Abstract
The present case provides direct evidence of human herpesvirus 6 reactivation in resected lymph node tissue in a patient with drug-induced hypersensitivity syndrome. This case clearly demonstrates that appropriate pathological evaluation of lymphadenopathy for drug-induced hypersensitivity syndrome, which mimics malignant lymphoma in clinical, radiological, and pathological findings, is required.
CASE REPORT
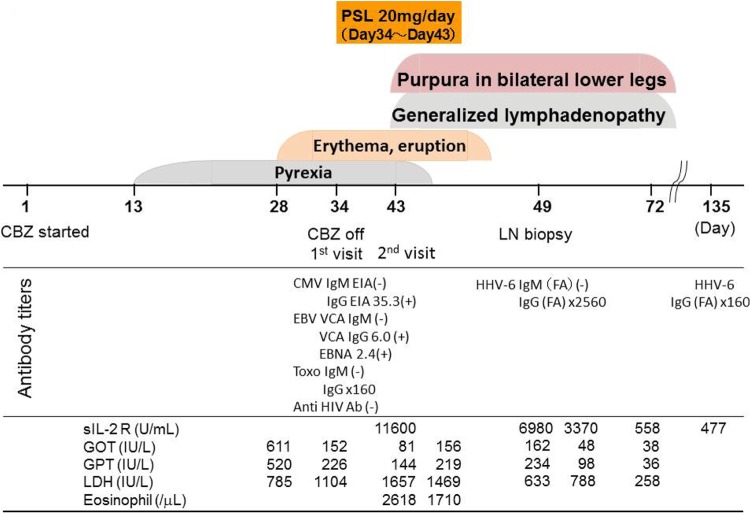
A 57-year-old woman was referred to our department because of generalized erythema involving mainly her face and upper trunk, with a morbilliform eruption for a week, persistent pyrexia, and a sore throat for the preceding 3 weeks. She had been treated for bipolar disorder and had a history of atopic dermatitis and bronchial asthma in her childhood. She had been on lorazepam, famotidine, and loxoprofen for a decade, and carbamazepine had been started 1 month prior to coming to our department (Fig. 1, day 1). She was allergic to soybeans, corn, eggs, and rice. She had not been in contact with any sick persons. At her first visit to our department (day 34), physical examination showed mild periorbital edema and expanding erythema from her face to both lower legs, with a morbilliform eruption, but no mucosal ulcers or erosions were noted. Thus, she was tentatively diagnosed as having an adverse cutaneous reaction to carbamazepine; therefore, carbamazepine was stopped, and oral prednisolone therapy was started at a dose of 20 mg/day. One week after starting the prednisolone therapy (Fig. 1, day 43), her sore throat and high-grade fever had almost disappeared, and some aspects of the cutaneous lesions, such as pigment deposition and nonpalpable purpura, had improved. Of note, at this time, she noticed generalized lymphadenopathy and returned to our department.
Fig 1.
Clinical course of the present case. PSL, prednisolone; CBZ, carbamazepine; LN, lymph node; CMV, cytomegalovirus; EIA, enzyme immunoassay; FA, fluorescent antibody; EBV, Epstein-Barr virus; VCA, viral capsid antigen; EBNA, Epstein-Barr virus nuclear antigen; Toxo, Toxoplasma gondii; Ab, antibody; sIL-2 R, soluble interleukin 2 receptor; GOT, glutamate oxaloacetate transaminase; GPT, glutamate pyruvate transaminase; LDH, lactate dehydrogenase.
At her 2nd visit to our department (day 43), physical examination showed generalized superficial lymphadenopathy (supraclavicular, cervical, axillary, and inguinal), and each node was 20 mm in size, firm, nonfixed, circumscribed, and rubbery, suggesting lymphoma. Laboratory examination revealed marked elevations of her white blood cell count (37,400/μl, with 1% basophils, 7% eosinophils, 25% neutrophils, 44% lymphocytes, 14% atypical lymphocytes, and 3% monocytes), lactate dehydrogenase (1,657 IU/liter), and soluble interleukin 2 receptor (11,600 U/ml). Moderate elevations of serum hepatic biliary enzymes were also noted (aspartate aminotransferase, 81 IU/liter; alanine aminotransferase, 144 IU/liter; alkaline phosphatase, 264 IU/liter; γ-glutamyl transpeptidase, 371 IU/liter). Two sets of blood cultures were negative, and no evidence of a recent infectious mononucleosis (IM) syndrome (Epstein-Barr virus) or IM-like syndrome (cytomegalovirus, Toxoplasma gondii, human immunodeficiency virus) was detected (Fig. 1).
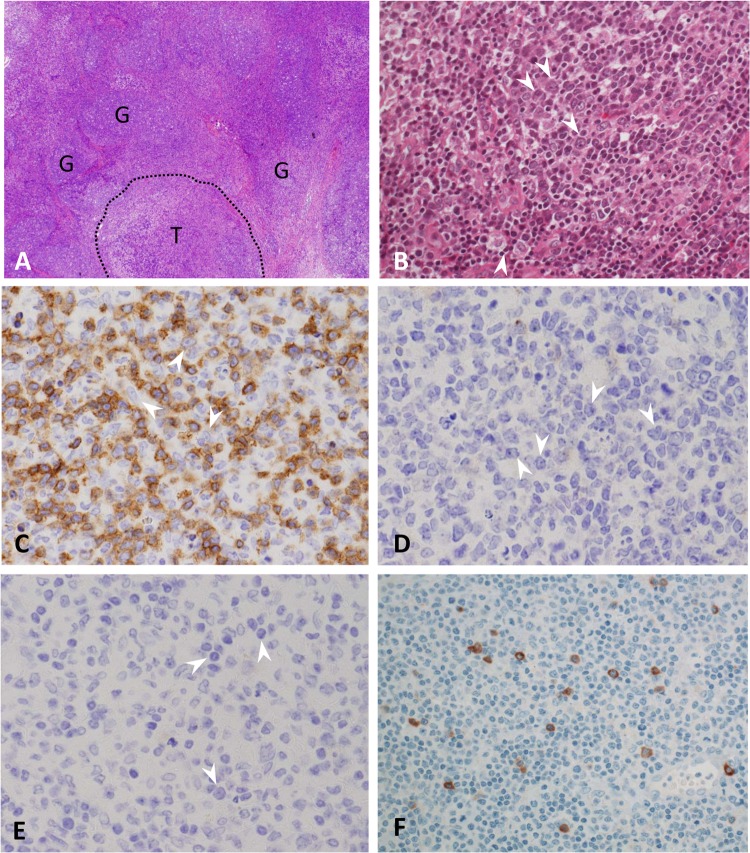
On day 48, she was admitted to another hospital with suspected malignant lymphoma, and whole-body computed tomography showed the superficial lymphadenopathy. A lymph node biopsy of the right axilla was performed the next day. After hematoxylin-eosin staining, the histological examination showed enlarged nodes that contained an immunoblastic reaction predominantly affecting the T zones of the node, sparing the follicles (Fig. 2A, labeled “G” [for germinal center]), which resulted in expanding T zones (Fig. 2A, labeled “T”) possessing a pleomorphic infiltration with many blast-type cells (Fig. 2B, white arrowheads), plasma cells, histiocytes, and eosinophils. Although the blast-type cells showed morphological features indistinguishable from those of Reed-Sternberg cells, they were negative for CD3 (Fig. 2C, white arrowheads), CD30 (Fig. 2D, white arrowheads), and CD15 (Fig. 2E, white arrowheads).
Fig 2.
Hematoxylin-eosin staining. (A) Histological examination of the enlarged right axillary lymph nodes shows enlarged nodes that contain an immunoblastic reaction predominantly affecting the T zones of the node and sparing the follicles (labeled “G” for germinal center), resulting in expanding T zones (labeled “T”). The area contains a pleomorphic infiltration with many blast-type cells (B) (white arrowheads) with abundant CD3-positive T lymphocytes in the background (C). The blast-type cells seen in panel B show morphological features indistinguishable from those of Reed-Sternberg cells but are negative for CD3 (C) (white arrowheads), CD30 (D) (white arrowheads), and CD15 (E) (white arrowheads). (F) Immunohistochemical staining for HHV-6 was positive in atypical Reed-Sternberg-like cells. Immunohistochemical staining was performed using an indirect immunoperoxidase technique on paraffin-embedded sections. A monoclonal antibody (clone C3108-103; final dilution, 1/100; Chemicon, Inc., Temecula, CA, USA) against the 101-kDa virion protein (a major viral antigen) of HHV-6 was used.
Three weeks after withdrawal of carbamazepine, the lymphadenopathy resolved spontaneously, suggesting reactive lymphadenopathy. Indeed, in the present case, immunohistochemical staining for human herpesvirus 6 (HHV-6) was positive only in the Reed-Sternberg-like cells (Fig. 2F), and the serum titer for HHV-6 IgG antibody (fluorescent-antibody technique) taken on day 49 (Fig. 1) showed marked elevation (2,560-fold) but was negative for HHV-6 IgM. HHV-6 genomes in the resected lymph node tissues was quantitated using a real-time PCR method (1) and showed 84.6 copies/μg DNA in the tissues, suggesting that aberrant propagation of HHV-6 occurred in the tissues.
Based on this pattern after immunohistochemical staining, together with a high serum titer of HHV-6 IgG antibody, as well as the presence of high fever, liver dysfunction, and a hematological disorder (leukocytosis, >11,000/μl; atypical lymphocytes, >5%; eosinophilia, >1,500/μl), the patient was diagnosed with drug-induced hypersensitivity syndrome (DIHS), satisfying all of the criteria for DIHS proposed by a Japanese severe cutaneous adverse reaction group, which includes HHV-6 reactivation (2, 3). She also had a high drug reaction with eosinophilia and systemic symptom (DRESS) score of 8 (score for definite case, >5) according to the scoring system for DRESS cases proposed by Kardaun et al. (4). DIHS and DRESS are now considered equivalent clinical conditions.
Saltzstein and Ackerman (5) stated that anticonvulsant-drug-associated lymphadenopathy closely mimics various malignant lymphomas, both clinically and pathologically, and described a pleomorphic reaction by various inflammatory cells (6), as in the present case. In a review of the literature, pathological evaluation of drug-induced lymphadenopathy has been rarely reported, but the following four patterns seem evident: (i) a Hodgkin's disease pattern (frank lymphoma, pseudo-pseudolymphoma) (5), (ii) a Hodgkin's disease-like pattern (pseudolymphoma) (5, 7, 8), (iii) an angioimmunoblastic-lymphadenopathy pattern (6, 9) (the so-called angioimmunoblastic T cell lymphoma), and (iv) a benign lymphoid hyperplasia pattern with preservation of the lymph node architecture. Especially in patients with the Hodgkin's disease-like pattern, normal lymph node architecture may be preserved, and large, atypical Reed-Sternberg-like cells stain as CD30− CD15− or CD30+ CD15− (10). These findings might be misdiagnosed as malignant lymphoma by an unwary physician.
Lymphatic proliferation resembling Hodgkin's disease in the setting of long-term, low-dose methotrexate for rheumatic disease is well recognized, because a subset of these tumors regresses following withdrawal of methotrexate therapy (10), which is associated with Epstein-Barr virus reactivation. Similarly, Shiohara et al. (3) described the relationship between human herpesvirus 6 and drug-induced hypersensitivity syndrome, the so-called DIHS (11), by detection with an HHV-6 DNA PCR of peripheral blood.
HHV-6 infects nearly all humans by the age of 2 years, and it has been implicated in several diseases, such as Kikuchi's lymphadenitis and IM-like syndrome in patients who are negative for Epstein-Barr virus and cytomegalovirus. A few previous reports showed HHV-6 reactivation by detection of viral DNA in DIHS patients' embedded, skin-biopsied specimens using the PCR method and/or in situ hybridization (12) or determination of HHV-6 DNA loads in peripheral leukocytes (2). However, no report has identified HHV-6 in resected lymph node specimens obtained from patients with DIHS (13)/DRESS. In this regard, this is the first report showing HHV-6 expression within atypical Reed-Sternberg-like cells and/or the detection of HHV-6 propagation in resected lymph node specimens by a quantitative PCR method, providing direct evidence that reactivation of HHV-6 plays a part in the pathogenesis of lymphadenopathy. Further immunohistochemical examinations of resected specimens for varicella-zoster virus, Epstein-Barr virus, cytomegalovirus, and human herpesvirus 7, considered to be associated with DIHS, were negative in our patient.
The present case provides direct evidence of HHV-6 reactivation in resected lymph node tissue in a patient with DIHS. Appropriate pathological evaluation of lymphadenopathy for DIHS/DRESS, which clinically mimics malignant lymphoma, should be seriously considered before any therapy directed at lymphoma is instituted.
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1. Collot S, Petit B, Bordessoule D, Alain S, Touati M, Denis F, Ranger-Rogez S. 2002. Real-time PCR for quantification of human herpesvirus 6 DNA from lymph nodes and saliva. J. Clin. Microbiol. 40:2445–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ushigome Y, Kano Y, Hirahara K, Shiohara T. 2012. Human herpesvirus 6 reactivation in drug-induced hypersensitivity syndrome and DRESS validation score. Am. J. Med. 125:e9–10 doi:10.1016/j.amjmed.2011.10.027 [DOI] [PubMed] [Google Scholar]
- 3. Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. 2007. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br. J. Dermatol. 156:1083–1084 [DOI] [PubMed] [Google Scholar]
- 4. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, Roujeau JC. 2007. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br. J. Dermatol. 156:609–611 [DOI] [PubMed] [Google Scholar]
- 5. Saltzstein SL, Ackerman LV. 1959. Lymphadenopathy induced by anticonvulsant drugs and mimicking clinically pathologically malignant lymphomas. Cancer 12:164–182 [DOI] [PubMed] [Google Scholar]
- 6. Yates P, Stockdill G, McIntyre M. 1986. Hypersensitivity to carbamazepine presenting as pseudolymphoma. J. Clin. Pathol. 39:1224–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vittorio CC, Muglia JJ. 1995. Anticonvulsant hypersensitivity syndrome. Arch. Intern. Med. 155:2285–2290 [PubMed] [Google Scholar]
- 8. Kumari R, Timshina DK, Thappa DM. 2011. Drug hypersensitivity syndrome. Indian J. Dermatol. Venereol. Leprol. 77:7–15 [DOI] [PubMed] [Google Scholar]
- 9. Rytaned DA, Castleman B, Dorfman RF. 1975. Case 31-1975. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. N. Engl. J. Med. 293:292–297 [DOI] [PubMed] [Google Scholar]
- 10. Kamel OW, Weiss LM, van de Rijn M, Colby TV, Kingma DW, Jaffe ES. 1996. Hodgkin's disease and lymphoproliferations resembling Hodgkin's disease in patients receiving long-term low-dose methotrexate therapy. Am. J. Surg. Pathol. 20:1279–1287 [DOI] [PubMed] [Google Scholar]
- 11. Shiohara T, Inaoka M, Kano Y. 2006. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol. Int. 55:1–8 [DOI] [PubMed] [Google Scholar]
- 12. Suzuki Y, Inagi R, Aono T, Yamanishi K, Shiohara T. 1998. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch. Dermatol. 134:1108–1112 [DOI] [PubMed] [Google Scholar]
- 13. Shiohara T, Kano Y, Takahashi R, Ishida T, Mizukawa Y. 2012. Drug-induced hypersensitivity syndrome: recent advances in the diagnosis, pathogenesis and management. Chem. Immunol. Allergy 97:122–138 [DOI] [PubMed] [Google Scholar]