Abstract
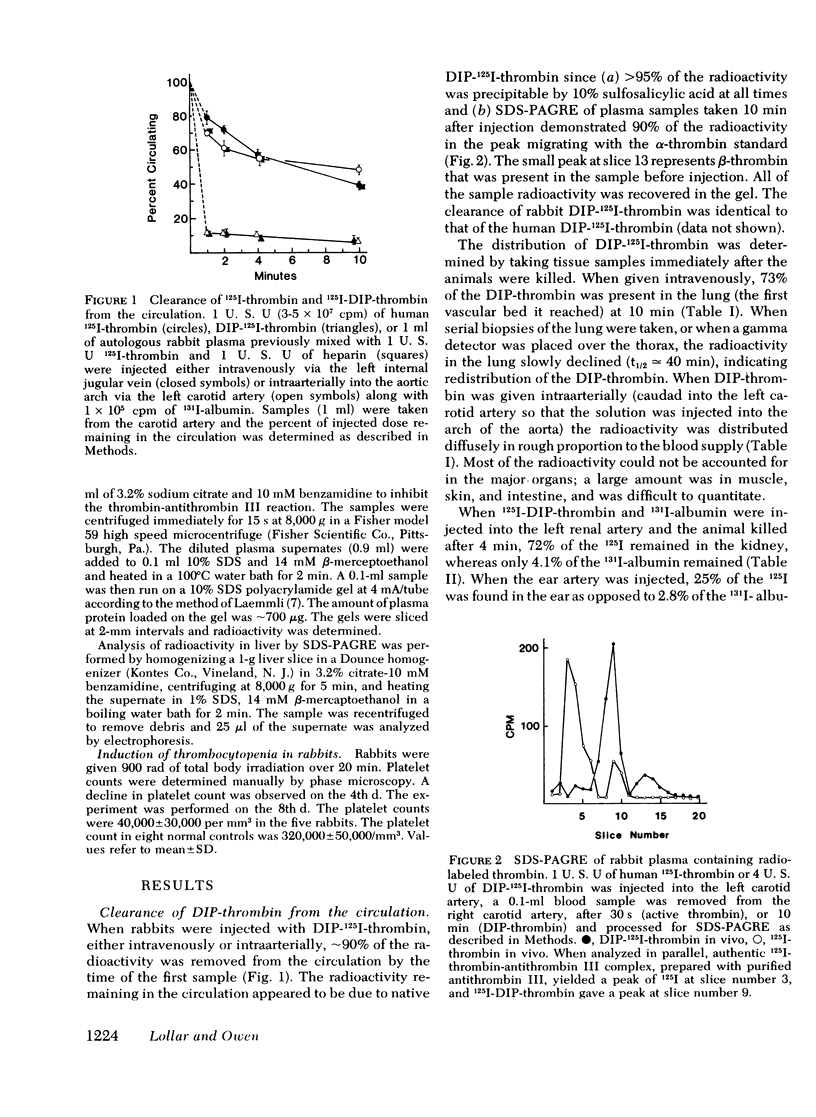
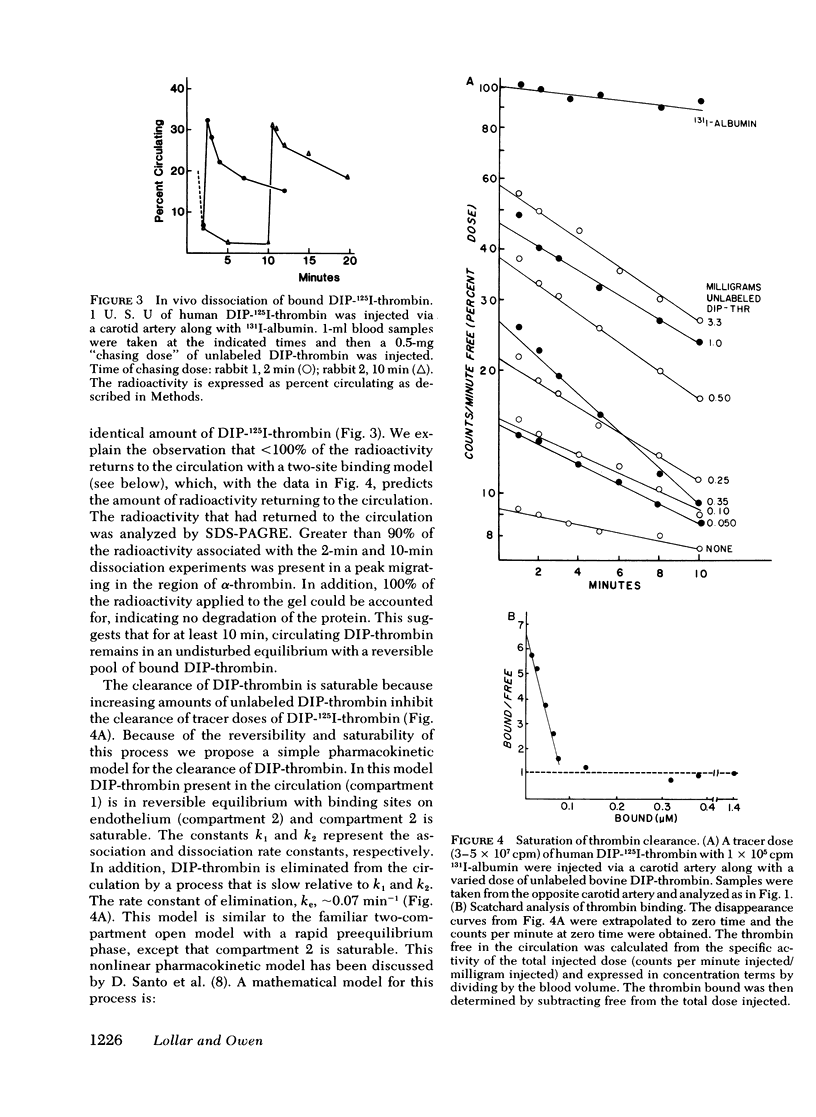
The clearance of 125I-thrombin and diisopropylphosphoryl-125I-thrombin (DIP-thrombin) from the circulation in rabbits was studied. When given either intraarterially or intravenously, DIP-thrombin, which is active-site blocked, was ∼90% cleared from the circulation by 1 min, the time of earliest sampling, indicating a large first-pass effect. DIP-thrombin given intravenously is found predominantly in the lungs, whereas DIP-thrombin injected into the aortic arch is distributed diffusely in approximate proportion to the blood supply. Renal artery, femoral artery, ear artery, left atrium, and portal vein infusions demonstrate that kidney, muscle, ear, heart, and liver, respectively, can remove DIP-thrombin from the circulation. These data imply that the clearance of DIP-thrombin is not a function of a specific organ but of the vascular bed per se. The clearance of DIP-thrombin was reversible since injection of 0.5 mg of unlabeled DIP-thrombin 10 min after the injection of a tracer dose of DIP-125I-thrombin resulted in the rapid reappearance of the DIP-125I-thrombin into the circulation. In addition, the clearance of DIP-thrombin was saturable, i.e., clearance of DIP-125I-thrombin was inhibited by unlabeled DIP-thrombin in a dose-dependent fashion. In vivo Scatchard analysis of the saturation of the clearance process demonstrated that DIP-thrombin can be removed by binding to high-affinity binding sites, since dissociation constants (KD) of 10 and 13 nM were obtained for human and bovine DIP-thrombin, respectively.
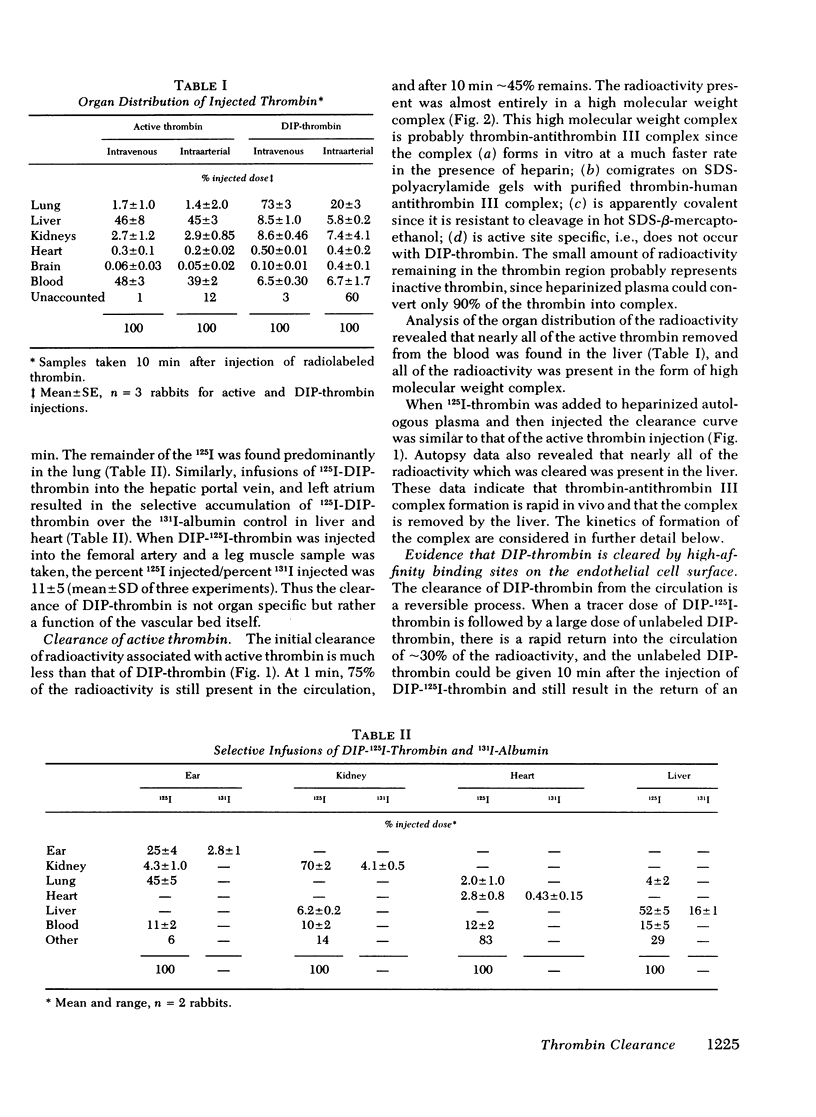
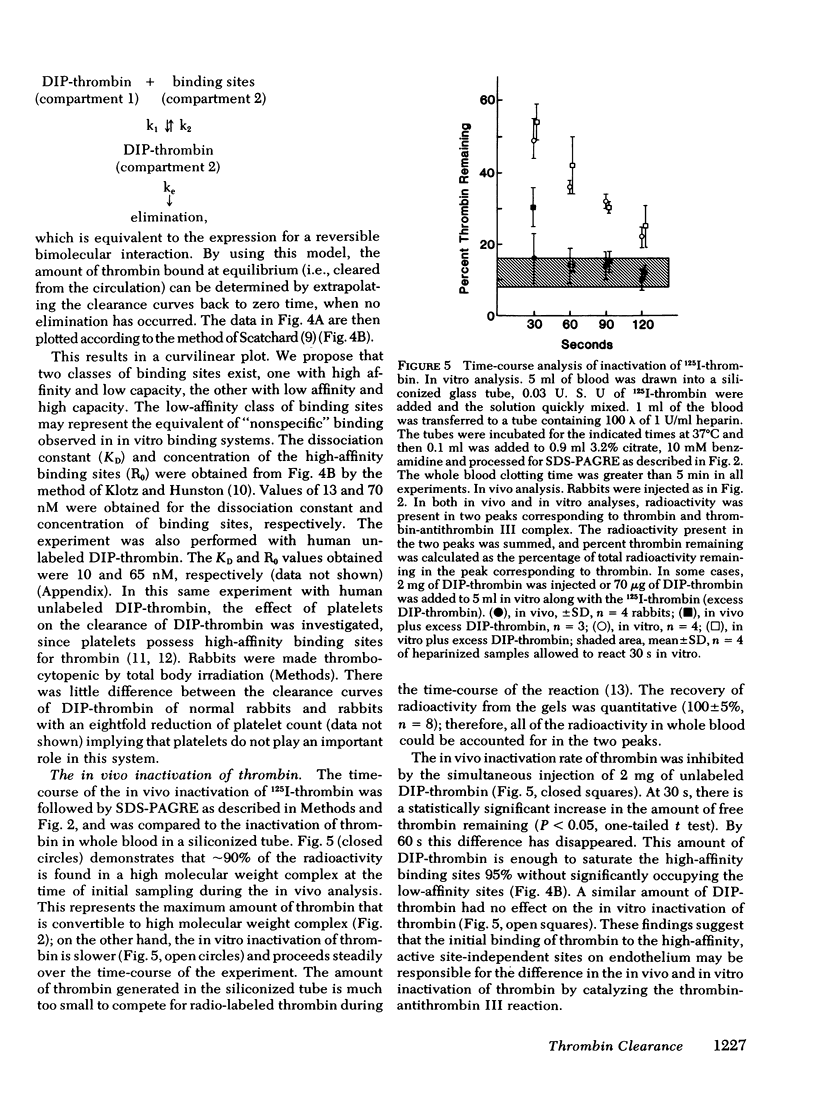
In contrast to DIP-thrombin, ∼75% of the radioactivity associated with active thrombin remained in the circulation at 1 min. By 10 min 55% of 125I-thrombin had been removed from the circulation, and essentially all of the radioactivity can be accounted for in the liver. Sodium dodecyl sulfate-polyacrylamide gel radioelectrophoresis of plasma samples taken after injection of 125I-thrombin demonstrated that all of the active thrombin was converted to covalent thrombin-antithrombin III complex by the time of initial sampling (30 s). The in vitro conversion of 125I-thrombin to thrombin-antithrombin III complex was considerably slower (50±5% conversion at 30 s). The simultaneous injection of excess unlabeled DIP-thrombin inhibited the rate of formation of 125I-thrombin-antithrombin III complex formation in vivo (but not in vitro), which suggests that the binding of active thrombin to the high affinity binding sites is required for the rapid inactivation of thrombin in vivo.
We propose that (a) thrombin in the circulation binds to active site-independent high-affinity binding sites on the endothelial cell surface; (b) the inactivation of thrombin by antithrombin III is faster in vivo than in vitro because the high-affinity binding sites, present in a high concentration in the microcirculation, catalyze the reaction; (c) thrombin-antithrombin III complexes are selectively removed by the liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awbrey B. J., Hoak J. C., Owen W. G. Binding of human thrombin to cultured human endothelial cells. J Biol Chem. 1979 May 25;254(10):4092–4095. [PubMed] [Google Scholar]
- Buonassisi V. Sulfated mucopolysaccharide synthesis and secretion in endothelial cell cultures. Exp Cell Res. 1973 Feb;76(2):363–368. doi: 10.1016/0014-4827(73)90388-1. [DOI] [PubMed] [Google Scholar]
- Danishefsky I., Ahrens M., Klein S. Effect of heparin modification on its activity in enhancing the inhibition of thrombin by antithrombin III. Biochim Biophys Acta. 1977 Jun 23;498(1):215–222. doi: 10.1016/0304-4165(77)90101-5. [DOI] [PubMed] [Google Scholar]
- Griffith M. J. Covalent modification of human alpha-thrombin with pyridoxal 5'-phosphate. Effect of phosphopyridoxylation on the interaction of thrombin with heparin. J Biol Chem. 1979 May 10;254(9):3401–3406. [PubMed] [Google Scholar]
- Griffith M. J., Kingdon H. S., Lundblad R. L. Inhibition of the heparin-antithrombin III/thrombin reaction by active site blocked-thrombin. Biochem Biophys Res Commun. 1979 Apr 13;87(3):686–692. doi: 10.1016/0006-291x(79)92013-8. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Regoeczi E. The inactivation of thrombin and plasmin by antithrombin III in the presence of sepharose-heparin. Thromb Res. 1977 May;10(5):645–660. doi: 10.1016/0049-3848(77)90047-0. [DOI] [PubMed] [Google Scholar]
- Jesty J. The kinetics of formation and dissociation of the bovine thrombin.antithrombin III complex. J Biol Chem. 1979 Oct 25;254(20):10044–10050. [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Schmer G., Hermodson M. A., Teller D. C., Davie E. W. Characterization of human, bovine, and horse antithrombin III. Biochemistry. 1976 Jan 27;15(2):368–373. doi: 10.1021/bi00647a020. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li E. H., Orton C., Feinman R. D. The interaction of thrombin and heparin. Proflavine dye binding studies. Biochemistry. 1974 Nov 19;13(24):5012–5017. doi: 10.1021/bi00721a023. [DOI] [PubMed] [Google Scholar]
- Machovich R., Blaskó G., Pálos L. A. Action of heparin on thrombin-antithrombin reaction. Biochim Biophys Acta. 1975 Jan 30;379(1):193–200. doi: 10.1016/0005-2795(75)90022-7. [DOI] [PubMed] [Google Scholar]
- Machovich R. Mechanism of action of heparin through thrombin on blood coagulation. Biochim Biophys Acta. 1975 Nov 18;412(1):13–17. doi: 10.1016/0005-2795(75)90334-7. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Wasiewski W. W., Fenton J. W., 2nd, Detwiler T. C. Equilibrium binding of thrombin to platelets. Biochemistry. 1976 Nov 2;15(22):4886–4893. doi: 10.1021/bi00667a021. [DOI] [PubMed] [Google Scholar]
- Owen W. G. Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta. 1975 Oct 20;405(2):380–387. doi: 10.1016/0005-2795(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Pomerantz M. W., Owen W. G. A catalytic role for heparin. Evidence for a ternary complex of heparin cofactor thrombin and heparin. Biochim Biophys Acta. 1978 Jul 21;535(1):66–77. doi: 10.1016/0005-2795(78)90033-8. [DOI] [PubMed] [Google Scholar]
- Shuman M. A., Majerus P. W. The measurement of thrombin in clotting blood by radioimmunoassay. J Clin Invest. 1976 Nov;58(5):1249–1258. doi: 10.1172/JCI108579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. F. The heparin-thrombin complex in the mechanism of thrombin inactivation by heparin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):111–117. doi: 10.1016/s0006-291x(77)80171-x. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]



