Abstract
Access to sputum smear microscopy in high-tuberculosis (TB)-burden regions is limited by a scarcity of microscopes and experienced technicians. We evaluated the accuracy of CellScope, a novel digital fluorescence microscope that may expand access to microscopy. The study utilized smear microscopy slides prepared from sputum specimens submitted by consecutive adults with ≥2 weeks of cough who were admitted to Mulago Hospital (Kampala, Uganda). Conventional light-emitting diode (LED) fluorescence microscopy (FM) and mycobacterial culture were performed by experienced technicians. Two U.S.-based postgraduate researchers without prior microscopy experience restained, imaged, and interpreted the slides using CellScope. We assessed whether sensitivity and specificity of CellScope-based LED FM was noninferior to conventional LED FM by using a preselected margin of inferiority of 15%. Of 525 patients included, 72% were HIV seropositive and 39% had culture-confirmed TB. The proportions of positive results were similar with CellScope and conventional LED FM (34% versus 32%, respectively; P = 0.32), and agreement was substantial. CellScope accuracy was within the noninferiority margin for both sensitivity (63% versus 70%; difference, −7%; 95% confidence interval [CI], −13% to −1%) and specificity (85% versus 92%; difference, −7%; 95% CI, −12% to −3%). A subanalysis of 43 slides evaluated by each CellScope reader found substantial interreader reliability (custom-weighted kappa, 0.65) and variable intrareader reliability (custom-weighted kappa, 0.11 versus 0.48). CellScope offers promise for expanding microscopy services. Future studies should evaluate the device when operated by health workers in low-resource settings, the feasibility of image transmission and analysis by experienced microscopists, and the accuracy of automated image analysis algorithms.
INTRODUCTION
Tuberculosis (TB) continues to be responsible for more deaths than any other infectious disease besides HIV/AIDS (1). In Africa, where the burden of TB is greatest, approximately 40% of individuals who fall ill with the disease go undiagnosed (2). Sputum smear microscopy is capable of detecting the majority of infectious TB cases, and mathematical models suggest that expanding access to high-quality smear microscopy may improve individual outcomes (3) and reduce TB prevalence and incidence (4). Although recently developed molecular detection methods are becoming available in some diagnostic centers (5), the costs and infrastructure requirements of current tests are prohibitive for most peripheral clinics in high-burden countries (6). Therefore, efforts to improve the quality and expand the reach of microscopy continue to be a global priority (7).
In low-income countries, smear microscopy typically involves direct visualization of stained smears by experienced laboratory technicians using conventional light microscopes. In contrast, in high-income countries, microscopy in the related discipline of pathology increasingly involves the use of digital images viewed on high-resolution monitors, an approach that maintains or improves diagnostic accuracy (8, 9). Compact, long-lasting, light-emitting diodes (LEDs) (10), complementary metal oxide semiconductor (CMOS) image sensors with high sensitivity and large pixel counts (11), decreased size and increased speed of microprocessors, and more-efficient and compact batteries provide an opportunity for expanding access to TB diagnostic services through portable, low-cost digital microscopes.
Here, we report on the diagnostic accuracy of sputum smear microscopy for pulmonary TB with CellScope, a novel digital fluorescence microscope, in comparison to conventional LED fluorescence microscopy (FM). We hypothesized that the capacity to enlarge and enhance objects of interest with CellScope-based digital LED FM would allow individuals without prior smear microscopy experience to read slides with diagnostic accuracy similar to that of experienced laboratory technicians using conventional LED FM.
MATERIALS AND METHODS
Device description.
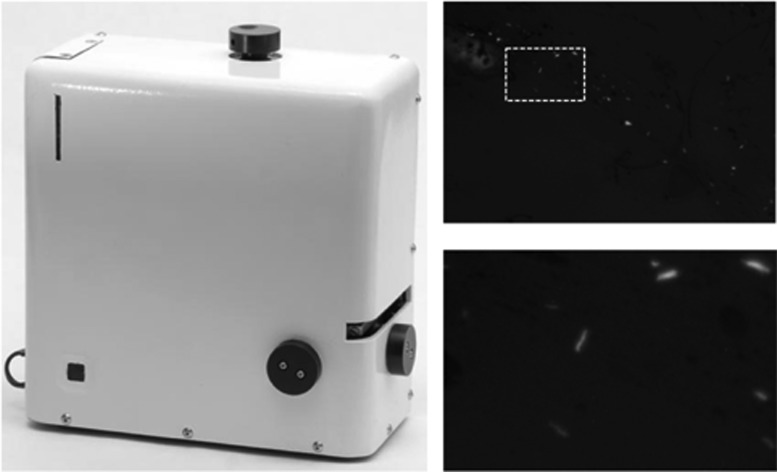
CellScope is a digital fluorescence microscope (12). For this evaluation, we used a stand-alone, portable, battery-powered prototype designed at the University of California, Berkeley, and manufactured by The Pilot Group (Monrovia, CA) that incorporates LEDs and a light sensor typical of commercial mobile phone cameras within an enclosed plastic case (Fig. 1). The case included a slide-loading tray and knobs for manual adjustment of slide position and focus. We connected the platform via a USB 2.0 cable to a low-cost laptop computer (Intel Classmate PC laptop; EliteGroup, Taiwan) featuring a 1,024- by 600-pixel LCD display. Custom software on the laptop enabled live on-screen visualization of the sputum smears, including adjustment of exposure, gain, and other imaging features. Images were able to be saved as 8-bit tagged image file format (TIFF) files for later analysis, and high-frequency image sampling enabled images to be enlarged with proper interpolation for viewing at apparent magnifications of up to ×3,500.
Fig 1.
CellScope (left) is a small (20- by 20- by 10-cm), light (3-kg), portable, battery-powered digital fluorescence microscope. The system is built around a 0.4-numerical-aperture (NA) ×20 microscope objective providing 0.76-μm resolution and a 0.64- by 0.49-mm sample-referenced field of view. Fluorescence excitation is provided by a 1-W, 460-nm-wavelength LED via a 0.65-NA condenser. Digital images captured by the device (top right) can be enlarged with proper interpolation for viewing at apparent magnifications of up to ×3,500 (bottom right).
Sample selection.
This study included smear microscopy slides prepared from a morning sputum specimen submitted by 585 consecutive adults (age ≥ 18 years) with cough for ≥2 weeks' duration who were admitted to Mulago Hospital (Kampala, Uganda) between September 2007 and June 2008. Details of patient enrollment and evaluation for the parent study have been published previously (13, 14). The morning sputum specimen was initially analyzed at the Uganda National Tuberculosis Reference Laboratory (NTRL), which has participated in a biannual external quality assurance program for smear microscopy administered by the World Health Organization (WHO) since 2005. Experienced NTRL technicians prepared direct smears on glass slides, stained them using auramine O, interpreted results using LED FM (Lumin; LW Scientific, Lawrenceville, GA) in accordance with standard algorithms (13, 15, 16), and stored the slides in opaque boxes. The technicians then processed the remaining sputum sample by the N-acetyl-l-cysteine (NALC)-NaOH method for culture on solid Lowenstein-Jensen medium and/or Bactec 960 MGIT liquid medium (13, 16).
Digital fluorescence microscopy.
Stored slide boxes were transported to a laboratory at the University of California, Berkeley, where all aspects of the present study were completed between May and July 2011. Two postgraduate researchers (A. Tapley and C. Reber) without prior microscopy experience and blinded to the original LED FM and culture results reexamined smears using identical CellScope devices. With assistance from CellScope engineers and microscopists in the Mycobacteriology Section at the San Francisco Department of Public Health Laboratory, each reader received 3 h of training on device operation and 6 h of training and practice on staining, slide reading, and acid-fast bacillus (AFB) identification.
The readers restained slides in batches (≤20 slides) (17) using the 2-min modified auramine O stain kit (Scientific Device Laboratory, Des Plaines, IL) (18) and imaged and interpreted slides within 24 h of staining according to a standardized protocol. We randomly divided 535 consecutive slides equally between the readers. Each reader restained and analyzed their assigned slides using CellScope once and scored each slide as positive or negative based on the detection of AFB within one 2-cm-wide smear length. The remaining 50 slides, representing a sample size based on convenience, were restained and analyzed twice by each reader and scored using a modification of the International Union Against Tuberculosis and Lung Disease (IUATLD)/WHO semiquantitative grading system for LED FM (16). Modification of the scoring system primarily involved scaling appropriately for estimated differences between the area read by a typical ×20 fluorescence microscope and by CellScope.
For the purposes of blinding, a study coordinator not involved in slide reading implemented a relabeling system. We collected reading time for all slides. The CellScope digital images have 0.76-μm nominal resolution (equivalent to a standard 0.4-numerical-aperture [NA] ×20 microscope objective), are digitally sampled above the Nyquist criterion, and are presented to the user at magnifications of ≥×500. The individual image field of view is 0.644 by 0.486 mm.
Statistical analysis.
We performed data analysis using STATA 10.0 (StataCorp LP, College Station, TX). We evaluated whether the diagnostic accuracy of LED FM performed by inexperienced readers using CellScope was noninferior to that of LED FM performed by experienced technicians using a conventional LED fluorescence microscope. The sample size was determined by the number of patient slides available for the analysis. Assuming 60% sensitivity and 95% specificity of LED FM and 40% prevalence of culture-positive TB (19), we projected 80% power to determine if the sensitivity and specificity of CellScope were noninferior to those of conventional LED FM, given a prespecified noninferiority margin of 15% and a one-sided test of correlated proportions at a 5% significance level (power analysis and sample size; NCSS, Kaysville, UT). Although limited by the sample size, the margin of noninferiority was considered acceptable given that CellScope was performed by users without prior microscopy experience, mimicking the circumstances of clinics and communities lacking access to conventional LED FM and/or experienced technicians.
We calculated the sensitivity and specificity of microscopy techniques in reference to mycobacterial culture results (i.e., two cultures per patient), compared them using McNemar's paired test of proportions, and reported sensitivity and specificity differences with exact binomial 95% confidence intervals (CI). Significance was defined as a P value of <0.05.
For the subset of slides read twice by each reader, we evaluated both interreader and intrareader reliability in the semiquantitative scores. Drawing on the IUATLD external quality assessment guidelines' definitions for slide-grading errors, we categorized pairs of results for each slide as no error, minor error, or major error based on the degree of discrepancy between the scores assigned (20). The subtotals for each category were then weighted 100%, 50%, and 0%, respectively, and the weighted values were used to generate a kappa statistic, the magnitude of which reflects the strength of agreement (21). The level of agreement was assessed on the basis of kappa values of ≤0.20 (poor), 0.21 to 0.40 (fair), 0.41 to 0.60 (moderate), 0.61 to 0.80 (substantial), and ≥0.81 (almost perfect) (22). We used bootstrapping with 1,000 replications to calculate bias-corrected 95% CIs for each kappa value.
Ethics statement.
All patients provided written informed consent. Institutional review boards at Makerere University, Mulago Hospital, the Uganda National Council for Science and Technology, and the University of California, San Francisco, approved the human subject aspects of the study protocol. The institutional review board at the University of California, Berkeley, deemed that the CellScope phase of the study did not constitute human subject research because the slides had been deidentified.
RESULTS
Study population.
Of the 585 total patients in the study, 60 patients (10%) lacked culture results and were excluded from the analysis. Among the remaining 525 patients who provided sputum, 246 (47%) were women. The median age of the patient population was 32 years (interquartile range [IQR], 27 to 39 years). A total of 380 (72%) patients were HIV seropositive, with a median CD4+ T-lymphocyte count of 55 cells/μl (IQR, 19 to 175 cells/μl). Mycobacterium tuberculosis was cultured from 207 (39%) patients.
Diagnostic accuracy of CellScope and conventional LED FM.
Among the 525 slides, the proportions of positive results were similar with CellScope performed by inexperienced readers and conventional LED FM performed by experienced technicians (34% versus 32%, respectively; difference, −1.7%; 95% CI, −5.3% to −1.9%). There was substantial agreement between the two techniques (84%; unweighted kappa, 0.64; 95% CI, 0.57 to 0.71).
Using culture as a reference standard, the difference in sensitivity between CellScope LED FM performed by inexperienced readers and conventional LED FM performed by experienced technicians was within the prespecified 15% margin of noninferiority (63% versus 70%, respectively; difference, −7%; 95% CI, −13% to −1%) (Table 1). Similarly, the difference in specificity was also within the prespecified margin of noninferiority (85% versus 92%, respectively; difference, −7%; 95% CI, −12% to −3%). CellScope sensitivities were similar for both readers (reader 1, 61%; reader 2, 66%; difference, −5%; 95% CI, −18% to +8%), whereas specificity was significantly higher for reader 1 than reader 2 (90% versus 80%, respectively; difference, 10%; 95% CI, 2% to 18%).
Table 1.
Diagnostic accuracy of LED FM by CellScope versus by conventional techniquea
| Diagnostic measure | Value (%) with each LED FM technique |
Difference (%) (95% CI) | P value | |
|---|---|---|---|---|
| Conventional | CellScope | |||
| Sensitivity (no. correctly identified as positive/total no. of positive results) | 70 (144/207) | 63 (130/207) | −7 (−13 to −1) | 0.029 |
| Specificity (no. correctly identified as negative/total no. of negative results) | 92 (294/318) | 85 (271/318) | −7 (−12 to −3) | 0.001 |
n = 525. A total of 207 samples had positive culture results, and 318 samples had negative culture results.
Inter- and intrareader reliability of sputum microscopy with CellScope.
Slides from seven of the 60 patients excluded from the analysis for missing culture results were among the 50 semiquantitatively scored slides, leaving slides from 43 patients for analysis of inter- and intrareader reliability. In assessing CellScope interreader reliability, we found substantial agreement (custom-weighted kappa, 0.65; 95% CI, 0.31 to 0.85) between readers (Table 2). There were 7 minor grading errors (scanty versus a negative, 2+, or 3+ score assigned) but only one major grading error (negative versus a 1+, 2+, or 3+ score assigned).
Table 2.
Interreader comparison of semiquantitative scores for LED FM with CellScopea
| Reader 2 score | Reader 1 score |
Total | ||||
|---|---|---|---|---|---|---|
| Negative | Scanty | 1+ | 2+ | 3+ | ||
| Negative | 29 | 5b | 34 | |||
| Scanty | 2b | 2 | ||||
| 1+ | 1 | 2 | 3 | |||
| 2+ | 1c | 1 | 2 | |||
| 3+ | 2 | 2 | ||||
| Total | 32 | 6 | 2 | 1 | 2 | 43 |
n = 43.
Minor error, scanty score versus a negative, 2+, or 3+ score.
Major error, negative score versus a 1+, 2+, or 3+ score.
We assessed intrareader reliability by comparing the two semiquantitative scores assigned by each reader. The agreement between the two readings was moderate for reader 1 (weighted kappa, 0.48; 95% CI, 0.17 to 0.69), with no major grading errors and 13 minor grading errors (Table 3). Of the 13 minor grading errors, 12 were discrepancies between scanty and negative scores. However, the agreement between the two readings was poor for reader 2 (weighted kappa, 0.11; 95% CI, −0.20 to 0.43), with 9 major and 6 minor grading errors (Table 4).
Table 3.
Reader 1 intrareader comparison of semiquantitative scores for LED FM with CellScopea
| 2nd reading of reader 1 | 1st reading of reader 1 |
Total | ||||
|---|---|---|---|---|---|---|
| Negative | Scanty | 1+ | 2+ | 3+ | ||
| Negative | 25 | 5b | 30 | |||
| Scanty | 7b | 1 | 1b | 9 | ||
| 1+ | 1 | 1 | ||||
| 2+ | 1 | 1 | ||||
| 3+ | 2 | 2 | ||||
| Total | 32 | 6 | 2 | 1 | 2 | 43 |
n = 43.
Minor error, scanty score versus a negative, 2+, or 3+ score.
Table 4.
Reader 2 intrareader comparison of semiquantitative scores for LED FM with CellScopea
| 2nd reading of reader 2 | 1st reading of reader 2 |
Total | ||||
|---|---|---|---|---|---|---|
| Negative | Scanty | 1+ | 2+ | 3+ | ||
| Negative | 24 | 1b | 2c | 1c | 2c | 30 |
| Scanty | 6b | 6 | ||||
| 1+ | 1c | 1 | 1 | 3 | ||
| 2+ | 1c | 1 | ||||
| 3+ | 2c | 1 | 3 | |||
| Total | 34 | 2 | 3 | 2 | 2 | 43 |
n = 43.
Minor error, scanty score versus a negative, 2+, or 3+ score.
Major error, negative score versus a 1+, 2+, or 3+ score.
Slide examination time with CellScope.
The median slide examination time for LED FM with CellScope was 4.2 min (IQR, 2.3 to 6.0 min). The median examination time was 1.3 min (IQR, 0.5 to 3.2 min) for positive slides versus 4.6 min (IQR, 3.8 to 6.4 min) for negative slides (P < 0.001).
DISCUSSION
In this study, we found that users with limited prior microscopy experience were able to obtain diagnostic-quality images of stained sputum smears using CellScope, a novel portable digital fluorescence microscope. The sensitivity and specificity of CellScope were lower than those of conventional LED FM performed by experienced laboratory technicians but within our prespecified margin of inferiority. CellScope has strong potential to expand the reach of TB microscopy services in resource-limited settings.
Although a number of designs for novel digital microscopy devices have been recently reported (12, 23–25), to date, these studies have been primarily proof-of-concept in nature. By using a large sample size, well-accepted measures of diagnostic accuracy, and rigorous statistical methods, our study lays the groundwork for further development of technologies like CellScope that use digital FM for the diagnosis of TB. Our results suggest that CellScope may be used to obtain high-quality diagnostic images of microscopy slides in settings in which microscopy is not currently offered. Its small size (20 by 20 by 10 cm), light weight (3 kg), and durable plastic case make the device eminently portable. In addition, its 5-hour battery can be recharged as easily as a mobile phone, whether from an electrical outlet, a solar cell, or a vehicle battery.
As with more-conventional methods (26–28), we found that the diagnostic accuracy of LED FM with CellScope is operator dependent and is unlikely to be adequate without sufficient microscopy training and experience. However, CellScope's use of digital imaging may be leveraged to overcome the limited supply of trained microscopists in resource-limited settings. Digital images produced by CellScope can be transmitted via the mobile network to distant experts for evaluation, an approach that we are currently using in an ongoing study and that has been successfully demonstrated by others in several settings (29–31). Indeed, mobile phone coverage is extensive in most low-income countries in which TB is endemic (32–34), and CellScope images can also be easily stored for later transmission when a mobile network is unavailable. In addition, significant progress has been made in the development of reliable computer algorithms for the detection of AFB in digital images of sputum smears (11, 35), including one algorithm based on CellScope images that performed as well as human readers (36). Automated image analysis may facilitate on-the-spot diagnosis, improve sensitivity by enabling analysis of a larger number of fields than typically evaluated by a human microscopist, and improve specificity through rigid and reliable criteria for AFB identification. With its use of an external laptop or smart phone for image visualization, CellScope is well positioned to integrate computationally intensive image analysis into its current platform.
Our study has a number of potential limitations. First, to simulate use of CellScope by inexperienced personnel, we chose U.S.-based postgraduate researchers with no prior sputum microscopy experience to restain, image, and interpret slides using CellScope. One reader had substantially lower specificity and poor intrareader reliability, suggesting that digital imaging on its own does not overcome the need for trained and experienced human readers. In addition, further studies are needed to assess the comfort and proficiency of users from low-resource settings with obtaining digital smear images using CellScope. Second, the consequences of extended slide storage and restaining of slides prior to the readings with CellScope are unclear. Both CellScope readers reported that image interpretation was impeded frequently by excessive background fluorescence or fluorescent nonbacillary particulate matter, a finding also observed by other researchers analyzing restained sputum smear slides (37). Finally, the mean slide examination time by CellScope readers was over 4 min, compared to the 1- to 3-min range found in recent studies involving conventional LED FM (26, 27). It is possible that reading times were influenced by reader inexperience and the use of restained slides. Future studies will assess reading times when images are transmitted to expert microscopists and with the use of automated image analysis algorithms.
In summary, our prospective, blinded study found that inexperienced readers using CellScope, a novel, portable digital fluorescence microscope, can achieve levels of sensitivity and specificity within 15% of those achieved by experienced technicians using conventional LED FM. Although this level of accuracy is insufficient for diagnostic use in the field, the CellScope platform can be leveraged to circumvent many operator-related factors that limit the reach of conventional approaches to FM. In particular, future studies should address the feasibility and accuracy of image transmission over wireless networks to expert microscopists and/or integration of automated image analysis algorithms. Portable and low-cost digital fluorescence microscopes, such as CellScope, represent an important opportunity for achieving broader access to high-quality TB diagnostic testing.
ACKNOWLEDGMENTS
We thank the staff at the Uganda NTRL for performing conventional LED FM and mycobacterial culture for this study. We also thank the patients, administration, and staff of Mulago National Referral Hospital for making this research possible. We express our gratitude for the generous donations of time and expert advice provided by Sally Liska and Anna Babst of the San Francisco Public Health Laboratory and Marguerite Roemer and Carol Goodman (emeritus) of the clinical microbiology laboratory at San Francisco General Hospital.
The study was supported in part by grants from the National Institutes of Health (K23 AI080147 [to J.L.D.], K24 HL087713 and R01 HL 090335 [to L.H.], and K23 HL094141 [to A.C.]), the Vodafone Americas Foundation (to D.A.F.), and the Blum Center for Developing Economies (to D.A.F.). This work was also supported by the National Center for Research Resources (KL2 RR024130).
Two authors (N.S. and D.A.F.) are on a pending patent related to the CellScope technology.
Footnotes
Published ahead of print 3 April 2013
REFERENCES
- 1. Lawn SD, Zumla AI. 2011. Tuberculosis. Lancet 378:57–72 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization 2012. Global tuberculosis report 2012. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3. Keeler E, Perkins MD, Small P, Hanson C, Reed S, Cunningham J, Aledort JE, Hillborne L, Rafael ME, Girosi F, Dye C. 2006. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 444(Suppl 1):49–57 [DOI] [PubMed] [Google Scholar]
- 4. Dowdy DW, Chaisson RE, Moulton LH, Dorman SE. 2006. The potential impact of enhanced diagnostic techniques for tuberculosis driven by HIV: a mathematical model. AIDS 20:751–762 [DOI] [PubMed] [Google Scholar]
- 5. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans CA. 2011. GeneXpert—a game-changer for tuberculosis control? PLoS Med. 8:e1001064 doi:10.1371/journal.pmed.1001064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stop TB. Partnership, World Health Organization 2010. The global plan to stop TB: 2011–2015. World Health Organization, Geneva, Switzerland [Google Scholar]
- 8. Ozluk Y, Blanco PL, Mengel M, Solez K, Halloran PF, Sis B. 2012. Superiority of virtual microscopy versus light microscopy in transplantation pathology. Clin. Transplant. 26:336–344 [DOI] [PubMed] [Google Scholar]
- 9. Rocha R, Vassallo J, Soares F, Miller K, Gobbi H. 2009. Digital slides: present status of a tool for consultation, teaching, and quality control in pathology. Pathol. Res. Pract. 205:735–741 [DOI] [PubMed] [Google Scholar]
- 10. Hänscheid T. 2008. The future looks bright: low-cost fluorescent microscopes for detection of Mycobacterium tuberculosis and Coccidiae. Trans. R. Soc. Trop. Med. Hyg. 102:520–521 [DOI] [PubMed] [Google Scholar]
- 11. Fontaine R. 2011. Recent innovations in CMOS image sensors, p 1–5 In Proceedings of the 22nd Annu. Advanced Semiconductor Manufacturing Conference IEEE/SEMI, Saratoga Springs, NY [Google Scholar]
- 12. Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. 2009. Mobile phone based clinical microscopy for global health applications. PLoS One 4:e6320 doi:10.1371/journal.pone.0006320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cattamanchi A, Huang L, Worodria W, den Boon S, Kalema SN, Katagira W, Byanyima P, Yoo S, Matovu J, Hopewell PC, Davis JL. 2011. Integrated strategies to optimize sputum smear microscopy: a prospective observational study. Am. J. Respir. Crit. Care Med. 183:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cattamanchi A, Davis JL, Worodria W, den Boon S, Yoo S, Matovu J, Kiidha J, Nankya F, Kyeyune R, Byanyima P, Andama A, Joloba M, Osmond DH, Hopewell PC, Huang L. 2009. Sensitivity and specificity of fluorescence microscopy for diagnosing pulmonary tuberculosis in a high HIV prevalence setting. Int. J. Tuberc. Lung Dis. 13:1130–1136 [PMC free article] [PubMed] [Google Scholar]
- 15. Kent P, Kubica G. 1985. Public health mycobacteriology—a guide for the level III laboratory. U.S. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 16. World Health Organization 1998. Laboratory services in tuberculosis control. Part II. Microscopy. World Health Organization, Geneva, Switzerland [Google Scholar]
- 17. Kam KM, Yip C-W, Tang HS, Van Deun A. 2009. Bulk acid-fast staining of sputum smears: time to end a taboo. Int. J. Tuberc. Lung Dis. 13:1119–1123 [PubMed] [Google Scholar]
- 18. Hendry C, Dionne K, Hedgepeth A, Carroll K, Parrish N. 2009. Evaluation of a rapid fluorescent staining method for detection of mycobacteria in clinical specimens. J. Clin. Microbiol. 47:1206–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins M, Aziz MA, Pai M. 2007. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6:570–581 [DOI] [PubMed] [Google Scholar]
- 20. Aziz MA, Ba F, Becx-Bleumink M, Humes R, Iademarco M. 2002. External quality assessment for AFB smear microscopy. Association of Public Health Laboratories, Washington, DC [Google Scholar]
- 21. Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174 [PubMed] [Google Scholar]
- 22. Guyatt G, Rennie D, Meade M, Cook D. 2008. Users' guides to the medical literature: a manual for evidence-based clinical practice, 2nd ed McGraw-Hill Professional, New York, NY [Google Scholar]
- 23. Miller AR, Davis GL, Oden ZM, Razavi MR, Fateh A, Ghazanfari M, Abdolrahimi F, Poorazar S, Sakhaie F, Olsen RJ, Bahrmand AR, Pierce MC, Graviss EA, Richards-Kortum R. 2010. Portable, battery-operated, low-cost, bright field and fluorescence microscope. PLoS One 5:e11890 doi:10.1371/journal.pone.0011890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCall B, Pierce M, Graviss EA, Richards-Kortum R, Tkaczyk T. 2011. Toward a low-cost compact array microscopy platform for detection of tuberculosis. Tuberculosis 91:S54–S60 [DOI] [PubMed] [Google Scholar]
- 25. Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ. 2011. Miniaturized integration of a fluorescence microscope. Nat. Methods 8:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albert H, Manabe Y, Lukyamuzi G, Ademun P, Mukkada S, Nyesiga B, Joloba M, Paramasivan CN, Perkins MD. 2010. Performance of three LED-based fluorescence microscopy systems for detection of tuberculosis in Uganda. PLoS One 5:e15206 doi:10.1371/journal.pone.0015206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shenai S, Minion J, Vadwai V, Tipnis T, Shetty S, Salvi A, Udwadia Z, Pai M, Rodrigues C. 2011. Evaluation of light emitting diode-based fluorescence microscopy for the detection of mycobacteria in a tuberculosis-endemic region. Int. J. Tuberc. Lung Dis. 15:483–488 [DOI] [PubMed] [Google Scholar]
- 28. Cuevas LE, Yassin MA, Al-Sonboli N, Lawson L, Arbide I, Al-Aghbari N, Sherchand JB, Al-Absi A, Emenyonu EN, Merid Y, Okobi MI, Onuoha JO, Aschalew M, Aseffa A, Harper G, Anderson de Cuevas RM, Kremer K, van Soolingen D, Nathanson C-M, Joly J, Faragher B, Squire SB, Ramsay A. 2011. A multi-country non-inferiority cluster randomized trial of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis. PLoS Med. 8:e1000443 doi:10.1371/journal.pmed.1000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuijn CJ, Hoefman BJ, van Beijma H, Oskam L, Chevrollier N. 2011. Data and image transfer using mobile phones to strengthen microscopy-based diagnostic services in low and middle income country laboratories. PLoS One 6:e28348 doi:10.1371/journal.pone.0028348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frean J. 2007. Microscopic images transmitted by mobile cameraphone. Trans. R. Soc. Trop. Med. Hyg. 101:1053. [DOI] [PubMed] [Google Scholar]
- 31. Zimic M, Coronel J, Gilman RH, Luna CG, Curioso WH, Moore DAJ. 2009. Can the power of mobile phones be used to improve tuberculosis diagnosis in developing countries? Trans. R. Soc. Trop. Med. Hyg. 103:638–640 [DOI] [PubMed] [Google Scholar]
- 32. Teltscher S, Magpantay E, Gray V, Olaya D, Vallejo I. 2009. Measuring the information society: the ICT Development Index. Telecommunication Development Bureau, International Telecommunications Union, Geneva, Switzerland [Google Scholar]
- 33. Priftis M, Yang H, Zhenwei Qiang C, Kimura K. 2012. The little data book on information and communication technology. World Bank, Washington, DC [Google Scholar]
- 34. Bellina L, Missoni E. 2009. Mobile cell-phones (M-phones) in telemicroscopy: increasing connectivity of isolated laboratories. Diagn. Pathol. 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veropoulos K, Learmonth G, Campbell C, Knight B, Simpson J. 1999. Automated identification of tubercle bacilli in sputum. A preliminary investigation. Anal. Quant. Cytol. Histol. 21:277–282 [PubMed] [Google Scholar]
- 36. Chang J, Arbelaez P, Switz N, Reber C, Tapley A, Davis L, Cattamanchi A, Fletcher D, Malik J. 2012. Automated tuberculosis diagnosis using fluorescence images from a mobile microscope, p 345–352 In Ayache N, Delingette H, Golland P, Kensaku M. (ed), Proceedings of the 15th International Conference on Medical Image Computing and Computer Assisted Intervention, Nice, France Springer-Verlag, Berlin, Germany: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yip C-W, Chan MY, Cheung WF, Yu KW, Tang HS, Kam KM. 2012. Random blinded rechecking of sputum acid-fast bacilli smear using fluorescence microscopy: 8 years' experience. Int. J. Tuberc. Lung Dis. 16:398–401 [DOI] [PubMed] [Google Scholar]