Abstract
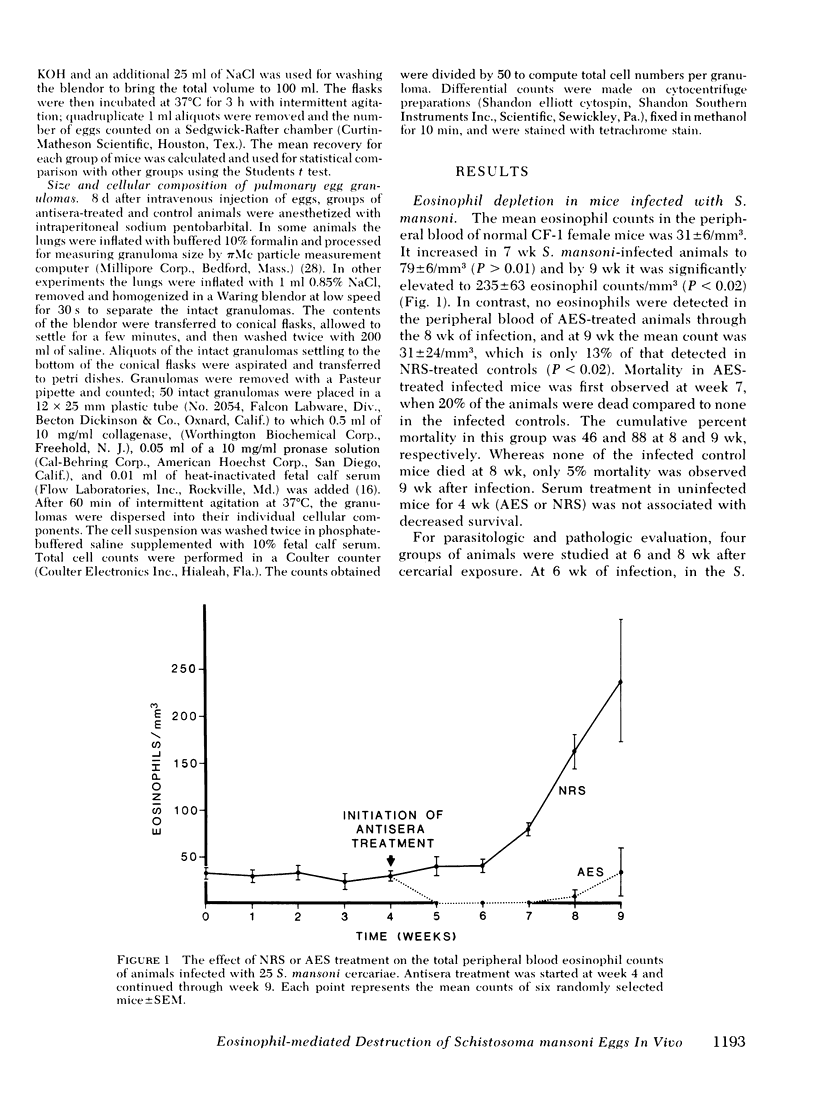
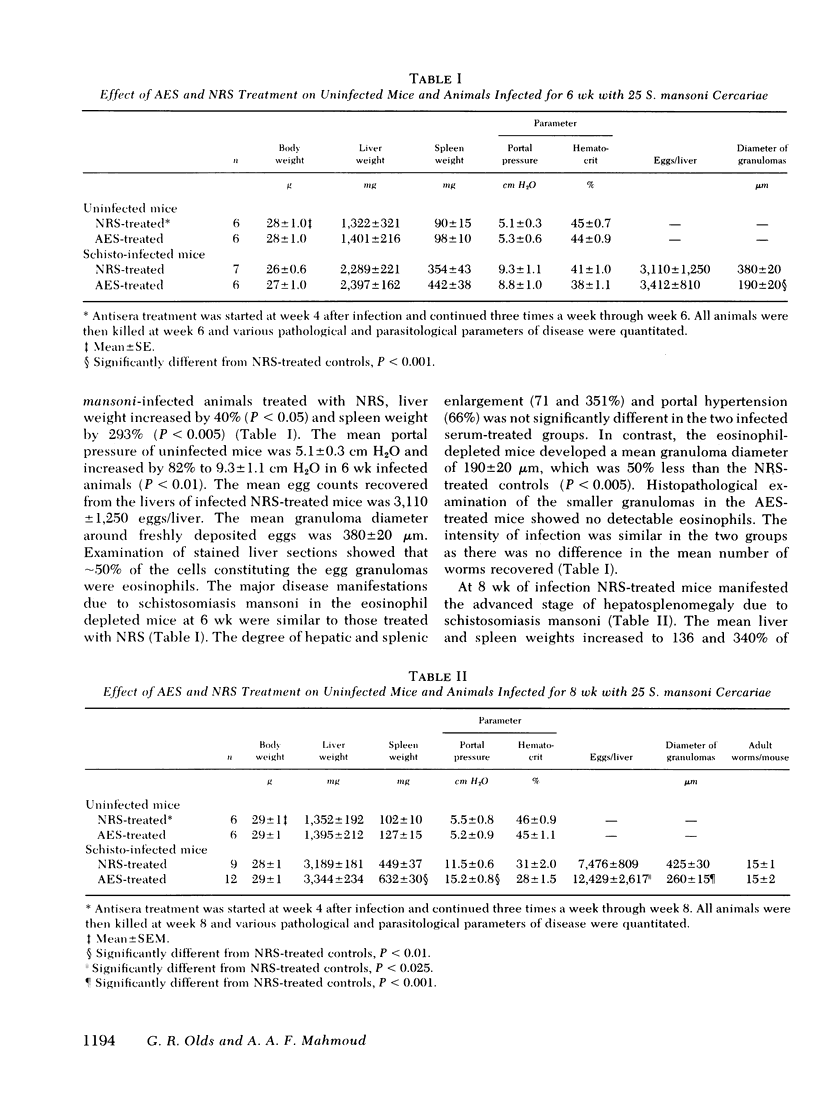
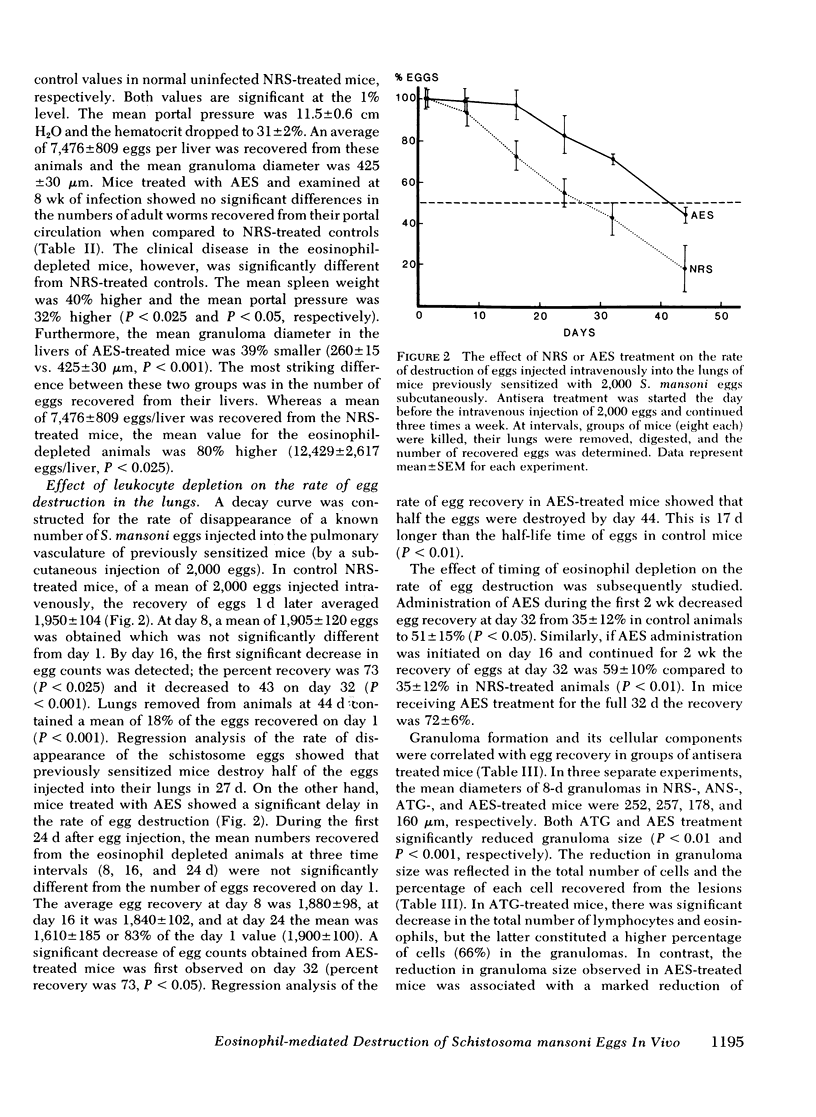
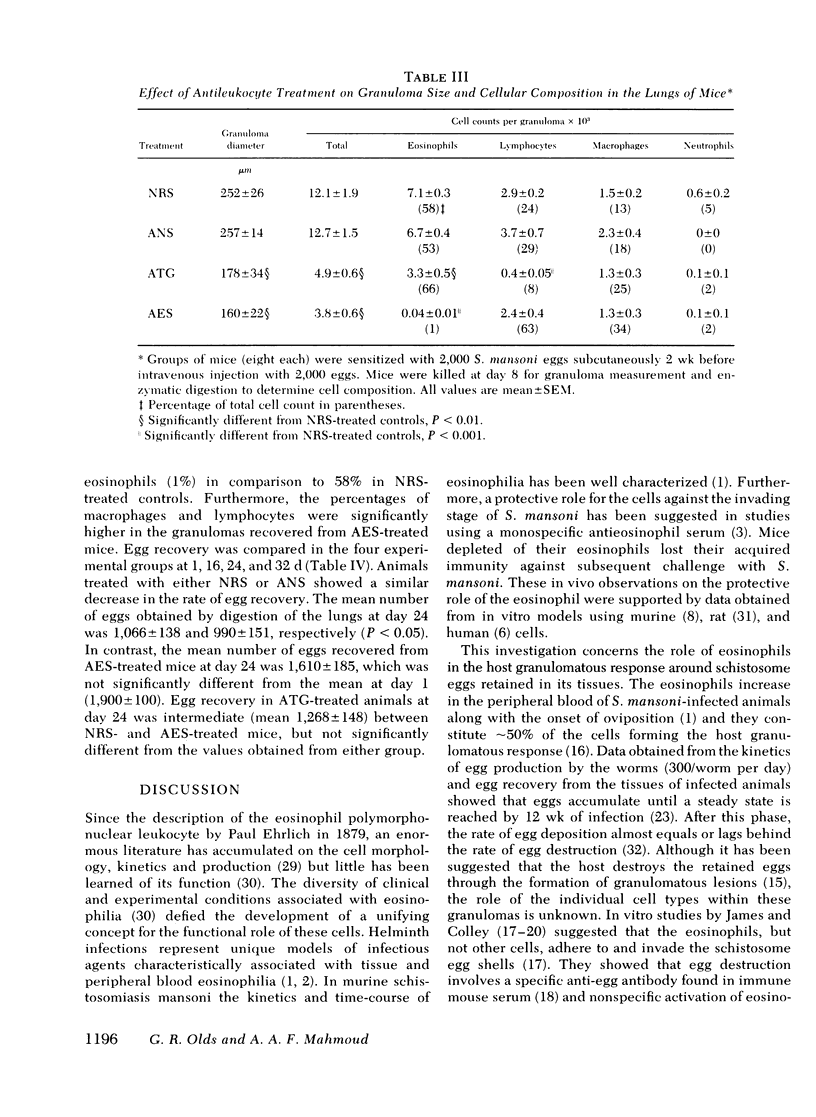
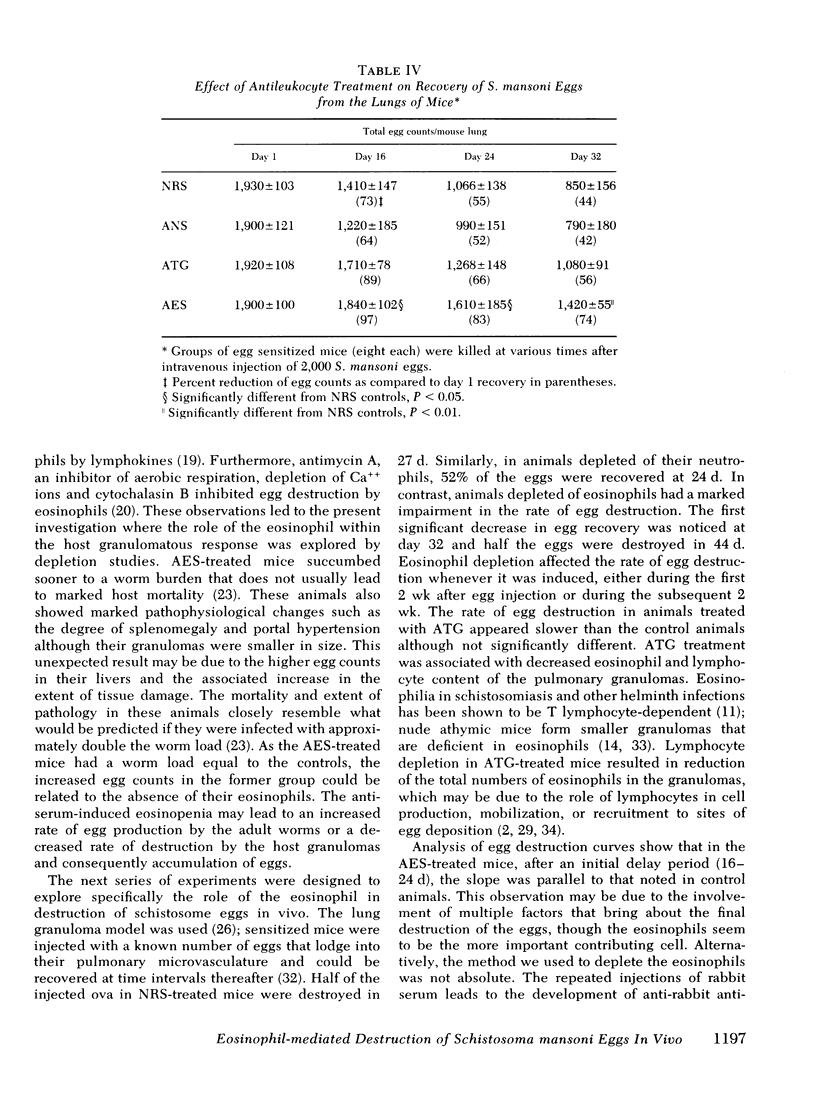
Eosinophils form 50% of cells in the host granulomatous response to Schistosoma mansoni eggs, but their functional role in these granulomas and their relation to egg destruction is unknown. We have studied the course of S. mansoni infection in mice treated with normal rabbit serum (NRS) or depleted of their eosinophils by monospecific anti-eosinophil serum (AES). At 6-wk of infection (after 2 wk of egg deposition) the AES-treated animals were similar to NRS-treated controls with the exception that hepatic granulomas in the AES-treated animals were 50% smaller and devoid of eosinophils. At 8 wk of infection, AES-treated mice had significantly higher mortality, spleen weight, portal pressure, and 80% more eggs retained in their livers. These data suggest that eosinophil depletion delayed egg destruction. We subsequently studied destruction of eggs injected into the pulmonary microvasculature of sensitized mice. 2,000 S. mansoni eggs were intravenously injected into the tail veins of mice treated with NRS, anti-neutrophil serum, AES or ATG (anti-thymocyte globulin); at time intervals the remaining eggs were recovered from the lungs by tissue digestion. Egg recovery from NRS- or anti-neutrophil serum-treated mice began to decrease by day 16 and the percent recovery of eggs at day 24 was 55 and 52%, respectively. In contrast, animals treated with AES had smaller lung granulomas that were devoid of eosinophils and a marked delay of egg destruction was seen. It took until day 44 for 50% of the eggs to be destroyed. In ATG-treated animals smaller granulomas were seen that had diminished lymphocytes and also 75% less eosinophils. ATG treatment apparently slowed egg destruction but was not statistically significant. Our data define the role of the eosinophils in destruction of schistosome eggs in vivo and delineates the protective function of these cells within the host granulomatous response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsden A. F., Boros D. L., Hood A. T. Etiology of the liver granulomatous response in Schistosoma mansoni-infected athymic nude mice. Infect Immun. 1980 Jan;27(1):75–80. doi: 10.1128/iai.27.1.75-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Boyer M. H., Beeson P. B. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970 Jun 1;131(6):1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Mahmoud A. A., Sher A., Rees P. H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975 Aug 28;256(5520):727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Vadas M. A., Wassom D. L., Dessein A., Hogan M., Sherry B., Gleich G. J., David J. R. Interactions between human eosinophils and schistosomula of Schistosoma mansoni. II. The mechanism of irreversible eosinophil adherence. J Exp Med. 1979 Dec 1;150(6):1456–1471. doi: 10.1084/jem.150.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Wassom D. L., Gleich G. J., Loegering D. A., David J. R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979 Jan;122(1):221–229. [PubMed] [Google Scholar]
- CHEEVER A. W., WARREN K. S. HEPATIC BLOOD FLOW IN MICE WITH ACUTE HEPATO-SPLENIC SCHISTOSOMIASIS MANSONI. Trans R Soc Trop Med Hyg. 1964 Sep;58:406–412. doi: 10.1016/0035-9203(64)90086-0. [DOI] [PubMed] [Google Scholar]
- Cheever A. W. A quantitative post-mortem study of Schistosomiasis mansoni in man. Am J Trop Med Hyg. 1968 Jan;17(1):38–64. doi: 10.4269/ajtmh.1968.17.38. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Anderson L. A. Rate of destruction of Schistosoma mansoni eggs in the tissues of mice. Am J Trop Med Hyg. 1971 Jan;20(1):62–68. doi: 10.4269/ajtmh.1971.20.62. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Mahmoud A. A., Warren K. S. Granulomatous hypersensitivity to Schistosoma mansoni eggs in thymectomized and bursectomized chickens. J Immunol. 1974 Sep;113(3):1064–1067. [PubMed] [Google Scholar]
- Duvall R. H., DeWitt W. B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967 Jul;16(4):483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Buchanan R. D., Colley D. G. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. I. Eosinophilia and immunologic responses to a schistosomal egg preparation. Am J Pathol. 1973 May;71(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Grove D. I., Mahmoud A. A., Warren K. S. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977 Mar 1;145(3):755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophi-mediated destruction of Schistosoma mansoni eggs in vitro. II. The role of cytophilic antibody. Cell Immunol. 1978 Jun;38(1):35–47. doi: 10.1016/0008-8749(78)90029-1. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophil-mediated destruction of S. mansoni eggs IV. Effects of several inhibitory substances on eosinophil function. Cell Immunol. 1978 Jun;38(1):59–67. doi: 10.1016/0008-8749(78)90031-x. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophil-mediated destruction of Schistosoma mansoni eggs III. lymphokine involvement in the induction of eosinophil functional abilities. Cell Immunol. 1978 Jun;38(1):48–58. doi: 10.1016/0008-8749(78)90030-8. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophil-mediated destruction of Schistosoma mansoni eggs. J Reticuloendothel Soc. 1976 Nov;20(5):359–374. [PubMed] [Google Scholar]
- Kassis A. I., Aikawa M., Mahmoud A. F. Mouse antibody-dependent eosinophil and macrophage adherence and damage to schistosomula of Schistosoma mansoni. J Immunol. 1979 Feb;122(2):398–405. [PubMed] [Google Scholar]
- Kazura J. W., Grove D. I. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis. Nature. 1978 Aug 10;274(5671):588–589. doi: 10.1038/274588a0. [DOI] [PubMed] [Google Scholar]
- MOORE D. V., SANDGROUND J. H. The relative egg producing capacity of Schistosoma mansoni and Schistosoma japonicum. Am J Trop Med Hyg. 1956 Sep;5(5):831–840. doi: 10.4269/ajtmh.1956.5.831. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Mandel A., Warren K., Webster L. T., Jr Niridazole. II. A potent long-acting suppressant of cellular hypersensitivity. J Immunol. 1975 Jan;114(1 Pt 2):279–283. [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Boros D. I. Production of a rabbit antimouse eosinophil serum with no cross-reactivity to neutrophils. J Exp Med. 1973 Jun 1;137(6):1526–1531. doi: 10.1084/jem.137.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Graham R. C., Jr Antieosinophil serum and the kinetics of eosinophilia in Schistosomiasis mansoni. J Exp Med. 1975 Sep 1;142(3):560–574. doi: 10.1084/jem.142.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. L., Grove D. I., Warren K. S. The Schistosoma mansoni egg granuloma: quantitation of cell populations. J Pathol. 1977 Jan;121(1):41–50. doi: 10.1002/path.1711210107. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., DiConza J. J., Gold J. A., Reid W. A. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977 Feb;118(2):594–599. [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., McLaren D. J., Smithers S. R. Complement-mediated killing of schistosomula of Schistosoma mansoni by rat eosinophils in vitro. J Exp Med. 1978 Jan 1;147(1):147–156. doi: 10.1084/jem.147.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O. Granuloma formation around Schistosoma mansoni, S. HAEMATOBIUM, AND S. japonicum eggs. Size and rate of development, cellular composition, cross-sensitivity, and rate of egg destruction. Am J Trop Med Hyg. 1970 Mar;19(2):292–304. doi: 10.4269/ajtmh.1970.19.292. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The pathogenesis of "clay-pipe stem cirrhosis" in mice with chronic schistosomiasis mansoni, with a note on the longevity of the schistosomes. Am J Pathol. 1966 Sep;49(3):477–489. [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. Eosinophil function and disorders. Adv Intern Med. 1974;19:1–25. [PubMed] [Google Scholar]
- von LICHTENBERG Host response to eggs of S. mansoni. I. Granuloma formation in the unsensitized laboratory mouse. Am J Pathol. 1962 Dec;41:711–731. [PMC free article] [PubMed] [Google Scholar]
- von Lichtenberg F. Experimental approaches to human schistosomiasis. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):79–87. doi: 10.4269/ajtmh.1977.26.79. [DOI] [PubMed] [Google Scholar]


