Abstract
Emergency departments (EDs) are an important diagnostic site for outpatients with potentially serious infections. EDs frequently experience high patient volumes, and crowding has been shown to negatively impact the delivery of early care for serious infections, such as pneumonia. Here, we hypothesized that other important factors in the early care of infectious diseases, the rate of blood culture contamination and the accurate detection of pathogens, would be sensitive to ED operational stress, as proper collection requires fastidious attention to technique and timing. We related all blood samples collected over 1 year and their rates of recovery of likely contaminants and pathogens to the number of patients being cared for in the ED at the time of sample collection. Likely pathogens and contaminants were classified through combined microbiological and manual chart review criteria. Zero-inflated Poisson regression was used to relate crowding to culture results. Blood samples were obtained from 7,586 patients over 82,521 adult and pediatric patient visits. The unadjusted rates of recovering a likely pathogen or a likely contaminant were 8.0% and 3.7%, respectively. Periods of increased crowding (3rd and 4th quartiles of hourly occupancy) were significantly associated (P < 0.01) with increased rates of contamination (relative risk, 1.23 compared to the least busy quartile). Collecting samples for culture during busy times was also associated with a reduced likelihood of recovering a likely pathogen (relative risk, 0.93 compared to the least busy quartile). ED crowding was associated with degraded performance of blood cultures, both increasing the rate of contamination and decreasing the diagnostic yield.
INTRODUCTION
Despite considerable efforts to reduce their incidence, contaminated blood cultures continue to be a significant clinical problem. Ongoing multicenter monitoring in the United States places the median rate of contamination at just under 3% (lower national quartile, 2.15%; upper quartile, 3.67%) (1). The clinical and financial impacts of contaminated cultures have been examined by numerous groups; the most recently published results indicate that among patients undergoing blood culture during emergency department (ED) evaluation and subsequent admission to the hospital, a false-positive culture adds an estimated $8,700 (2008 U.S. dollars) and an additional day of hospitalization (2). Excess contamination rates are driven primarily by a lack of operator fastidiousness during the collection process (3). As such, interventions such as dedicated phlebotomy teams, prepackaged blood culture kits, and the use of sterile gloves have helped decrease contamination rates (4, 5).
One clinical setting about which little is known regarding blood culture contamination is the emergency department (ED). EDs are an important and common site for initial diagnosis and treatment; more than 136 million visits took place in 2009, the most recent year for which Centers for Disease Control and Prevention data are available (6). Increasingly, EDs serve a diagnostic niche previously filled by both outpatient and inpatient venues. The expanding role of this clinical site for the initial diagnosis and treatment of many urgent conditions frequently results in crowded conditions. Although typically attributed to some combination of increased utilization (i.e., more patients seeking care) or decreased availability of inpatient beds, at least one recent report indicated that growing lengths of stay for patients in EDs are a result of increasing “practice intensity,” a concept that includes more extensive diagnostic testing and treatment prior to ultimate patient disposition (7). Previous work demonstrated that high levels of ED occupancy might negatively impact the care and outcome of a number of time-critical infectious conditions (8–10).
Here, we propose that the rate of blood culture contamination might be an especially sensitive marker for high-occupancy conditions in EDs. High-visibility events, such as acute myocardial infarction or stroke, often prompt caregiver teams to assemble and redirect resources and attention within the ED workflow. Because the timeliness and outcome of care of these events are commonly used as performance metrics, extensive quality monitoring and improvement programs might also be present. In contrast, blood samples for culture might be drawn by a lone operator in relative isolation, often under time pressure to expedite the skin preparation and sampling process, perhaps by using blood already drawn or preexisting vascular access sites. As blood culture results can take several days to return and because of frequent retrospective uncertainty about the precise circumstances under which blood samples for cultures are collected, operators might receive little or no feedback to improve future performance. We therefore hypothesized that the rate of blood culture contamination in our ED would be susceptible to variations in the intensity of clinical activity, which is defined here as “occupancy” (the total number of patients in the ED during any given time). We considered this hypothesis using operational and microbiological data from an academic tertiary ED that has 80,000 annual visits.
MATERIALS AND METHODS
Emergency department characteristics and determination of activity.
The University of Michigan Health System (UMHS) Emergency Department has 80,000 annual visits from adults and children. All faculty are board certified through the American Board of Emergency Medicine and oversee fellows, residents, and physician assistants. Approximately 30% of the patients seen are admitted to the hospital, and 2.6% are admitted to an intensive care unit (ICU). In our ED, blood samples for culture are most frequently drawn by emergency medical technicians, less frequently by nurses, and least frequently by physicians. During the study period, nurse-to-patient ratios in critical care areas were 2:3 and were 1:4 in the remainder of the department. Technician-to-patient ratios were 1:9.
The number of new patient arrivals, patient length of stay (LOS) in the ED, and the patient triage acuity score for each hour between 31 October 2010 and 30 October 2011 were gathered from the ED clinical information system at UMHS (Centricity 7.5.x; General Electric Healthcare, Piscataway, NJ) as we described previously (11, 12). Hourly occupancy was chosen over more complex crowding metrics and was reconstructed from arrival and LOS data (13). The results of all blood cultures performed during the study period were extracted from the UMHS Clinical Microbiology Laboratory information system. Data sets from these two sources were merged using patient date of birth and ED date of service, which was possible for 99.6% of patients. All work was approved by the University of Michigan Medical School Institutional Review Board.
Blood sample collection and interpretation.
The UMHS policy for blood sample collection required initial skin preparation with one alcohol pad, followed by three iodophor swabs, and a 1-min delay prior to venipuncture or line access. Since the reporting of the blood culture source is not standardized in our institutional medical record system, identification of venipuncture versus line access was not reliable enough to be included in our analysis. We defined one set of blood cultures as one aerobic bottle and one anaerobic bottle. All cultures were analyzed using the BacT/Alert FAN system for the detection of positive blood cultures and the Vitek 2 system for the identification of isolates (bioMérieux, Durham, NC). The time of blood sample acquisition was assumed to be the time stamped on the blood culture requisition.
The culture results were screened for contamination based on the Centers for Disease Control and Prevention/National Healthcare Safety Network definition for laboratory-confirmed bloodstream infection along with the interpretations of an infectious diseases clinician (P.N.M.) and a microbiologist (D.W.N.) based on standard practice guidelines (7). Cultures recovering diphtheroids (Corynebacterium spp. not diphtheriae), Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci, viridans group streptococci, Aerococcus spp., or Micrococcus spp. were further investigated by manual chart review of the ED visit and associated hospitalization of the patient from whom the blood sample for culture was recovered. These cultures were ultimately labeled as “likely contaminant” when a manual chart review showed that the blood culture report prompted either no additional antimicrobials, discontinuation of existing antibiotic therapy, or a specific mention in the medical record that the result represented a contaminant or likely contaminant. All cultures showing bacterial growth that did not meet these criteria were deemed to have identified a “likely pathogen.”
As the primary endpoint of this study was to identify factors associated with the recovery of a contaminant organism or pathogen at the time of blood culture, it was possible for a blood culture to contain both a likely pathogen and a likely contaminant. For example, a set returning both Klebsiella pneumoniae and a diphtheroid would be considered to have returned simultaneously a likely pathogen and a likely contaminant.
Statistical analysis.
ED operational and blood culture data merge routines were written in PERL (3). Summary statistics are presented as medians and percentages, with P values of <0.05 considered statistically significant. In all regression analyses, the experimental unit was the culture set, rather than the patient, as a single patient typically had multiple culture sets. We performed two-sample proportion tests to compare the contamination/pathogen recovery rates and Poisson regression to assess the relationship between ED activity measures and the likelihood of contamination. The preliminary analysis using Vuong's nonnested hypothesis test confirmed a greater number of negative cultures than would be expected from a typical Poisson distribution, so zero-inflated Poisson models were used. Hourly activity measures were binned into quartiles (e.g., the least busy 25% of operational hours per year). Acknowledging the uncertain delays between a blood sample actually being collected and the assignment of a time stamp for submission to the clinical laboratory, we performed analysis of risk of contamination as a function of recorded collection time and that time lagged by 1 or 2 h. In these analyses, a 2-h lag produced a significantly improved goodness of fit as noted by minimizing the Akaike information criterion (AIC), and thus this lag was used in the subsequent analysis. The statistical analysis was performed using R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
During the study period, 82,521 patient encounters occurred in the UMHS ED (63,533 adults and 18,988 children). Of these, 26,695 resulted in hospitalization, with 2,136 patients admitted to the intensive care unit and 993 patients taken directly to surgery. Among all patients, 7,586 had blood samples submitted for culture, with a total of 12,696 distinct blood culture samples collected in the ED. This represented 22.9% of the 55,476 blood culture samples processed at the entire University of Michigan Health System during the study period. In pediatric patients, single samples were collected from 1,354 of 1,490 patients (90.0%), whereas in adult patients, single samples were collected from 1,386 of 6,078 patients (22.8%). The median time between ED arrival and blood sample acquisition was 97 min (interquartile range [IQR], 51 to 167 min), a delay similar to what we reported previously in the care of patients with sepsis in this ED (12). The mean duration between the documented time of specimen collection and delivery to the laboratory was 38 min (IQR, 16 to 40 min). Details of patient demographics are shown in Table 1.
Table 1.
Clinical features of patients undergoing blood culture in the emergency department
| Patient featurea | Total no. of patients seen in the ED | No. of patients whose blood was cultured | No. (%) of likely pathogens recovered | No. (%) of likely contaminants recovered |
|---|---|---|---|---|
| Age | ||||
| 0–60 days | 758 | 142 | 5 (3.5) | 4 (2.8) |
| 61 days to 2.9 yr | 5,640 | 421 | 39 (9.2) | 8 (1.9) |
| 3–17.9 yr | 12,590 | 927 | 46 (5.0) | 15 (1.6) |
| 18–49 yr | 36,236 | 2,069 | 149 (7.2) | 76 (3.7) |
| 50–59 yr | 10,691 | 1,260 | 94 (7.5) | 51 (4.0) |
| 60–69 yr | 7,583 | 1,219 | 132 (10.8) | 63 (5.2) |
| 70–79 yr | 4,929 | 853 | 89 (10.4) | 32 (3.8) |
| >80 yr | 4,094 | 677 | 52 (7.7) | 36 (5.3) |
| All ages | 82,521 | 7,568 | 606 (8.0) | 285 (3.8) |
| Disposition | ||||
| Hospitalized | 26,695 | 6,113 | 532 (8.7) | 234 (3.8) |
| Admitted to general care | 23,566 | 5,284 | 406 (7.7) | 187 (3.5) |
| Admitted to ICU | 2,136 | 728 | 106 (14.6)b | 42 (5.8) |
| Transferred to OR | 993 | 101 | 11 (10.9) | 5 (5.0) |
| Discharged home | 52,701 | 1,078 | 36 (3.3) | 17 (1.6) |
| Left prior to evaluation | 2,310 | 23 | 2 (8.7) | 0 (0.0) |
| Died prior to discharge | 61 | 8 | 3 (37.5) | 1 (12.5) |
| Transferred to other facility | 403 | 4 | 0 (0.0) | 0 (0.0) |
| Other | 342 | 342 | 42 (12.2) | 33 (9.6) |
OR, operating room; ICU, intensive care unit.
P < 0.05; proportions were compared to reference groups of 18- to 49-year-olds and admissions to general care.
Among patients who had blood samples for culture drawn, 865 (11.4%) had at least one culture positive for bacterial growth, 606 (8.0% of total) of which grew likely pathogens, while 285 (3.7% of total) were deemed likely contaminated. Note that because of our definitions, cultures recovering true pathogens and contaminants were not mutually exclusive: 26 patients had cultures classified in this way. Of all individual samples for culture collected in the ED, 1,188 (9.4%) were positive for bacterial growth, 913 (7.2%) of which grew true pathogens, while 296 (2.3%) were deemed contaminated, giving a gross false-positive rate of 24.9%. In a subset analysis of the number of samples collected per visit, patients with multiple cultures were more likely to have positive cultures (7.8%) than patients with single cultures (5.1%) (P < 0.001), although there was no difference in the contamination rates (2.2% versus 2.6%, respectively; P > 0.05). When the proportions of cultures recovering true pathogens or contaminants were compared between age categories or disposition status, with age 18 to 49 years or admission to general care as reference categories, respectively, patients admitted to an ICU had an increased likelihood of recovery of a likely pathogen (P = 0.01). A total of 109 unique bacterial species were identified, with coagulase-negative staphylococci, Staphylococcus aureus, and Escherichia coli recovered most frequently. Details of the blood culture microbiology results are shown in Table 2.
Table 2.
Organisms recovered from blood cultures performed during emergency department visits
| Organism | No. of organisms recovered |
|---|---|
| Likely pathogens | |
| Staphylococcus aureus | 161 |
| Escherichia coli | 152 |
| Coagulase-negative staphylococcia | 124 |
| Klebsiella pneumoniae | 96 |
| Pseudomonas aeruginosa | 33 |
| Streptococcus pneumoniae | 33 |
| Alpha-hemolytic Streptococcus | 32 |
| Enterobacter cloacae | 29 |
| Streptococcus mitis | 18 |
| Klebsiella oxytoca | 16 |
| Other species (89 distinct species) | 232 |
| Likely contaminants | |
| Coagulase-negative staphylococcia | 254 |
| Isolates not undergoing full identificationb | 79 |
| Enterococcus faecalis | 47 |
| Micrococcus species | 31 |
| Bacillus cereus | 12 |
| Vancomycin-resistant E. faecium | 12 |
| Abiotrophia/Granulicatella | 5 |
| Nutritionally variant Gram-positive coccus | 5 |
| Ochrobactrum anthropi | 4 |
| Vancomycin-resistant E. faecalis | 4 |
| Enterococcus durans | 2 |
| Lactobacillus species | 1 |
| Lactococcus species | 1 |
| Microbacterium species | 1 |
Designated as a likely contaminant or a likely pathogen based on chart review.
Includes all results that were not investigated following laboratory designation of contaminant.
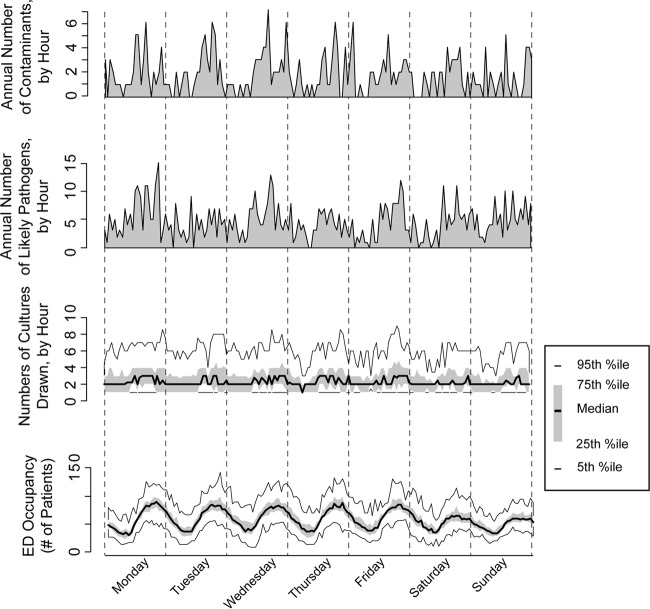
Hourly ED occupancy showed a typical cyclic variation (Fig. 1). Blood culture activity, including the number of samples collected, number positive, and number contaminated, showed similar periodicity (P < 0.001 versus no temporal variation) and, as expected, indicated that during busy times more samples for culture were collected. Therefore, as ED occupancy increased, the proportion of patients having blood cultures performed decreased (P < 0.01) (Fig. 2A). There were no differences among either adult or pediatric patients in regard to the number of sets tested per visit as a function of ED occupancy (P > 0.05 for each population) (Fig. 2B).
Fig 1.
Diurnal variability in emergency department (ED) blood culture activity. (Bottom) Hourly variation in ED occupancy (the number of patients in the ED during any hour) over the week. Results are reported as the 5th, 25th, median, 75th, and 95th percentile values over the 1-year study period. (Second from bottom) Number of samples for culture collected per hour, using the same plotting convention. (Second from top) Cumulative number of cultures recovering a likely pathogen during any hour of the week across the study year. (Top) Cumulative number of cultures recovering a likely contaminant during any hour of the week across the study year.
Fig 2.
The impact of hourly emergency department (ED) occupancy on culture activity and outcome. (A) Fraction of patients undergoing blood culture during any hour of the year as a function of hourly ED occupancy, plotted as the 5th, 25th, 50th, 75th, and 95th percentiles. (B) Distribution of the number of sets sent for each patient. (C) Rate of recovering a contaminant species from a blood culture. (D) Fraction of blood cultures recovering a pathogen.
Positive blood cultures, both real and contaminated, are uncommon events, and thus the timing of their occurrence is not normally distributed. Therefore, we used zero-inflated Poisson regression, rather than the more usual logistic regression, to relate the rate of culture contamination with the level of ED patient volume at the time of blood sampling. As ED occupancy increased, the risk of a blood sample being contaminated increased as well. When considered in a simple bivariate way, the relative risk of a positive culture returning a contaminant was 1.22 (95% confidence interval [CI], 0.91 to 1.63) in the 3rd quartile of overall activity versus that in the least busy quartile and 1.25 (95% CI, 0.95 to 1.60) for the 4th quartile versus the 1st quartile. When the data were considered with the more complete zero-inflated Poisson model, the relationship between ED occupancy level and contamination became even more pronounced (P < 0.01) (Fig. 2C). Only specimens collected during the busiest quarter of ED operational hours were in excess of the 75th percentile contamination rate of the College of American Pathologists.
As we had initially hypothesized, blood samples for culture collected in the busiest half of all hours of the year were significantly more likely to be contaminated than those collected in the least busy quarter. What was unexpected was that during the busiest times, the likelihood of recovering a likely pathogen decreased. Compared to that in the least busy quartile, the relative risk of a positive culture collected in the 3rd quartile of activity returning a likely pathogen was 0.94 (95% CI, 0.86 to 1.03). Similarly, the relative risk in the busiest quartile was 0.93 (95% CI, 0.86–1.01). As with the contamination rate, consideration of the data in the zero-inflated Poisson model revealed a stronger and more statistically significant effect (P < 0.01) (Fig. 2D).
DISCUSSION
In the current work, we relate ED operational activity to two markers of blood culture quality, the rate of contamination and the frequency of recovering a true pathogen. We found that both of these measures suffered during busy hours of operation. These findings suggest several important features regarding ED-based blood culture testing.
First, the likelihood of blood culture contamination increased significantly with increased patient volume, particularly in the top 25% of busy times during the study year. Increases in the contamination rate suggest technical lapses on the part of caregivers collecting samples for culture. Prior to the conduct of this study, a widely held belief at the UMHS was that collection in the ED was disproportionately likely to contaminate blood cultures. The current results indicate that this is not true. In fact, during low occupancy times, the contamination rate in the department was commensurate with health system-wide rates and, except for the busiest times of the year, was also on par with that for other health systems in general as reported by the College of American Pathologists (1). Nevertheless, the increase in the contamination rate as a function of ED occupancy represents an important issue for future study and intervention. In particular, a question raised by our results is whether time pressure prompted an increase in drawing blood samples from preexisting intravenous catheters. Our laboratory information system accommodates annotating the site of collection of samples. However, during the study period, the site of collection was infrequently recorded and, when noted, was done so with ad hoc abbreviations and comments that were unreliable for formal analysis. Accordingly, it is not possible to state confidently whether samples were more likely to be drawn from existing catheters during high-occupancy periods.
Second, although the fraction of patients from whom blood samples for culture were collected decreased with increasing activity in the ED, the likelihood of recovering a pathogen decreased. Given the retrospective nature of our work, we are unable to comment on the indications for testing in any particular patient or the appropriateness of testing decisions. One potential explanation is that during high-occupancy times, culture bottles were filled incompletely, reducing the likelihood of recovering a pathogen. However, the observed effect of high occupancy is also consistent with a loosening of indications for blood culture during busy times. To partially address this possibility, we hypothesized that during high-occupancy times, samples for culture would be acquired from progressively less ill patients. Use of the emergency triage score as a marker for general illness severity did not reveal any differences in culture practice, although this is a very coarse metric for assessing severity of illness (data not shown).
The reduced quality of blood cultures during periods of operational stress might have some role as a surrogate marker for the degradation of sterile technique in other ED processes. For example, although anecdotally the ED is a clinical location at a high risk for central line-associated bloodstream infection (CLABSI), data to support this claim are very sparse; in a prospective surveillance study of ED-placed central venous catheters, our group was unable to substantiate an increased risk (14). However, CLABSIs are infrequent enough and their attribution sufficiently vague that blood cultures might serve a useful role in tracking unit “clean culture” more effectively, and this hypothesis warrants further study. In addition, that a cornerstone microbiological test was shown to be subject to operational stress might have implications for epidemic management as well, where EDs will play a central role not only in treatment but also in diagnosis.
The primary limitation of this work is the mapping of ED occupancy to the ill-defined concept of crowding. At first impression, our data suggest that the appropriateness of utilization and technical acquisition of blood samples for culture in the ED are negatively impacted due to overextended providers, which might or might not be true. Important covariates not available to us in this analysis relate to departmental staffing details, including the number of providers actually present at any given time during the study period and the reliable annotation of which type of provider collected each sample. Scheduling physicians, nurses, and technicians in a clinical environment with such strong cyclical activity is complex, as is retrospectively estimating with accuracy the number and type of providers working at any hour of the year. At our institution, retrospectively determining the presence of a nonphysician caregiver can only be estimated through payroll records, and in our experience, these are not sufficiently reliable for research use. As staffing levels for all types of providers increase during periods of high patient occupancy, it is inappropriate to conclude that our findings indicate only staffing inadequacies.
Similarly, attribution of a culture to a specific provider was not possible due to the lack of a consistent practice for chain-of-evidence-type tracing of samples from the bedside to the laboratory. Establishing a more reliable means of informing individual providers of their blood culture contamination and true-positive rates would be of value in developing improved quality assurance strategies.
In conclusion, the frequency with which ED blood cultures recovered a likely contaminant increased and the likelihood of recovering a pathogen decreased during times of high occupancy.
Footnotes
Published ahead of print 20 March 2013
REFERENCES
- 1. Bekeris L, Tworek J, Walsh M, Valenstein P. 2005. Trends in blood culture contamination: a College of American Pathologists Q-Tracks study of 356 institutions. Arch. Pathol. Lab. Med. 129:1222–1225 [DOI] [PubMed] [Google Scholar]
- 2. Gander R, Byrd L, DeCrescenzo M, Hirany S, Bowen S, Baughman J. 2009. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J. Clin. Microbiol. 47:1021–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Washer L, Chenoweth C, Kim H. 2013. Blood culture contamination: a randomized trial evaluating the comparative effectiveness of three skin antiseptic interventions. Infect. Control Hosp. Epidemiol. 34:15–21 [DOI] [PubMed] [Google Scholar]
- 4. Kim N, Kim M, Lee S, Yun N, Park S, Kim H, Kim N, Kim E, Park W, Oh M. 2011. Effect of routine sterile gloving on contamination rates in blood culture: a cluster randomized trial. Ann. Intern. Med. 154:145–151 [DOI] [PubMed] [Google Scholar]
- 5. Weinbaum F, Lavie S, Danek M, Sixsmith D, Heinrich G, Mills S. 1997. Doing it right the first time: quality improvement and the contaminated blood culture. J. Clin. Microbiol. 35:563–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Center for Health Statistics 2012. National hospital ambulatory medical care survey: 2009 emergency department summary tables. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 7. Pitts S, Pines J, Handrigan M, Kellerman A. 2012. National trends in emergency department occupancy, 2001 to 2008: effect of inpatient admissions versus emergency department practice intensity. Ann. Emerg. Med. 60:679–686 [DOI] [PubMed] [Google Scholar]
- 8. Carr B, Kaye A, Wlebe D, Gracias V, Schwab C, Patrick P. 2007. Emergency department length of stay: a major risk factor for pneumonia in intubated blunt trauma patients. J. Trauma 63:9–12 [DOI] [PubMed] [Google Scholar]
- 9. Chalfin D, Trazeciak S, Likourezo A, Baumann B, Dellinger R. 2007. Impact of delayed transfer of critically ill patients from the emergency department to intensive care unit. Crit. Care Med. 35:1477–1483 [DOI] [PubMed] [Google Scholar]
- 10. Pines J, Locallo A, Hollander J, Baxt W, Lee H, Phillips C, Metlay J. 2007. The impact of emergency department crowding measures on time to antibiotics for patients with community acquired pneumonia. Ann. Emerg. Med. 50:510–516 [DOI] [PubMed] [Google Scholar]
- 11. Meurer W, Smith B, Losman E, Sherman D, Yaksich J, Jared J, Malani P, Younger J. 2009. Real-time identification of serious infection in geriatric patients using clinical information system surveillance. J. Am. Geriatr. Soc. 57:40–45 [DOI] [PubMed] [Google Scholar]
- 12. Nelson J, Smith B, Jared J, Younger J. 2011. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann. Emerg. Med. 57:500–504 [DOI] [PubMed] [Google Scholar]
- 13. McCarthy M, Aronsky D, Jones I, Miner J, Band R, Baren J, Desmond J, Baumlin K, Ding R, Shesser R. 2008. The emergency department occupancy rate: a simple measure of emergency department crowding? Ann. Emerg. Med. 51:15–24 [DOI] [PubMed] [Google Scholar]
- 14. Diaz K, Kelly S, Smith B, Malani P, Younger J. 2012. Prospective study of central venous catheters placed in a tertiary care emergency department: indications for use, infectious complications, and natural history. Am. J. Infect. Control 40:65–67 [DOI] [PubMed] [Google Scholar]