Abstract
Background
Stress sensitivity and HPA axis activity may be relevant to the development and expression of psychotic disorders. Cortisol secretion has been associated with positive symptoms both in patients with psychotic disorders and in young people at clinical risk for psychosis. Herein, we aimed to replicate these findings, to determine which positive symptoms may be associated with cortisol levels, and to explore any associations with affective symptoms and impaired stress tolerance.
Methods
Thirty-one clinical high risk patients were evaluated in cross-section for associations between salivary cortisol levels upon clinic entry at 11 am, demographic variables, and clinical symptoms.
Results
Salivary cortisol levels were unrelated to medication exposure or demographics, except for higher levels in the ten females studied. Salivary cortisol bore no relationship to overall positive symptom severity but was associated with anxiety, as well as with suspiciousness and impaired stress tolerance, which were themselves highly intercorrelated.
Conclusions
Cortisol secretion in the context of a putative novel social situation (i.e. clinic entry) may be a biological correlate of suspiciousness, impaired stress tolerance and affective symptoms in individuals vulnerable to developing psychosis. These associations are consistent with findings from experience sampling studies in individuals at risk for psychosis as well as basic studies of animal models of schizophrenia.
Keywords: Cortisol, Psychosis, Schizophrenia, Risk, Prodrome, Prodromal
1. Introduction
For several decades, it has been theorized that stress sensitivity and activity of the hypothalamic pituitary adrenal (HPA) axis, as indexed by cortisol secretion, may be relevant to the development and expression of psychotic disorders such as schizophrenia (Rosenthal, 1970; Walker and Diforio, 1997). Studies from the early seventies showed that cortisol secretion escalates in the period leading up to psychosis relapse (Sachar et al., 1970) and exacerbation (Franzen, 1971). In the past decade, Walker and colleagues have demonstrated that cortisol secretion is related in cross-section to positive symptom severity in both patients with schizophrenia (Walder et al., 2000) and in young people at heightened clinical risk for the disorder (Walker et al., 2001), with more recent research (Walker et al., 2010) suggesting that elevated basal salivary cortisol secretion may have predictive value for development of psychotic disorder. Cortisol secretion is an easily assayed biological correlate of the stress sensitivity that is hypothesized to play a role in the generation of positive symptoms (Corcoran et al., 2003), a theorized mechanism supported by both neuroimaging (Garner et al., 2005; Soliman et al., 2008) and preclinical (Lodge and Grace, 2006) studies. The findings by Walker and colleagues are in need of additional support, as another research group has reported different findings, namely an association of plasma morning cortisol secretion not with positive symptoms, as measured with the Brief Psychiatric Rating Scale, but instead with “hassles” and affective symptoms of depression and anxiety (Thompson et al., 2007a).
Herein, we aimed to determine if we could replicate the findings of association of cortisol secretion with positive symptoms in at-risk individuals (Walker et al., 2001), with similar but albeit more limited methodology, sampling salivary cortisol and utilizing symptom measures designed specifically for clinical high risk patients. We also explored whether cortisol secretion was related to specific positive symptoms and/or to affective symptoms and increased sensitivity to everyday stress or “hassles”.
2. Experimental/materials and methods
2.1. Participants
Participants were a subgroup of patients consecutively enrolled early in Phase I (2002–2003) of the Recognition and Prevention (RAP) Program clinical high risk research program in New York. The RAP program designates a hierarchy of risk states, based 1) solely on negative symptoms, 2) on attenuated positive symptoms and 3) finally on the presence of a schizophrenia-like psychosis (Cornblatt et al.,2003). This study was circumscribed to this second group, entitled clinical high risk positive on the basis of positive symptoms (CHR+), as these are most analogous to the ultra high risk patients and schizotypal patients in which cortisol secretion has been previously studied (Walker et al., 2001; Thompson et al., 2007a; 2007b; Walker et al., 2010). The presence of a CHR+risk state was determined using modified criteria (Lencz et al., 2003) from the Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms (SIPS/SOPS; Miller et al., 2003), and is analogous to the Attenuated Positive Symptom Syndrome (APSS) described in the SIPS/SOPS, except that duration of the prodromal state is not limited to the previous year. Exclusion criteria included Axis I diagnoses of psychotic disorder, schizophrenia spectrum disorders, and bipolar disorder, as well as mental retardation (IQ<70), current substance dependence, lack of fluency in English and any history of neurological, neuroendocrine or medical conditions which may affect brain functioning. All procedures were approved by the Institutional Review Board at the Zucker Hillside Hospital, as well as by the Institutional Review Board at the New York State Psychiatric Institute, where the lead author was in research training. Adult participants and parents of minors provided written informed consent, whereas minors provided written assent only.
2.2. Demographic and clinical characteristics
Participants were queried as to demographic characteristics (age, sex, parental socioeconomic status) (the higher SES among two custodial parents — ranked from 5 (low) to 1 (high) (Hollingshead, 1957)). Prescription of antipsychotic or antidepressant medication, as reported by the participant, was each coded as yes/no. The Scale of Prodromal Symptoms (SOPS; Miller et al., 2003) was employed to measure severity of specific and overall positive symptoms, negative symptoms, and a “general symptom” entitled “impaired tolerance to normal stress”, which queries as to sensitivity to normal everyday stressors and hassles. (Anchors for “prodromal” ratings of this item include “thrown off by unexpected happenings in the usual day”; “increasingly challenged by daily experiences” and “avoids or is overwhelmed by stressful situations that arise during the day” (Miller et al., 2003).) Other SOPS “general” symptoms evaluated in an exploratory fashion included sleep impairment and motor abnormalities. Anxiety was assessed with the Beck Anxiety Inventory (Beck et al., 1998), whereas depressive symptoms were determined using a composite score on the Beck Depression Inventory (Beck et al., 1996) and the Children’s Depression Inventory (Kovacs, 1985). Participants ages sixteen and younger were administered the CDI and those ages seventeen and older the BDI. To ensure comparability of these two measures, a total depression score was calculated as a percentage of the maximum possible score. As in previous studies of cortisol in clinical high risk patients (Walker et al., 2001; Thompson et al., 2007a), cortisol was assayed one day before clinical measures were administered.
2.3. Cortisol assays
Salivary cortisol measures were obtained at clinic arrival at 11 am, at the onset of a day of clinical and neuropsychological assessment. Samples were collected using Sarstedt (Germany) polyester swabs without preparation. Participants were instructed to place the swab in their mouth, between their lower lip and gum, and to keep it there until it was wet, a process supervised by research staff. When deemed saturated, the swab was placed in a Salivette plastic vial and capped and labeled. Within hours, the samples were stored at −25 °C at the Zucker Hillside Hospital. Samples were then transported on dry ice to the Nathan Kline Institute, where they were weighed, centrifuged and analyzed by the Analytical Psychopharmacology Laboratory under the supervision of T.A. Cooper. (Of note, this methodology differs from that of Walker and colleagues, who use a passive drip collection technique to assay salivary cortisol.)
Salivary cortisol secretion is a reliable indicator of free cortisol in plasma, the biologically active hormone (van Eck et al., 1996), and salivary cortisol assays have been employed as an index of HPA axis activity in several hundred studies (Nejtek, 2002; Schwartz et al., 1998; Silver et al., 1983). Salivary cortisol levels have been correlated with serum and 24-hour urine cortisol levels in children and adults (Bober et al., 1988; Burke et al., 1985; Woodside et al., 1991; Hermus et al., 1993). In adolescents, salivary cortisol sampling is the method of choice as venipuncture has been associated with dropout rates as high as 67% (Susman et al., 1997), especially among more impaired individuals. The high interreliability of cortisol levels across subsequent days when assayed at the same time within individuals is such that one day of sampling is likely sufficient (Carrion et al., 2002).
2.4. Statistical analysis
Descriptive statistics were employed to represent demographic, clinical and endocrine data. The relationship of demographic variables and medication status (yes/no) to symptoms and cortisol values was explored to identify any potentially confounding demographic variables. Correlational analyses were done to examine the associations among cortisol values with symptoms, using Spearman’s rho, and scatterplots were examined for outliers (given the limited sample size). Partial correlations were done with any potentially confounding variables, including demographics or medication status. Alpha was set at .05 for the hypothesized association of salivary cortisol levels with overall positive symptom severity, as well as for the analyses of salivary cortisol with specific positive and affective symptoms, and stress sensitivity. Bonferroni correction was done for six exploratory tests of association: salivary cortisol levels with three symptoms (negative symptoms, sleep impairment, and motor abnormalities), and intercorrelations among the three symptoms associated with cortisol (suspiciousness, impaired stress tolerance, anxiety).
3. Results
There were 31 clinical high risk (CHR+) patients (21 males; 10 females), 71% white, ascertained from both urban and suburban areas of Queens and counties of Long Island, New York, who arrived promptly to the clinic at 11 am and for whom sufficient saliva was available for assay of cortisol. The ranges and means (SD) of the baseline “11 am” salivary cortisol levels, demographic variables and clinical symptom ratings in this cohort are presented in Table 1, as well as their correlations with salivary cortisol values. Among demographic variables, only sex was related to cortisol secretion, as females had a higher mean cortisol value than males (2.3 (SD 1.3)ng/ml vs. 1.4 (SD 1.0)ng/ml; p=.04); no symptoms varied by sex (data not shown). None of the other demographic variables (e.g. socioeconomic status, age) were associated with cortisol secretion (Table 1) or symptoms (data not shown). The prescription of antipsychotics (13%) was unrelated to salivary cortisol levels (yes: 1.4 (SD 0.7)ng/ml vs. no: 1.8 (SD 1.2)ng/ml; p=.57) but the prescription of antidepressants (27%) was associated with higher cortisol values at a trend level (yes: 2.3 (SD 1.5)ng/ml vs. no: 1.5 (SD 0.9); p=.09).
Table 1.
Endocrine, demographic and clinical characteristics of the CHR+cohort.
| Range | Mean (SD) | Associations with cortisol (Spearman’s rho) | |
|---|---|---|---|
| 11 am salivary cortisol values (ng/ml) | 0.3–5.0 | 1.7 (1.1) | |
| Age | 12–18 | 15.6 (2.0) | −.01 |
| Socioeconomic status | 1–5 | 2.2 (.89) | −.02 |
| Hypothesized associations | |||
| SOPS total positive symptom scores | 3–13 | 7.7 (3.4) | −.05 |
| SOPS unusual thought content | 0–5 | 1.5 (1.7) | .08 |
| SOPS suspiciousness | 0–5 | 2.8 (1.4) | .38* |
| SOPS grandiosity | 0–3 | .37 (.85) | −.30 |
| SOPS perceptual disturbances | 0–5 | 1.7 (1.8) | −.12 |
| SOPS conceptual disorganization | 0–5 | 1.4 (1.3) | −.11 |
| SOPS D4 “Impaired Tolerance to Normal Stress” | 0–5 | 2.1 (1.8) | .42* |
| Beck Anxiety Inventory scores | .02–.71 | .21 (.16) | .38* |
| Composite depression scores | .04–.57 | .30 (.14) | .36 |
| Exploratory | |||
| SOPS total negative symptoms | 1–22 | 9.3 (4.9) | .07 |
| SOPS D1 “Sleep Disturbance” | 0–5 | 2.2 (2.0) | .17 |
| SOPS D4 “Motor Disturbances” | 0–5 | 0.9 (1.2) | .10 |
Uncorrected alpha<.05.
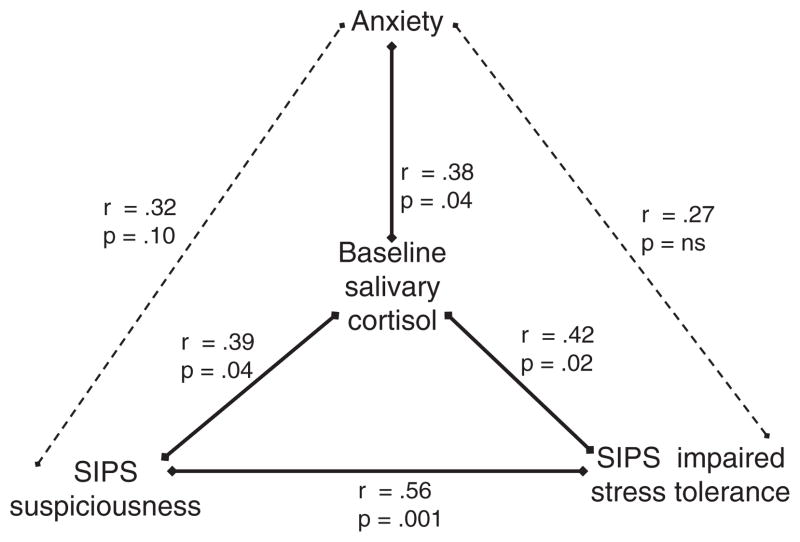
Although cortisol secretion bore no relationship to overall positive symptom severity (Spearman’s rho=−.05; p=.80) it was associated specifically with suspiciousness (Spearman’s rho=.39; p=.04) (Table 1, Fig. 1). Among affective symptoms, cortisol secretion was related to both anxiety (Spearman’s rho=.38, p=.04) and impaired stress tolerance (Spearman’s rho=.42, p=.02) (Table 1, Fig. 1). The inclusion of antipsychotics (yes/no) and antidepressants (yes/no) in regression models did not change the strength of these associations of cortisol with suspiciousness, anxiety or impaired stress tolerance (data not shown).
Fig. 1.
Associations of morning salivary cortisol with symptoms (Spearman rho).
Among these clinical features – suspiciousness, anxiety and impaired stress tolerance – that were associated with salivary cortisol secretion, suspiciousness was highly correlated with impaired stress tolerance (Spearman’s rho=.57, p=.001) (see Fig. 1), even with Bonferroni adjustment for multiple comparisons (alpha=.05/6=.008). These several associations held even with adjustment for age and sex (data not shown). In exploratory analyses, cortisol secretion bore no association with negative symptoms, sleep impairment or motor abnormalities (Fig. 1). (Due to the small size of this sample, there was insufficient power to explore a relationship between cortisol levels and eventual conversion to psychosis.)
4. Discussion
In this cross-sectional study of a cohort of clinical high risk patients, cortisol secretion, as measured by salivary assay, was not associated with overall positive symptoms, despite our hypothesis that it would be, but was related to suspiciousness, anxiety and impaired stress tolerance (and with depression at a trend level). The association with affective symptoms is consistent with findings by Thompson et al., 2007a, in which morning cortisol was assayed in a similarly ascertained cohort but from serum (rather than saliva) samples. As in this 2007 study by Thompson and colleagues, single assays of cortisol secretion were unrelated to concurrent total positive symptom severity. However, we uniquely examined the association of cortisol secretion to specific positive symptoms, and found an association with suspiciousness; both cortisol secretion and suspiciousness were also correlated with the SOPS item “impaired tolerance to normal stress.”
As saliva was assayed at clinic arrival, the resulting cortisol levels may be more an index of response to novelty than of baseline HPA axis activity. Of interest, the mean “11 am” salivary cortisol level at clinic entry in this study of 1.7 (SD 1.1)ng/ml is nearly identical to the mean “11 am” salivary cortisol observed in healthy adolescents upon arrival at a laboratory for psychological research in another study, which is 1.7 (SD 0.8)ng/ml (Klimes-Dougan et al., 2001), despite some differences in methodological details. The psychological correlates of this relatively heightened endocrine response to novelty, especially involving contact with other people in an evaluative context, would be expected to include the arousal of anxiety and stress sensitivity. Elevated salivary cortisol secretion has been associated with shyness in young children (Kagan et al., 1988), internalizing problems in adolescents (Klimes-Dougan et al., 2001), and anxiety and withdrawal behavior across development (Flinn and England, 1997). Among individuals with vulnerability for psychosis, this anxiety and heightened cortisol secretion in a social evaluative context would be expected to be accompanied by the cognate of suspiciousness, through the use of externalizing appraisals (Garety et al., 2001).
These associations of cortisol secretion with affective and positive symptoms (paranoia) are consistent with findings from momentary assessment technology studies with individuals with vulnerability to psychosis (i.e. affected probands in remission and unaffected family members), in which experience to everyday stress elicits negative emotions, enhanced salivary cortisol secretion and subtle psychotic experiences, including paranoia (Myin-Germeys et al., 2001; Myin-Germeys and van Os, 2007; Simons et al., 2008; Collip et al., 2011a, 2011b). As in the current study of clinical high risk patients, the association of cortisol secretion with positive symptoms in individuals at genetic high risk for schizophrenia could not be accounted for by its association with affective symptoms (Collip et al., 2011a, 2011b). Of interest, such stress sensitivity (assessed with momentary assessment technology) may be an endophenotype of psychosis proneness, as it cosegregates in twin studies (Lataster et al., 2008), and its association may be moderated by allelic variations in schizophrenia susceptibility genes i.e. brain-derived neurotrophic factor (BDNF) and catechyl-O-methyl transferase (COMT),(Simons et al., 2008; van Winkel et al., 2008), particularly, as in the case of COMT, in patients with schizophrenia spectrum disorders (Collip et al., 2011b).
The neural mechanisms by which enhanced stress sensitivity can lead to positive symptoms have been elaborated by Grace and colleagues using animal models of schizophrenia (Lodge and Grace, 2006). The ventral subiculum is a central component in mediating stress responses and in context-dependent processing. The ventral subiculum determines the proportion of dopaminergic neurons in the ventral striatum that can be phasically activated by the pedunculopontine tegmental nucleus (PPTg), which conveys the “signal” of sensory stimuli. In a schizophrenia model, abnormally increased activity in the ventral subiculum would lead to increased striatal dopamine activity, which has been associated with both positive symptoms in patients (Laruelle and Abi-Dargham, 1999), and with cortisol release in response to a stressor in individuals with psychosis proneness (psychometric negative schizotypes) (Soliman et al., 2008). As theorized by Heinz (2002): “stress-induced or chaotic activation of dopamine release may attribute incentive salience to otherwise irrelevant stimuli and thus be involved in the pathogenesis of delusional mood and other positive symptoms”.
It cannot be ruled out, however, that stress sensitivity, and its correlates in affective symptoms and suspiciousness, may characterize a group of clinical high risk patients who meet putatively prodromal criteria but who are not among those individuals who eventually develop psychotic disorder. Although Walker et al. (2010) identified increased cortisol secretion as a predictor of conversion to psychosis in 56 clinical high risk patients, Thompson et al. (2007b) found the opposite, albeit in a smaller sample — specifically increased cortisol secretion, and more severe depressive and anxiety symptoms, in clinical high risk patients who appeared to be “false positives” in terms of psychosis development. Differences in sample ascertainment and methodology may account for the discrepant findings in those two studies. Our sample size was too small to address the question of predictive value of cortisol secretion for “conversion” to psychosis.
Limitations of this study are its cross-sectional design, limited assay of salivary cortisol secretion (one sample only), small sample size and absence of a comparison group. A single assay of cortisol might not have covered the intra-individual variability that characterizes cortisol measurements and may have contributed to Type II error, obscuring a potential association between salivary cortisol and total positive symptoms that was found by other investigators in both clinical high risk (Walker et al., 2001) and genetic high risk cohorts (Collip et al., 2011a). The superior methodology used by Walker and colleagues includes multiple assays of salivary cortisol over several hours beginning at 9 am to capture diurnal variation in basal secretion of cortisol, whereas our single salivary assay at 11 am may simply elicit a stress response to a novel experience. Walker and colleagues also use a more standardized methodology, which includes providing specific instructions to participants as to refraining from caffeine, alcohol, dairy products and nonprescription medications, as well as brushing teeth within 30 min prior to sampling. By contrast, in the current study, no restrictions were placed on smoking, caffeine intake, and eating, and there was no documentation for females of phase of the menstrual cycle. Of note, methodological differences make it difficult to compare the mean salivary cortisol level for high-risk patients in this study (1.7 (SD 1.1)ng/ml) with that of Walker and colleagues, who find overall means of ~4.5 ng/ml for salivary cortisol assessed sequentially at 9 am, 10 am and then 11 am in clinical high risk adolescents (Mittal et al., 2007; Walker et al., 2010). Our study was also likely underpowered, such that there was insufficient variance among positive symptoms to detect an association with cortisol, save for suspiciousness, which was more severe. Also, minor physical anomalies were not assessed, which have been associated with cortisol secretion in clinical high risk patients (Mittal et al., 2007).
These limitations can be addressed in future studies of clinical high risk patients and matched controls, which should use momentary assessment technology with concurrent salivary cortisol assay, as developed and utilized by Myin-Germeys and colleagues, to clarify the in vivo temporal relationship of cortisol secretion to symptoms in CHR patients (Myin-Germeys et al., 2001; Myin-Germeys and van Os, 2007; Simons et al., 2008; Collip et al., 2011a). Cortisol reactivity in CHR patients could also be examined in response to a social stressor in the laboratory, as well as its correlates in other parameters, including momentary affect and positive symptoms, and autonomic variables (e.g. heart rate variability, vagal tone, etc.).
In conclusion, these data add to the growing literature that stress sensitivity and affective symptoms in clinical high risk patients have a biological correlate in the HPA axis, relevant for development of treatment strategies to target this stress sensitivity.
Acknowledgments
Role of the funding source
Funding for this study was provided by the NIMH grants K23MH066279 (CC), MH61523 (BC), MH60575 (Intervention Research Center grant, PI: John M. Kane, M.D.), NARSAD (CC), and the Sackler Institute for Developmental Psychobiology at Columbia (CC). The NIMH, NARSAD and Sackler Institutes had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
Cheryl Corcoran designed the study and wrote the protocol under the guidance of Dolores Malaspina and Barbara Cornblatt. Cortisol samples were assayed and stored by Danielle McLaughlin. Andrea Auther did all patient assessments. Christopher Smith completed the data analyses in the study. Cheryl Corcoran wrote the manuscript that was primarily edited by Barbara Cornblatt. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest.
Contributor Information
C.M. Corcoran, Email: cc788@columbia.edu.
C. Smith, Email: christopher.smith@bellevue.nychhc.org.
D. McLaughlin, Email: dmclaugh@lij.edu.
A. Auther, Email: aauther@lij.edu.
D. Malaspina, Email: Dolores.Malaspina@NYUMC.ORG.
B. Cornblatt, Email: cornblat@lij.edu.
References
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67 (3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steerm RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1998;56 (6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Bober JF, Weller EB, Weller RA, Tait A, Fristad MA, Preskorn SH. Correlation of serum and salivary cortisol levels in prepubertal school-aged children. J Am Acad Child Adolesc Psychiatry. 1988;27 (6):748–750. doi: 10.1097/00004583-198811000-00014. [DOI] [PubMed] [Google Scholar]
- Burke PM, Reichler RJ, Smith E, Dugaw K, McCauley E, Mitchell J. Correlation between serum and salivary cortisol levels in depressed and nondepressed children and adolescents. Am J Psychiatry. 1985;142 (9):1065–1067. doi: 10.1176/ajp.142.9.1065. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry. 2002;51 (7):575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol Med. 2011a;41 (11):2305–2315. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- Collip D, van Winkel R, Peerboom O, Lataster T, Thewissen V, Lardinois M, Drukker M, Bart PFR, van Os J, Myin-Germeys I. COMT Val158Met-stress interaction in psychosis: role of background psychosis risk. CNS Neurosci Ther. 2011b;17 (6):612–619. doi: 10.1111/j.1755-5949.2010.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29 (4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Correll CU, Auther AM, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29 (4):633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Social economics of childhood glucocorticoid stress response and health. Am J Phys Anthropol. 1997;102 (1):33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Franzen G. Serum cortisol in chronic schizophrenia. Changes in the diurnal rhythm and psychiatric mental status of withdrawal of drugs. Psychiatr Clin. 1971;4 (4):237–246. [PubMed] [Google Scholar]
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31 (2):189–195. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58 (5):417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia—psychopathological and behavioral correlates. Eur Psychiatry. 2002;17:9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- Hermus AR, Pieters GF, Borm GF, Verhofstad AA, Smals AG, Benraad TJ, Kloppenborg PW. Unpredictable hypersecretion of cortisol in Cushing’s disease: detection by daily salivary cortisol measurements. Acta Endocrinol. 1993;128 (5):428–432. doi: 10.1530/acta.0.1280428. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor Index of Social Position. 1957. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240 (4849):167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13 (3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21 (4):995–998. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lataster T, Wichers M, Jacobs N, Mengelers R, Derom C, Thiery E, Van Os J, Myin-Germeys I. Does reactivity to stress cosegregate with subclinical psychosis? A general population twin study. Acta Psychiatr Scand. 2008;119 (1):45–53. doi: 10.1111/j.1600-0447.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, Auther AM, Correll CU, Cornblatt B. The assessment of “prodromal schizophrenia”: unresolved issues and further directions. Schizophr Bull. 2003;29 (4):717–728. doi: 10.1093/oxfordjournals.schbul.a007041. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacologia. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29 (4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007;61 (10):1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. doi: 10.1016/j.cpr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Nejtek VA. High and low emotion events influence emotional stress perceptions and are associated with salivary cortisol response changes in a consecutive stress paradigm. Psychoneuroendocrinology. 2002;27 (3):337–352. doi: 10.1016/s0306-4530(01)00055-5. [DOI] [PubMed] [Google Scholar]
- Rosenthal D. Genetic Theory and Abnormal Behavior. McGraw-Hill; New York: 1970. [Google Scholar]
- Sachar EJ, Kanter SS, Buie D, Engle R, Mehlman R. Psychoendocrinology of ego disintegration. Am J Psychiatry. 1970;126 (8):1067–1078. doi: 10.1176/ajp.126.8.1067. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman DJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Dev. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Silver AC, Landon J, Smith DS, Perry LA. Radioimmunoassay of cortisol in saliva with the “GammaCoat” kit. Clin Chem. 1983;29:1869–1870. [PubMed] [Google Scholar]
- Simons C, Wichers M, Derom C, Thiery E, Myin-Germeys I, Krabbendam L, van Os J. Subtle gene–environment interactions driving paranoia in daily life. Genes Brain Behav. 2008;8 (1):5–12. doi: 10.1111/j.1601-183X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- Soliman A, O’Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A. Stress-induced dopamine release in humans at risk of psychosis: a [11C] raclopride PET study. Neuropsychopharmacology. 2008;33 (8):2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behavior, and behavioral and psychological problems in young adolescents: a longitudinal perspective. J Res Adolesc. 1997;7:81–105. [Google Scholar]
- Thompson KN, Phillips LJ, Komesaroff P, Yuen HP, Wood SJ, Pantelis C, Velakoulis D, Yung AR, McGorry PD. Stress and HPA-axis functioning in young people at ultra high risk for psychosis. J Psychiatr Res. 2007a;41 (7):561–569. doi: 10.1016/j.jpsychires.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Thompson KN, Berger G, Phillips LJ, Komesaroff P, Purcell R, McGorry PD. HPA axis functioning associated with transition to psychosis: combined DEX/CRH test. J Psychiatr Res. 2007b;41 (5):446–450. doi: 10.1016/j.jpsychires.2005.11.010. [DOI] [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58 (5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Stefanis N, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene–stress interaction. Schizophr Bull. 2008;34 (6):1095–1105. doi: 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder DJ, Walker EF, Lewine RJ. Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol Psychiatry. 2000;48 (12):1121–1132. doi: 10.1016/s0006-3223(00)01052-0. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104 (4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13 (3):721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol. 2010;119 (2):401–408. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside DB, Winter K, Fisman S. Salivary cortisol in children: correlations with serum values and effect of psychotropic drug administration. Can J Psychiatry. 1991;36 (10):746–748. doi: 10.1177/070674379103601011. [DOI] [PubMed] [Google Scholar]