Abstract
Obesity is associated with pathological cardiac remodeling and risk of heart failure (HF). Adipocytokines (ADKs) may mediate the increased risk of cardiovascular disease associated with excess adiposity. Yet data relating ADKs to cardiac remodeling phenotypes are sparse. We related two circulating ADKs, resistin and adiponectin, to three important echocardiographic markers of cardiac remodeling, left ventricular mass (LVM), left atrial diameter (LAD), and LV fractional shortening (LVFS) in 2,615 participants (mean age 61 years, 55% women) in the Framingham Offspring Study. Adiponectin concentrations were inversely related to LVM in multivariable linear regression models adjusting for key clinical correlates including BMI (regression coefficient per s.d.-increment in ln-adiponectin = −3.37, P = 0.02; P for trend across quartiles = 0.02). Adiponectin was not associated with LAD or LVFS (P > 0.56). Resistin concentrations were inversely related to LVFS (regression coefficient per s.d.-increment in ln-resistin = −0.01, P = 0.03; P for trend across quartiles = 0.04). Resistin was not associated with LVM or LAD (P > 0.05). In our moderate-sized, community-based sample, higher circulating concentrations of adiponectin and resistin were associated with lower LVM and lower LVFS, respectively. In conclusion, these associations identify potential mechanisms by which excess adiposity may mediate adverse cardiac remodeling and HF risk.
Introduction
Heart failure (HF) is a growing global health problem that is associated with substantial morbidity and mortality (1,2). The American College of Cardiology/American Heart Association HF guidelines espouse a pathophysiologic model that recognizes the fundamental importance of cardiovascular risk factors and cardiac remodeling in the pathogenesis of HF (3). Identification and treatment of risk factors, particularly in high-risk patients with adverse cardiac remodeling, may help to delay or prevent HF (4).
Obesity, a highly prevalent risk factor related to pathological cardiac remodeling and HF, has emerged as a preventable risk factor for HF (5,6). The mechanisms underlying the relation between obesity and HF have been incompletely elucidated and include the promotion of hypertension, dyslipidemia, and diabetes (5,6). Other mechanisms linking increased adiposity to cardiac structural and functional abnormalities include the elaboration of regulatory proteins called adipocytokines (ADKs) by fat depots, especially the visceral fat compartment (7). ADKs may cause cardiac remodeling through their proinflammatory and proatherosclerotic influences, adverse effects on endothelial function, neurohormonal balance and atherosclerosis (8,9), and direct myocardial actions (10). Whereas numerous ADKs have been identified, adiponectin and resistin are two key mediators with systemic effects.
On the basis of existing data linking obesity, insulin resistance, and HF (11), we hypothesized that circulating ADKs are associated with adverse cardiac remodeling. We tested this hypothesis by relating circulating levels of adiponectin and resistin to three important echocardiographic markers of cardiac remodeling, left ventricular mass (LVM), left atrial diameter (LAD), and LV fractional shortening (LVFS), in a moderate-sized, community-based sample.
Methods and Procedures
The design and methods of the Framingham Offspring Study have been described elsewhere (12). In brief, the Framingham Offspring Study began in 1971 as a community-based, observational, prospective study of cardiovascular disease and its risk factors. The study enrolled 5,124 participants who were the children of the Original Framingham Heart Study cohort, and their spouses. Participants undergo an evaluation at the Heart Study approximately once every 4–8 years.
For the present investigation, we focused on the 3,532 attendees of examination cycle 6 (1996–1998) who underwent routine echocardiography. Participants were excluded from this analysis if they had prevalent myocardial infarction or HF at examination cycle 6. Participants missing adiponectin or resistin (n = 736) at examination 7 (1999–2001) were also excluded. On the basis of data showing an association between advanced kidney disease and increased adiponectin and resistin production, participants with serum creatinine >2 mg/dl (n = 16) were also excluded, leaving 2,615 participants for this investigation (13). All study participants provided written informed consent, and the study protocol was approved by the Boston University Medical Center Review Board.
At each Heart Study examination, participants undergo a physician-administered history and physical examination, anthropometry, and laboratory evaluation of cardiovascular risk factors. BMI was calculated as the weight in kilograms divided by the square of height in meters (kg/m2). Diabetes was defined as either a fasting plasma glucose ≥126 mg/dl or treatment with blood glucose-lowering medication (14). Criteria used for defining myocardial infarction and HF in the Framingham Heart Study have been described elsewhere (15).
Adiponectin and resistin were measured from venous blood samples obtained from participants after an overnight fast at examination cycle 7. Samples were frozen at −80 °C until the assays were performed. Laboratory methods for blood lipids, plasma glucose, and serum creatinine assays have been published previously (16). Plasma resistin and total adiponectin were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) (17). The intra-assay coefficients of variation were 5.8% for adiponectin and 9.0% for resistin.
All participants underwent resting two-dimensionally guided M-mode echocardiography at the 6th examination cycle, using a standardized protocol (18). LV septal wall thickness, posterior LV wall thickness, and LV end-diastolic diameter were measured at end-diastole. LV end-systolic diameter and LAD were measured at end-systole. All measurements were obtained in accordance with the American Society of Echocardiography guidelines using a leading-edge-to-leading-edge technique (18). LVM was determined using the formula 0.8 (1.04 (LV end-diastolic diameter + LV septal wall thickness + LV posterior wall thickness)3 − (LV end-diastolic diameter)3) + 0.6 g (19). LVFS was calculated as (LV end-diastolic diameter − LV end-systolic diameter)/(LV end-diastolic diameter) (17). Excellent inter- and intraobserver reproducibility for M-mode measurements of LAD, LVM, and LVFS have been reported at the Framingham Heart Study and in other investigations (20,21). For the present investigation we focused on three measures: LVM (an aggregate indicator of LV hypertrophy), LAD (a marker of atrial pressure or volume overload and diastolic function), and LVFS (which reflects LV systolic function).
We logarithmically transformed concentrations of adiponectin and resistin to normalize their skewed distributions. We related adiponectin and resistin concentrations (natural log-transformed continuous variables; independent variable) to LVM, LAD, and LVFS (dependent variables) using multivariable linear regression analyses pooling men and women. Sexes were pooled to maximize statistical power, given that we did not observe effect modification by sex upon formal testing (P > 0.05 for association of all echocardiographic variables with both markers) nor did we observe any significant interaction between sex and adiponectin or resistin in multivariable regression models including LVM, LAD, and LVFS.
We evaluated models adjusting for age and sex, and multivariable models that adjusted for age, sex, diabetes, BMI, antihypertensive treatment, systolic blood pressure, total cholesterol-to-HDL ratio, and smoking. These variables were identified a priori on the basis of their cross-sectional associations with LA enlargement, LV hypertrophy, or LV dysfunction (22–26). Analyses examining the relations of adiponectin and resistin to sex-standardized echocardiographic variables yielded similar results to those obtained from sex-adjusted models, and so the latter are presented. We also conducted a secondary analysis including participants with a serum creatinine >2 mg/dl and found that inclusion of individuals with advanced kidney disease did not alter our results (13).
We also used ANOVA to examine linear trends in LVM, LAD, and LVFS across quartiles of adiponectin and resistin. All analyses were performed using SAS software (SAS, Cary, NC) and a two-sided P value <0.05 was considered statistically significant.
RESULTS
Clinical and echocardiographic characteristics of our study sample are shown in Table 1. The sample was middle aged to elderly, with a modest prevalence of vascular risk factors. Adiponectin levels correlated inversely with BMI (Spearman correlation coefficient = −0.29 P < 0.0001), whereas resistin was positively correlated (Spearman correlation coefficient = 0.163, P < 0.0001).
Table 1.
Clinical and echocardiographic characteristics of the study sample
| Variable | Men (n = 1,174) | Women (n = 1,441) |
|---|---|---|
| Clinical featuresa | ||
| Age, years | 61.0 ± 9.5 | 61.3 ± 9.5 |
| Systolic blood pressure, mm Hg | 128 ± 17 | 126 ± 20 |
| BMI, kg/m2 | 28.7 ± 4.6 | 27.6 ± 5.8 |
| Total cholesterol/HDL ratio | 4.5 ± 1.4 | 3.6 ± 1.1 |
| Antihypertensive treatment, % | 34.1% | 29.7% |
| Smoking, % | 13.3% | 13.5% |
| Diabetes, % | 11.9% | 8.5% |
| Echocardiographic featuresb | ||
| LA dimension, mm | 42.0 ± 4.9 | 37.4 ± 4.5 |
| LVFS, % | 36.0 ± 5.2 | 38.2 ± 5.4 |
| LV mass, g | 188.9 ± 41.0 | 138.3 ± 29.7 |
| Adipokine measuresa | ||
| Adiponectin, μg/ml | 7.4 ± 4.4 | 12.5 ± 6.8 |
| Resistin, ng/ml | 14.2 ± 7.1 | 14.2 ± 7.6 |
Values are mean ± s.d. or percentages.
HDL, high-density lipoprotein; LA, left atrial; LV, left ventricular; LVFS, LV fractional shortening.
Clinical characteristics as well as resistin and adiponectin measurements are from examination cycle 7.
Echocardiographic measurements are from examination cycle 6.
Plasma adiponectin levels were inversely associated with LVM in age- and sex-adjusted models (regression coefficient per s.d.-increment in ln-adiponectin = −8.45; P < 0.001). This association remained significant after adjustment for covariates (regression coefficient per s.d.-increment in ln-adiponectin = −3.37; P = 0.02). Adiponectin levels were inversely associated with LAD (regression coefficient = −0.08; P < 0.001) but not LVFS (regression coefficient = 0.002; P = 0.44) in age- and sex-adjusted models. Adjustment for additional clinical covariates attenuated the association of adiponectin with LAD (P= 0.92).
In multivariable models examining linear trends of LVM, LAD, and LVFS across adiponectin quartiles (Table 2), plasma adiponectin was related inversely to LVM (P for trend <0.02; Figure 1) but not with LAD or LVFS (P > 0.5 for both).
Table 2.
Echocardiographic measures according to quartiles of adiponectin
| Echo measure | Quartile I | Quartile II | Quartile III | Quartile IV | P value for trend |
|---|---|---|---|---|---|
| Age- and sex-adjusted | |||||
| LV mass (g) | 164.7 | 163.3 | 157.4 | 153.1 | <0.001 |
| LAD (mm) | 40.0 | 39.8 | 39.3 | 38.8 | <0.001 |
| FS (%) | 37.3 | 37.0 | 37.5 | 37.5 | 0.34 |
| Multivariable-adjusteda | |||||
| LV mass (g) | 161.1 | 161.6 | 158.6 | 156.4 | 0.02 |
| LAD (mm) | 39.5 | 39.6 | 39.4 | 39.5 | 0.94 |
| FS (%) | 37.3 | 37.0 | 37.5 | 37.4 | 0.47 |
BP, blood pressure; FS, fractional shortening; LAD, left atrial diameter; LV, left ventricular.
Values are least square means from models adjusting for age, sex, antihypertensive treatment, systolic BP, BMI, total cholesterol:HDL ratio, diabetes, and smoking.
Figure 1.
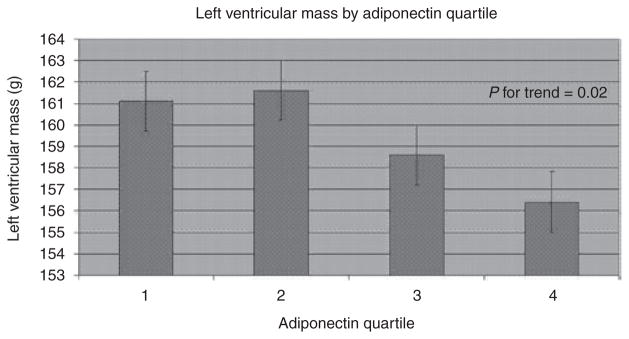
Multivariable-adjusted left ventricular mass by quartile of adiponectin. Bars represent s.e.
Plasma resistin levels were inversely related to LVFS (regression coefficient per s.d.-increment in ln-resistin = −0.01; P = 0.01) and positively related to LVM (regression coefficient = 5.62; P = 0.005) in age- and sex-adjusted models. Upon multivariable adjustment, the relation of resistin to LVM was rendered statistically nonsignificant (P = 0.92) but the inverse relation to LVFS was maintained (regression coefficient per s.d.-increment in ln-resistin = −0.01; P = 0.03). Resistin was not related to LAD in either model (P > 0.15).
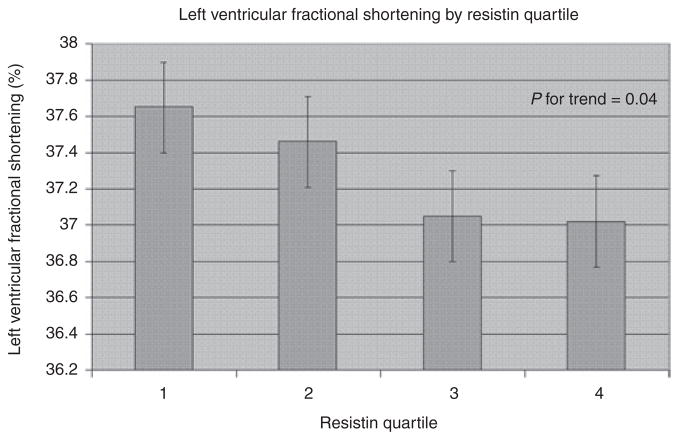
In models examining linear trends of sex-adjusted LVM, LAD, and LVFS across resistin quartiles (Table 3), plasma resistin was inversely related to LVFS (Figure 2).
Table 3.
Echocardiographic measures according to quartiles of resistin
| Echo measure | Quartile I | Quartile II | Quartile III | Quartile IV | P value for trend |
|---|---|---|---|---|---|
| Age- and sex-adjusted | |||||
| LV mass (g) | 156.0 | 158.0 | 161.7 | 162.6 | 0.001 |
| LA size (mm) | 39.2 | 39.6 | 39.5 | 39.7 | 0.06 |
| FS (%) | 37.7 | 37.5 | 37.0 | 37.0 | 0.02 |
| Multivariable–adjusteda | |||||
| LV mass (g) | 158.8 | 158.7 | 160.5 | 159.6 | 0.53 |
| LA size (mm) | 39.6 | 39.7 | 39.3 | 39.4 | 0.14 |
| FS (%) | 37.7 | 37.5 | 37.0 | 37.0 | 0.04 |
BP, blood pressure; FS, fractional shortening; LA, left atrial; LV, left ventricular.
Values are least square means from models adjusting for age, sex, antihypertensive treatment, systolic BP, BMI, total cholesterol:HDL ratio, diabetes, and smoking.
Figure 2.
Multivariable-adjusted left ventricular fractional shortening by quartile of resistin. Bars represent s.e.
DISCUSSION
Accumulating evidence suggests that lower adiponectin and higher resistin are associated with increased risk of cardiovascular disease (27,28). We observed that higher levels of circulating adiponectin were associated with lower LVM, suggesting a protective effect. In contrast, we found that higher levels of resistin were associated with reduced LV systolic function, as measured using LVFS. The magnitude of these associations was modest and their clinical significance is unclear, given the cross-sectional design. We did not observe an association of either resistin or adiponectin with LAD in multivariable-adjusted analyses.
In addition to its insulin-sensitizing effects, adiponectin has anti-inflammatory and antifibrotic properties, protects against endothelial dysfunction and atherosclerosis, reduces vascular smooth muscle proliferation (8) and sympathetic nervous system activation (9), and antagonizes angiotensin II (29). Adiponectin supplementation inhibits pressure-induced cardiac hypertrophy in obese and nonobese mouse models (29). Although little is known about the relation of adiponectin to ventricular hypertrophy in humans, several prior studies have reported an inverse relation of circulating adiponectin with LV wall thickness and LVM index (30–32). Our study confirms these findings in a moderate-sized, population-based sample, adjusting for important clinical variables not adjusted for in prior investigations.
Increased LAD is an echocardiographic marker of atrial remodeling that is commonly seen in association with LV hypertrophy, and likely reflects increased LV filling pressures (33,34). Increased circulating adiponectin was inversely associated with LAD in our study after adjustment for age and sex, but this relation was nullified after adjustment for additional clinical covariates, suggesting that BMI may mediate the relation of adiponectin to LAD. A prior investigation demonstrated that circulating adiponectin was positively associated with LAD index (30), but that study did not adjust for clinical covariates including hypertension and diabetes. Our observation that circulating adiponectin was not associated with LVFS is consistent with existing literature suggesting that adiponectin acts primarily as an inhibitor of cardiac hypertrophy (35). Although adiponectin levels have been associated with coronary heart disease in the community (36), data suggesting that circulating adiponectin is associated with LV function have come exclusively from subjects after myocardial infarction (37–39).
The underlying mechanisms for the association between higher levels of circulating resistin and greater risk of incident MI and HF are unclear (27,38). Resistin has been related, however, to both increased risk of coronary heart disease and to increased inflammatory markers known to be associated with increased risk of LV systolic dysfunction and HF (38,40–42). Our findings are consistent with the hypothesis that resistin may promote HF through development of LV systolic dysfunction.
Although higher circulating resistin was associated with greater LVM after age- and sex-adjustment, we did not note an association between resistin and either LVM or LAD after adjustment for important clinical covariates. Resistin levels correlated with BMI in our study, and the association between resistin and LVM observed before multivariable adjustment may reflect the established relation of BMI to LVM. Especially when viewed in light of data showing that adipose cells may regulate resistin secretion by other cell types, including inflammatory cells, additional studies are needed to elucidate potential links between obesity, inflammation, resistin, and cardiac remodeling (43–45).
Our study has several limitations. First, the observational nature of our investigation precludes any causal inferences. Second, measurements of resistin and adiponectin (cycle 7) were not contemporaneous with the echocardiographic examination (cycle 6). Third, diastolic function was not assessed during examination cycle 6. Fourth, our study sample consisted of middle aged to elderly participants almost exclusively of European descent. Additional studies are required to determine whether our results generalize to other age or ethnic groups.
In our community-based cohort, higher circulating concentrations of adiponectin and resistin were associated with lower LVM and lower LVFS, respectively. Our results, while observational, are consistent with the concept that ADKs may promote maladaptive cardiac remodeling, thereby providing a mechanistic link between obesity and HF.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart study (contract no. N01-HC-25195), 6R01-Ns 17950, 2 K24 HL04334, RO1HL080124, RO1DK80739 (R.s.V.), Framingham, MA.
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Heart Failure Society of America. HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:e1–e2. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 4.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 8.Kawanami D, Maemura K, Takeda N, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 9.Imai J, Katagiri H, Yamada T, et al. Cold exposure suppresses serum adiponectin levels through sympathetic nerve activation in mice. Obesity (Silver Spring) 2006;14:1132–1141. doi: 10.1038/oby.2006.130. [DOI] [PubMed] [Google Scholar]
- 10.Liao Y, Takashima S, Maeda N, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1975;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 13.Roubicek T, Bartlova M, Krajickova J, et al. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition. 2009;25:762–768. doi: 10.1016/j.nut.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Care Committee. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 (Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 15.Sorlie P. Cardiovascular diseases and death following myocardial infarction and angina pectoris: Framingham study 20-year follow-up. In: Kannel WB, Gordon T, editors. The Framingham Study: An Epidemiologic Investigation of Cardiovascular Disease. Government Printing Office; Washington, D.C: 1977. pp. 77–1247. [Google Scholar]
- 16.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 17.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 18.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 20.Wade MR, Chandraratna PA, Reid CL, Lin SL, Rahimtoola SH. Accuracy of nondirected and directed M-mode echocardiography as an estimate of left atrial size. Am J Cardiol. 1987;60:1208–1211. doi: 10.1016/0002-9149(87)90434-6. [DOI] [PubMed] [Google Scholar]
- 21.Sundström J, Sullivan L, Selhub J, et al. Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J. 2004;25:523–530. doi: 10.1016/j.ehj.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Hirschler V, Acebo HL, Fernandez GB, et al. Influence of obesity and insulin resistance on left atrial size in children. Pediatr Diabetes. 2006;7:39–44. doi: 10.1111/j.1399-543X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 23.Knutsen KM, Stugaard M, Michelsen S, Otterstad JE. M-mode echocardiographic findings in apparently healthy, non-athletic Norwegians aged 20–70 years. Influence of age, sex and body surface area. J Intern Med. 1989;225:111–115. doi: 10.1111/j.1365-2796.1989.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 24.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 25.Gu L, Pandey V, Geenen DL, Chowdhury SA, Piano MR. Cigarette smoke-induced left ventricular remodelling is associated with activation of mitogen-activated protein kinases. Eur J Heart Fail. 2008;10:1057–1064. doi: 10.1016/j.ejheart.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerdts E, Oikarinen L, Palmieri V, et al. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Hypertension. 2002;39:739–743. doi: 10.1161/hy0302.105683. [DOI] [PubMed] [Google Scholar]
- 27.Frankel DS, Vasan RS, D’Agostino RB, Sr, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnett MS, Lee CW, Kinnaird TD, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Ran J, Hirano T, Fukui T, et al. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metab Clin Exp. 2006;55:478–488. doi: 10.1016/j.metabol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Ohara T, Kim J, Asakura M, et al. Plasma adiponectin is associated with plasma brain natriuretic peptide and cardiac function in healthy subjects. Hypertens Res. 2008;31:825–831. doi: 10.1291/hypres.31.825. [DOI] [PubMed] [Google Scholar]
- 31.Ebinç H, Ebinç FA, Ozkurt ZN, et al. Impact of adiponectin on left ventricular mass index in non-complicated obese subjects. Endocr J. 2008;55:523–528. doi: 10.1507/endocrj.k07e-098. [DOI] [PubMed] [Google Scholar]
- 32.Kozakova M, Muscelli E, Flyvbjerg A, et al. Adiponectin and left ventricular structure and function in healthy adults. J Clin Endocrinol Metab. 2008;93:2811–2818. doi: 10.1210/jc.2007-2580. [DOI] [PubMed] [Google Scholar]
- 33.Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 34.Pritchett AM, Mahoney DW, Jacobsen SJ, et al. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 35.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 37.Shibata R, Izumiya Y, Sato K, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeishi Y, Niizeki T, Arimoto T, et al. Serum resistin is associated with high risk in patients with congestive heart failure–a novel link between metabolic signals and heart failure. Circ J. 2007;71:460–464. doi: 10.1253/circj.71.460. [DOI] [PubMed] [Google Scholar]
- 39.Biolo A, Shibata R, Ouchi N, et al. Determinants of adiponectin levels in patients with chronic systolic heart failure. Am J Cardiol. 2010;105:1147–1152. doi: 10.1016/j.amjcard.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohmori R, Momiyama Y, Kato R, et al. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46:379–380. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Bo S, Gambino R, Pagani A, et al. Relationships between human serum resistin, inflammatory markers and insulin resistance. Int J Obes (Lond) 2005;29:1315–1320. doi: 10.1038/sj.ijo.0803037. [DOI] [PubMed] [Google Scholar]
- 42.Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450–2457. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee RR, Lazar MA. Resistin: molecular history and prognosis. J Mol Med. 2003;81:218–226. doi: 10.1007/s00109-003-0428-9. [DOI] [PubMed] [Google Scholar]
- 44.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa R, Tanaka C, Sato M, et al. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010;398:723–729. doi: 10.1016/j.bbrc.2010.07.008. [DOI] [PubMed] [Google Scholar]