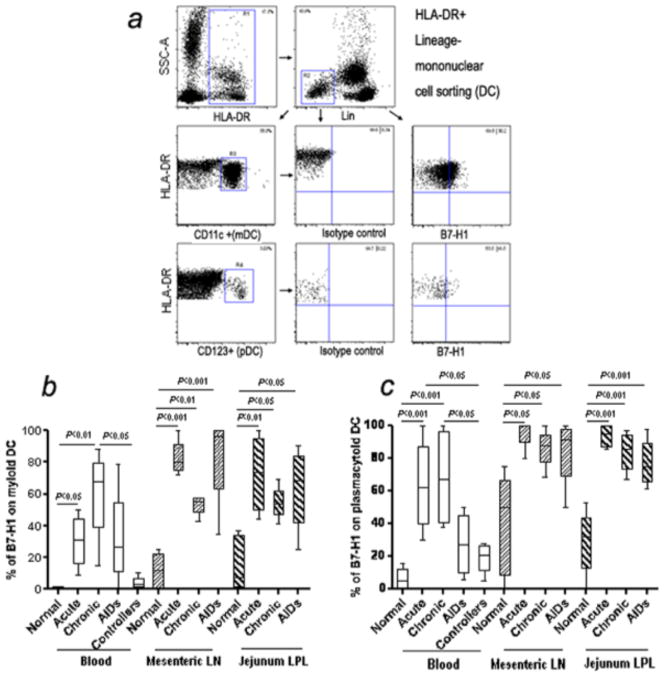
To evaluate a potential link between expression of B7-H1 on DCs and the immunopathology of SIV-infected rhesus macaques, we examined the two major DC subsets previously described (23,24) as Lineage negative myeloid DC (mDCs) (HLA-DR+CD11c+) or plasmacytoid DC (pDCs) (HLA-DR+CD123+) in PBMC from blood, or mononuclear cells isolated from mesenteric lymph node and jejunum lamina propria, and B7-H1 expression on mDCs or pDCs was examined (Fig. 1a). The percentage of mDCs and pDCs comprised between 5–60% and 1–5% of the Lin neg., HLA-DR+ fraction in normal (uninfected) blood respectively (data not shown). B7-H1 was constitutively expressed at low levels on both mDCs and pDCs in normal animals, but significantly up-regulated on DCs from blood, mesenteric lymph node and jejunum lamina propria after SIV infection (Fig. 1b and c). B7-H1+DCs were comparable between SIV-infected and uninfected animals. For mDCs, the percentage of B7-H1 positive mDCs in blood from acute and chronic stage were significantly higher than healthy controls (p<0.05), and expression levels of B7-H1 on mDCs were also markedly up-regulated in mesenteric lymph node and jejunum lamina propria in the acute, chronic, or AIDS stage animals (p<0.05). In addition, significant up-regulation of B7-H1 on mDCs was observed in the mesenteric lymph node (p<0.001) after SIV infection compared with controls (Fig. 1b). Similar to mDCs, up-regulation of B7-H1 on pDCs was also observed, including in blood, mesenteric lymph node and jejunum lamina propria-derived pDCs. Percentages of B7-H1+pDCs from jejunum lamina propria were significantly higher in SIV-infected animals than healthy controls (p<0.001) (Fig. 1c). Interestingly, there were no significant difference in frequency of B7-H1+ circulating mDCs or pDCs between controllers and normal controls. This data suggests that up-regulation of B7-H1 on both mDCs and pDCs correlates with SIV disease progression.
Figure 1.
(a) Rhesus macaque myeloid DC and plasmacytoid DC were identified in blood as HLA-DR+, lineage negative (Lin-) cells as depicted. PBMCs were first selected based on side scatter and HLA-DR+ (R1 gate) and further defined as HLA-DR+ Lin- cells (R2 gate). CD11c+HLA-DR+ (mDCs) and CD123+ HLA-DR+ (pDCs) events within R2 were defined in R3 and R4, respectively. B7-H1 expression on macaque mDCs or pDCs in blood is shown in representative histograms. (b and c) Expression of B7-H1 on myeloid DC and plasmacytoid DC from blood and mucosal tissues in SIV-infected macaques. Note marked and significant increases in B7-H1 expression in blood, mes LN, and jejunum pDCs and mDCs in SIV infected animals in acute and chronic infection. Mean values, SE, and statistically significant differences are shown.
Expression of PD-1 on T cells of SIV-infected macaques in situ
Recent studies have shown that the PD-1/B7-H1 pathway plays a major role in regulating T-cell exhaustion and unresponsiveness (25). Further, HIV-specific CD4 and CD8 T cells express high levels of PD-1 in blood of patients with high viremia (17–19). Here, immunohistochemistry and confocal microscopy were performed to compare PD-1 expression on T cells in lymph nodes between normal and infected macaques. As expected, routine histopathology demonstrated expanded germinal centers and margination of the T cell zones typical of lymph nodes in SIV infection, as compared to normal controls (Fig. 2a and b, respectively). However, while PD-1 expression was constitutively expressed in the small follicular regions of uninfected animals (Fig 2c), PD-1+ cells were markedly expanded throughout the expanded germinal centers and even in T cell zones of lymph nodes in SIV infected macaques (Fig. 2d). Three-color confocal microscopy confirmed expanded B cell germinal centers and macrophages interspersed with large numbers of PD-1+ cells that were of lymphocyte morphology and expressed neither B cell nor macrophage markers (Fig. 2e, f) Note PD-1 expression (green) was significantly increased on lymphocytes within these germinal centers in infected macaques (Fig. 2f) compared with controls (Fig. 2e).
Figure 2.

Microarchitecture of the lymph node and expression of PD-1 in tissues post SIV infection. (a,b) Representative Hematoxylin and Eosin (H&E) staining of lymph node sections from a normal (top left, a) and SIV-infected macaque (top right, b; 3 months p.i). Scale bar=100μm. (c and d) Representative PD-1 expression in lymph node of a normal (c) and SIV-infected macaque (d) demonstrating markedly expanded populations of PD-1+ cells in follicular regions after SIV infection (brown). Scale bar = 50 μm. (e) Lymph node from a representative normal macaque showing distribution of B cells (CD20+; red), macrophages (CD68+; blue) in relation to normal PD-1 expression within B cell zones and germinal centers (inset shown in Figure to the right as indicated). (f) Lymph node from a chronically infected macaque (3 mos) showing markedly expanded germinal centers and increased PD-1 expressing cells specifically within germinal centers. Note that PD-1 is expressed only on cells with a lymphocyte morphology and not on B cells or macrophages/DC. Colors indicate B cells (CD20+ red); macrophages (CD68+, blue) and PD-1 (green).
Expression of B7-H1 on DC correlates with PD-1 on T cells in SIV infection
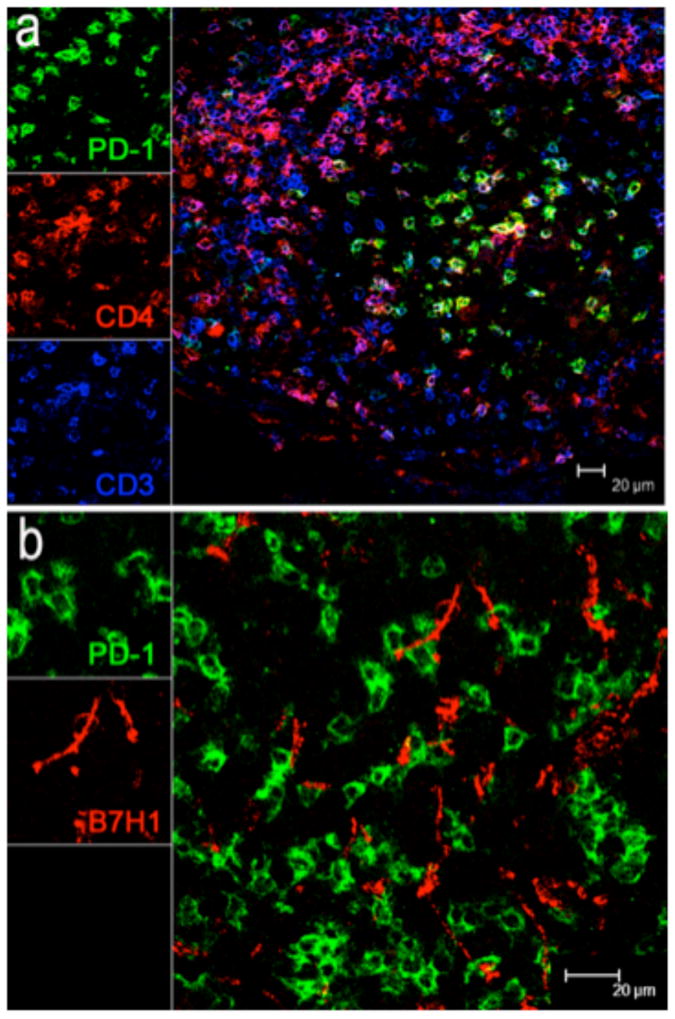
To identify whether B7-H1+ cells interact with PD-1+ T cells in lymphoid tissues from SIV-infected animals, lymph node sections were further analyzed by multi-fluorescent immunohistochemistry to determine their identity and distribution in situ. Co-labelling with CD4 confirmed most of the PD-1+ cells in lymphocytes were CD4+ T cells (Fig. 3a). Further, both B7-H1+ and PD-1+ cells were predominantly distributed within the germinal centers of follicles, yet these were markedly expanded in SIV infected animals (Fig. 2, 3). As shown in Fig. 3, most PD-1+ cells have a lymphocyte morphology and co-express CD3 and CD4. In contrast, B7-H1+ cells displayed obvious dendritic morphology consistent with DC, and were in close proximity to, and even occasionally surrounding PD-1+ T cells (Fig. 3b). The expanded number and distribution of PD-1+ CD4+ T cells in association with B7-H1+ DCs may facilitate greater interaction of these cells, and may regulate immune responses within these crucial secondary lymphoid tissues.
Figure 3.
(a) Distribution and co-expression of PD-1 on CD4+ T (CD3+) cells in lymph nodes of a representative SIV-infected macaque by confocal microscopy. Immunohistochemistry demonstrates PD-1 (green) expression is primarily co-expressed on CD4+ T cells in germinal centers in chronic SIV infection. (scale bar = 20μm). (b) Co-localization of B7-H1 (red) and PD-1 (green) expression in lymph node of a chronically infected macaque 3 months after infection. Note B7-H1+ cells (red) have a dendritic morphology and co-localize with PD-1+ cells (green) which have a lymphocyte morphology and shown to be CD4+ T cells in (a). B7-H1+ cells (red) are mostly found in follicular areas, and usually in close proximity and often direct contact with PD-1+CD4+ T cells. (scale bar = 20μm).
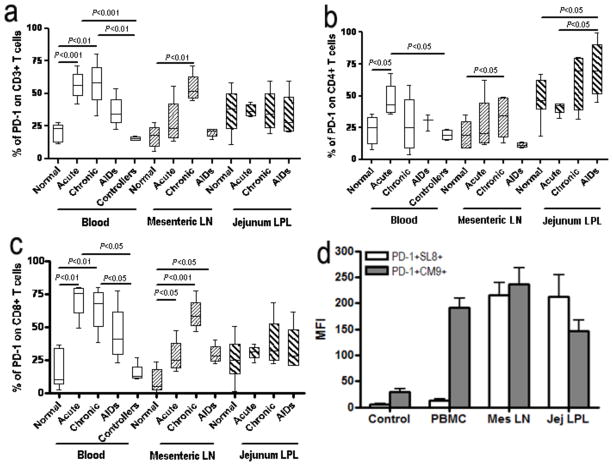
We next compared co-expression of PD-1 on total CD3+ T cells, CD4+, and CD8+ T cells in blood, mesenteric lymph nodes, and jejunum lamina propria (major target tissues for HIV) in acute, chronic and AIDS stages of SIV infection, and in blood from controllers by flow cytometry (Fig. 4). Prior to infection, about 21% of total CD3+ T cells in blood expressed PD-1, but this frequency was significantly elevated in acute and chronic stages of SIV infection (p<0.01). In mesenteric lymph nodes, PD-1+CD3+ T cells were significantly increased compared to normal controls (p<0.01)(Fig. 4a). Further, levels of PD-1 expression were elevated on CD4+ T cells in blood in acute, mesenteric lymph nodes in chronic, and jejunum in AIDS stages, compared with normal controls (p<0.05)(Fig. 4b). In fact, increases in PD-1 expression appeared to be limited to CD4+ T cells in the gut in SIV infection, as there was not a significant increase on CD8+ or total (CD3+) T cells (Fig. 4a, 4b and 4c). During the acute and chronic stage of infection, the frequency of PD-1 positive CD8+ T cells in blood and mesenteric lymph nodes was higher than in normal macaques (p<0.01), and this frequency was also higher in mesenteric lymph nodes in macaques with AIDS. The jejunum maintained high frequencies of PD-1 positive CD8+ T cells, yet did not show significant differences (Fig. 4c). In addition, SIV specific CD8+ T cells (gag CM-9 and tat SL-8 responsive) had high levels of surface PD-1 expression (Fig 4d). Notably, the frequency of PD-1 positive T cells in blood was similar between controllers and normal controls. Combined, these results clearly demonstrate up-regulation of PD-1 on both CD4+ and CD8+ T cell subsets in both mucosal and systemic lymphoid tissues of SIV-infected macaques, yet the dynamic responses of each appear to differ between tissues.
Figure 4.
PD-1 expression on T cells in blood, mesenteric LN and lamina propria in SIV-infected macaques. (a, b and c) Percentage of PD-1 expression on CD3+ (a), CD4+ (b) and CD8+ T cells (c) in normal, acute, chronic, and AIDS stage infection, and symptomatic AIDS. Note marked expansion of PD-1+ T cells occurs and persists in most tissues throughout SIV infection. (d) Surface PD-1 is predominantly expressed on SIV-specific CD8+ T cells clone (CM9 and SL-8) from chronic SIV-infected macaques. Mean values, SE, and statistically significant difference are shown.
Correlation of B7-H1 on circulating DC with PD-1 on T cells, CD4+ T cells, and viremia in SIV infection
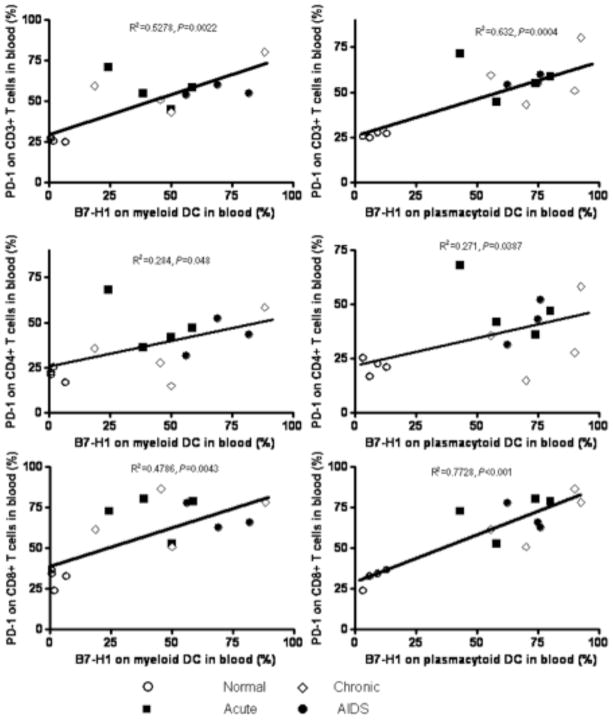
Since both B7-H1 expression on DCs and its receptor PD-1 on T cells were significantly up-regulated after SIV infection, we sought a correlation between B7-H1 expression on DCs and PD-1 on T cells in normal and SIV-infected animals. In blood, percentages of B7-H1+ mDCs significantly correlated with percentages of PD-1+ CD3+ T cells (R2=0.5278, p=0.0022), CD4+ T cells (R2=0.284, p=0.048) and CD8+ T cells (R2=0.4786, p=0.0043) (Fig. 5). Percentages of B7-H1+pDCs also positively correlated with PD-1+ CD3+ T cells (R2=0.632, p=0.0004), CD4+ T cells (R2=0.271, p=0.0387) and CD8+ T cells (R2=0.7728, p<0.001) (Fig. 5). Combined, this data indicates B7-H1 expression on mDCs and pDCs correlates with PD-1 expression on T cells in blood and major lymphoid targets of SIV infection.
Figure 5.
Positive correlations between B7-H1 levels on mDC (left panels) and pDC (right panels) with PD-1+ on T cell subsets including total T Cells (CD3+; top panels); CD4+ T cells (middle) and CD8+ T cells (bottom).
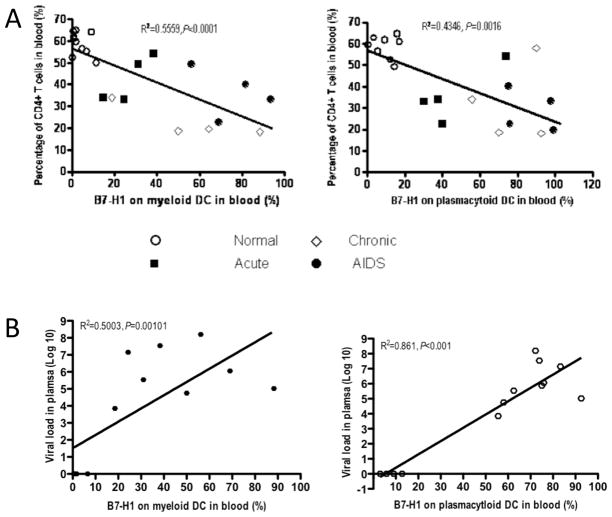
Since progression of HIV infection is associated with reductions in CD4+ T cells, increased viral loads, and immune activation, we sought correlations between B7-H1 expression on mDCs and/or pDCs, CD4+ T cells and viral loads in plasma. There was a highly significant inverse correlation between percentages of CD4 cells and both B7-H1+mDCs (R2=0.5559, p<0.0001) and pDCs (R2=0.4346, p<0.0016) (Fig. 6a). Similarly, there was a positive correlation between SIV viremia and both B7-H1+ mDCs (R2=0.5003, p<0.01) and pDCs (R2=0.861, p<0.001) (Fig. 6b). These data indicates B7-H1 expression on DCs is markedly increased in animals with declining levels of CD4+ T cells in SIV infection, and suggests B7-H1 expression on DCs may be involved in progression to AIDS.
Figure 6.
Correlations of B7-H1 levels on mDCs (left) and pDCs (right) with percentages of circulating CD4+ T cells and viral load in plasma of SIV-infected rhesus macaques. (a) Inverse correlation of percentage of B7-H1 on circulating DCs and CD4+ T cells in SIV-infected rhesus macaques. (b) Note a positive correlation of percentage of B7-H1 on circulating mDCs (filled hexagon), or pDCs (open hexgon) and SIV viral load in plasma in SIV-infected rhesus macaques.
Blockade of B7-H1 increases dendritic cell-mediated SIV-specific T cell responses
It is well known that DCs are potent APC, and DC maturation is associated with increased T cell stimulatory capacity. To determine if B7-H1 up-regulation on DCs is involved in suppression of T-cell responses, monocytes were obtained from PBMC and MoDC’s prepared as described in Materials and Methods including 6 days of inoculation with IL-4 and GM-CSF. The immature DCs were incubated with SIV lysate at day 6 and finally stimulated by maturation cocktail for 48 h. FACS analysis was performed to analyze maturation markers on immature DCs and antigen-loading mature DCs, respectively. As shown in Fig. 7a, mature DCs including the SIV antigen-loaded mature DC exhibited uniform up-regulation of CD80, CD83 and CD86, as compared with immature DCs, Meanwhile, B7-H1high expression on mature MoDCs was also induced, consistent with previously reports (14,26).
Figure 7.
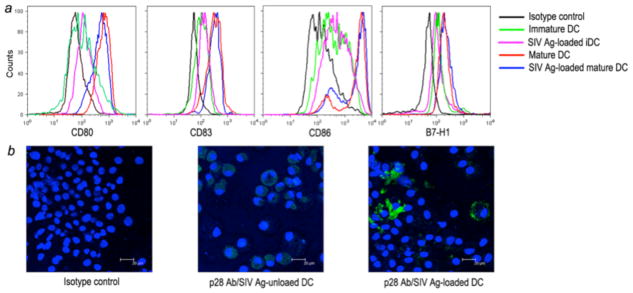
Identification of mature DCs and SIV antigen-loaded DCs by flow cytometry (top) and fluorescent microscopy (bottom). (a) Histogram of mature monocyte-derived macaque DCs. Monocyte-derived immature DCs (iDC) were cultured with maturation cocktail and phenotyped by FACS analysis of FITC-anti-CD80 or -CD86, PE-anti-CD83 or -B7-H1, and PerCP-anti-HLA-DR. (b) Immunofluorescent staining of SIV lysate pulsed DCs. Immature DCs were pulsed with SIV lysate for 24 hours, and then incubated for 2 days in DC maturation cocktail. SIV lysate-unloaded and -loaded DCs were cytospun onto slides, fixed and stained with a control antibody (left), or anti-p28 monoclonal antibody (green). Nuclei are stained in blue. Representative fields (scale bar = 20μm) SIV p28 antigen staining of loaded and unloaded DCs are shown.
To examine the effect of B7-H1 expression on activation of SIV-specific T cell responses by mature DCs, immature DCs (mDCs) were loaded with SIV lysate, and further matured in maturation cocktail. Matured (pDCs) DCs from infected macaques captured significant amounts of SIV antigen as detected by immunostaining for SIV p28 protein. Note intense staining (green) for SIV p28 was detected in cells (Fig. 7b). These SIV-loaded mature DCs were co-cultured with autologous SIV-primed T cells at different ratios and monitored for T cell activation by measuring IFN-γ release and T-cell proliferation.
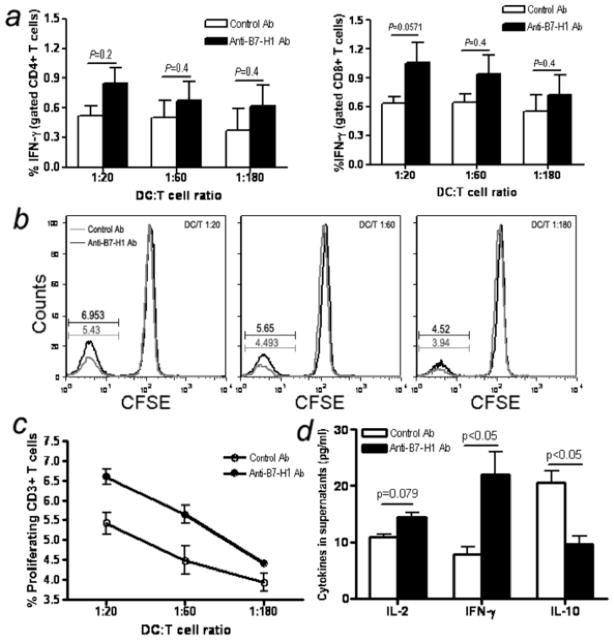
For measuring T-cell activation, intracellular IFN-γ producing CD4+ or CD8+ T cells were examined after co-culture of T cells with antigen loaded mature DCs. SIV antigen-loaded DCs consistently increased IFN-γ production from both CD4+ and CD8+ T cells upon addition of anti-B7-H1 Ab compared with irrelevant control antibody at different ratios (CD4+T-cell, p=0.2 for 1:20, p=0.4 for both 1:60 and 1:180; CD8+T-cell, p=0.0571 for 1:20, p=0.4 for both 1:60 and 1:180) (Fig. 8a). We also purified CD8+ T cells from PBMC in macaques and examined the effects of B7-H1 blockade on cytokine expression in purified CD8+ T cells alone. Adding anti-B7-H1 did not result in any effect on CD8+ T cell cytokine production (data not shown). This confirms the activation effect was due to blocking B7-H1 on MoDC rather than on CD8+ T cells. Thus, B7-H1 on MoDCs could inhibit T cell function through B7-H1 and PD-1 interactions, resulting in a decrease of IFN-γ production by T cells, but this inhibitory signal could be rescued by treatment with anti-B7-H1 of MoDCs..
Figure 8.
Effects of B7-H1 blockade on SIV-specific antigen function. (a) Loaded and unloaded DCs were added to 5×105 autologous T cells at shown ratios with control or anti-B7-H1 Ab added at dilutions indicated. Intracellular IFN-γ staining was performed 6 hours after incubation. Means ±SEM of triplicate data are shown. (b and c) Effects of B7-H1 blockade on SIV-specific T cell proliferation. Ranging doses of the loaded DCs were added to 105 autologous T cells, with control or anti-B7-H1 Ab added as indicated and T cell proliferation was assessed by CFSE dilution 6 days later. b, histogram of CFSE dilution. Means (±SEM) of triplicate data are provided. DCs and T cells alone (±SIV lysates) served as negative controls for both assays, which showed background levels less than the unloaded DC-T cells controls. (d) IL-2, IFN-γ and IL-10 cytokine levels in supernatants of T/DC co-culture at 60:1 after 6 days incubation. Bars reflect means of three independent data points.
We also investigated the effect of MoDCs B7-H1 blockade on SIV-specific CD3+ T cell proliferation by co-culture of CFSE-labeling CD3+ T cells and SIV antigen-loaded MoDCs at different ratios. As shown in Fig. 8b and c, the frequency of CFSE-negative CD3+ T cells increased significantly when treated with neutralizing B7-H1 Ab at all ratios tested compared with control Ab (P < 0.05 for 1:20 and 1:60). This suggests B7-H1 up-regulation on DCs is responsible for the decreased CD3+ T cell expansion observed in SIV-infected macaques. Further, B7-H1 expression may be associated with suppression of virus-specific T cell proliferation in vivo, and blockade of B7-H1 may restore the proliferative capacity of T-cells.
Finally, to determine SIV-specific cytokine release from T/DC cocultures following B7-H1 blockade, we activated autologous SIV-primed T cells with SIV antigen-loaded MoDCs at 60:1 ratio for 6 days, and cytokine levels in supernatants were compared. The data showed blockade of B7-H1 increased IL-2 production (P =0.079), and significantly enhanced IFN-γ (P < 0.05) and decreased IL-10 release (P < 0.05) (Fig. 8d). These results strongly suggest up-regulation of B7-H1 expression was associated with suppression of SIV-specific T-cell immune responses after SIV infection.
Discussion
To evade host immunity, HIV-1 uses numerous strategies to impair normal dendritic cell function including preventing activation of potentially protective antiviral immune responses by increasing IL-10 and decreasing IL-12 and IFN-γ production, as well as downregulating expression of surface co-stimulatory molecules (27–30). Utilizing the unique model of pathogenic SIV infection in nonhuman primates, we simultaneously compared expression of B7-H1 on DCs, and its receptor PD-1 on T cells, in the peripheral blood, lymph node, and GALT in various stages of SIV infection, and in blood of elite controllers. These findings demonstrate that up-regulation of B7-H1 on circulating mDCs and pDCs positively correlates with viremia and disease progression in SIV-infected rhesus macaques, and that DCs may be involved in mediating T-cell suppression and dysfunction through the B7-H1/PD-1 pathway in mucosal and secondary lymphoid tissues of infected hosts.
PD-1 expression on virus-specific CD8+ T cells is linked with impairment of immune function (18,19). Further, PD-1 is upregulated upon activation, and functional high-level expression is maintained even by seemingly “exhausted” CD8 T cells (17,25,31). PD-1 and its ligands have important roles in regulating immune defenses against tumors or infectious agents. Recent studies indicate that the interaction between B7-H1 and its receptor PD-1 mediate inhibition of T-cell responses, and negatively regulate cytokine production and proliferation of T cells (32,33). This inhibitory effect has been shown for CD4+ as well as for CD8+ T cells (34,35). Here, IHC analysis showed that PD-1 expression increased on T cells in general, and especially on CD4+ T cells within germinal centers of lymph nodes. Interestingly however, the percentages of PD-1 positive CD8+ T cells were similar to those of CD4+ T cells by flow cytometry. However, when the mean fluorescence intensity (MFI) was compared we found that the percentages did not correlate with the MFI values between CD4+ and CD8+ T cells in LN of chronically SIV-infected macaques. The mean percentage of PD-1+CD4+T cells was 27.17±1.09 but 51.48±9.525 for CD8+ T cells, whereas the MFI of PD-1 expression was 200±76.97 on CD4+ cells and 118.9±21.33 on CD8+ T cells. Thus, CD4+ T cells in germinal center clearly expressed higher levels of PD-1 than CD8+ cells, which is likely associated with more frequent DC/T cell interaction in this region as evidenced by co-localization of PD-1 on T cells and B7-H1 on DC (Fig. 3). The PD-1:PD-L pathway thus appears to be a key determinant of the outcome of infection, regulating the delicate balance between effective antimicrobial immune defenses and immune mediated tissue damage (36,37). Thus, these data support that up-regulation of B7-H1 on DCs and PD-1 on T cells contributes to suppression of T cell function during SIV infection. B7-H1 upregulation could also contribute to functional impairment of DCs as a mechanism of immune evasion by SIV. Several studies in vivo have found a greater effect of anti-B7-H1 blockade compared with anti-PD-1 or anti-PD-L2 blockade, and these suggest targeting B7-H1 could be a better strategy for treating chronic viral infections (31).
Dendritic cells are a heterogeneous population of APCs important for bridging the initiation and regulation of innate and adaptive immune responses. Myeloid DCs are phenotypically and functionally similar to monocyte-derived dendritic cells (MoDC), inducing Th1 cell responses (38). Plasmacytoid DCs are potent producers of alpha interferons in response to enveloped viruses, and appear to direct T-cell responses (39). Blockade of B7-H1 on MoDCs may thus restore SIV-specific T-cell function, and is supported here by the finding that B7-H1 expression on DCs is involved in functional T-cell suppression in SIV infection.
It has been reported that B7-H1 mRNA in lymphoid tissue and PBMCs increases in HIV-infected patients, that B7-H1 is inducible by IL-10, and that increased B7-H1 expression correlates with HIV-1 disease progression (22). B7-H1 expression on dendritic cells is also involved in the induction and maintenance of T cell anergy (40). Here, we show that B7-H1 expression is normally expressed at low levels on mDCs and pDCs in healthy macaques, but is significantly elevated in frequency on both B7-H1+ mDCs and pDCs in blood and mucosal tissues, and that levels inversely correlate with peripheral CD4+ T cell counts after SIV infection. However, B7-H1 expression on DC was low, and similar to uninfected controls in animals controlling infection. Further, immunohistochemistry demonstrated B7-H1+ cells and PD-1+ T cells co-localized in the hyperplastic/expanded follicles of lymph nodes of SIV-infected macaques. Although there is no single specific marker suitable for identifying DC in tissues by immunohistochemistry, B7-H1+ cells clearly displayed DC-like morphology in B7-H1/PD-1 double positive regions of the lymph node (Fig. 4). Combined, these data indicate interactions between B7-H1+DCs and PD-1+ T cells facilitate regulation of immune responses, and in particular, suppress T cell function during SIV infection, at least in organized lymphoid tissues. B7-H1 upregulation on DCs could also attribute to functional impairment of DCs as mechanism of immune evasion by SIV. Regardless, B7-H1/PD-1 interactions appear to be a key correlate of the outcome of SIV infection, and may be involved in regulating the delicate balance between effective immune defense and immune dysfunction.
In summary, this work represents the first description that B7-H1 upregulation on mDCs and pDCs parallels upregulation of PD-1 expression on T cells in mucosal and systemic lymphoid tissues, and inversely correlates with CD4+ T cell loss in SIV-infected rhesus macaques. Further, T cell effector function could be rescued by blocking B7-H1/PD-1 interaction in cells from SIV-infected macaques. The suppressive interaction between DCs and T cells involving the B7-H1/PD-1 pathway in HIV infection suggest that targeted therapies exploiting this pathway may represent a promising approach for enhancing T-cell immunity in infected patients.
Acknowledgments
Grant support: This work was supported by NIH grants AI49080, AI084793, and RR000164. We also thank the James B. Pendleton Charitable Trust Foundation for an instrumentation grant.
We thank Julie Bruhn, Calvin Lanclos, and Desiree Waguespachek for flow cytometry support and Janell LeBlanc, Kelsi Rasmussen, Maryjane Dodd, and Maury Duplantis for technical support.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 3.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 4.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 5.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura T, Gatanaga H, Borris DL, Connors M, Mitsuya H, Blauvelt A. Decreased stimulation of CD4+ T cell proliferation and IL-2 production by highly enriched populations of HIV-infected dendritic cells. J Immunol. 2003;170:4260–4266. doi: 10.4049/jimmunol.170.8.4260. [DOI] [PubMed] [Google Scholar]
- 7.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–67. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 8.Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 11.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 12.Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–390. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- 13.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, Dong H, Kwon ED, Novak AJ, Markovic SN, Pittelkow MR, Witzig TE, Ansell SM. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, Brockstedt DG, Dubensky TW, Jr, Chen L, Pardoll DM, Drake CG. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180:4836–4847. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 19.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 20.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P, Dong H, Maserati R, Shearer GM, Chen L, Clerici M. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101:2514–2520. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 23.Diop OM, Ploquin MJ, Mortara L, Faye A, Jacquelin B, Kunkel D, Lebon P, Butor C, Hosmalin A, Barre-Sinoussi F, Muller-Trutwin MC. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol. 2008;82:5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves RK, Fultz PN. Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology. 2007;365:356–368. doi: 10.1016/j.virol.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 26.Jasny E, Eisenblatter M, Matz-Rensing K, Tenner-Racz K, Tenbusch M, Schrod A, Stahl-Hennig C, Moos V, Schneider T, Racz P, Uberla K, Kaup FJ, Ignatius R. IL-12-impaired and IL-12-secreting dendritic cells produce IL-23 upon CD154 restimulation. J Immunol. 2008;180:6629–6639. doi: 10.4049/jimmunol.180.10.6629. [DOI] [PubMed] [Google Scholar]
- 27.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, Meyer L, Goujard C, Lebon P, Sinet M, Hosmalin A. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–310. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 29.Majumder B, Janket ML, Schafer EA, Schaubert K, Huang XL, Kan-Mitchell J, Rinaldo CR, Jr, Ayyavoo V. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005;79:7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 31.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 33.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman FJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 36.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008;180:4875–4884. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 39.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 40.Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 41.Veazey RS, I, Tham C, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]