Abstract
In clinical laboratories, diagnosis of imported malaria is commonly performed by microscopy. However, the volume of specimens is generally low and maintaining proficiency in reading blood smears, particularly at the species level, is challenging in this setting. To address this problem, the Provincial Laboratory for Public Health (ProvLab) in Alberta, Canada, implemented real-time PCR for routine confirmation of all smear-positive samples in the province. Here we report our experience over a 4-year period (2008 to 2012) with this new diagnostic algorithm. While detection of Plasmodium falciparum by microscopy alone was accurate, real-time PCR served as an important adjunct to microscopy for the identification of non-falciparum species. In 18% of cases, the result was reported as non-falciparum or the species could not be identified by microscopy alone, and in all cases, the species was resolved by real-time PCR. In another 4% of cases, the species was misidentified by microscopy. To enhance surveillance for malaria, we integrated our demographic, clinical, and laboratory data into a new system developed by the Canadian Network for Public Health Intelligence, called the Malaria System for Online Surveillance (SOS). Using this application, we characterized our patient populations and travel history to identify risk factors associated with malaria infection abroad.
INTRODUCTION
Malaria is the leading cause of febrile illness reported in travelers (1, 2) and requires prompt diagnosis and treatment. The majority of imported cases are caused by the species Plasmodium falciparum (3–8), which can cause a lethal infection in travelers with no immunity. About 50 million travelers are at risk of acquiring and importing malaria from the tropics and subtropics every year (9). Semi-immune immigrants and refugees are also at risk and can develop clinical malaria even years after leaving an area where malaria is endemic.
In most countries where malaria is nonendemic, malaria is a notifiable disease and cases are tracked by national surveillance programs or sentinel networks. Each year, 10,000 cases are reported globally, yet this likely represents a gross underestimate due to widespread underreporting (10, 11). In 2010, France reported the highest burden of imported malaria in Europe, with 2,438 cases (12), while the United States reported 1,688 cases (6). The number of cases in Canada is approximately 400 per year, but only 30 to 50% of cases are reported to public health agencies (5, 13).
In addition to case tracking, surveillance programs facilitate the identification of high-risk groups that can be targeted with specific prevention strategies. Epidemiological data from national and international surveillance programs consistently identify travelers visiting friends and relatives in their country of origin (VFRs) at greatest risk of acquiring travel-related malaria (6, 7, 10). Children are another important risk group, accounting for 15 to 20% of all imported cases (14, 15). Based on these data, public awareness and education campaigns that promote pretravel consultation and adherence to malaria prophylaxis can be tailored to these populations.
The impact of surveillance programs is intrinsically dependent on the accuracy of the malaria diagnosis. The most common diagnostic methods are microscopic analysis of blood smears and antigen detection with rapid diagnostic tests (RDT). While the sensitivity of these methods is generally sufficient to diagnose acute malaria cases, they have important limitations in settings where malaria is nonendemic. Microscopy requires skilled technologists, proficient in the identification of Plasmodium to the species level, yet the availability of expert microscopy in local or regional laboratories can be a challenge. RDTs are useful to rule out P. falciparum infections but lack sensitivity and specificity for the identification of the non-falciparum species (16).
Over the last decade, nucleic acid testing by PCR has emerged as a highly sensitive and specific diagnostic method. A number of PCR assays have been validated for the diagnosis of malaria in returning travelers, all demonstrating superior performance characteristics compared with microscopy (17). However, widespread implementation has been limited by the cost per test, the need for skilled labor, and the specific work flow requirements. In France, daily routine testing by conventional PCR was evaluated in comparison with microscopic methods over a period of 1 year (18). PCR testing was more efficient and provided a more accurate diagnosis than microscopy, particularly in cases with mixed infections.
In 2008, the Provincial Laboratory for Public Health (ProvLab) in Alberta was the first clinical microbiology lab in Canada to offer routine confirmation of malaria diagnosis and species identification by real-time PCR. Here we present an analysis of the impact of routine PCR confirmation on the accuracy of malaria diagnosis within a clinical laboratory setting. To support the integration of laboratory results with the national surveillance for malaria, data were analyzed using a new online application developed within the Canadian Network for Public Health Intelligence (CNPHI) platform called the Malaria System for Online Surveillance (Malaria SOS).
MATERIALS AND METHODS
Malaria diagnosis.
Frontline diagnosis was performed by microscopic examination of Giemsa-stained thick and thin smears at local and regional health facilities. In certain laboratories, samples were tested by RDT using the BinaxNOW kit (Alere, Canada). Patients were treated according to national guidelines. If the result was positive or there remained a strong clinical suspicion or travel history consistent with malaria, whole blood was forwarded to the Alberta ProvLab for confirmation by real-time PCR. Real-time PCR was performed as described previously (19) with Plasmodium consensus and species-specific primers and probes. Amplification of a region of the human β2-microglobulin gene served as an extraction and inhibition control. Samples were batch tested with a 1-week turnaround time. Submitters were notified immediately of any PCR results that were discordant with microscopy. The Alberta Ministry of Health was notified of cases, and data were entered in Malaria SOS for surveillance.
Malaria SOS.
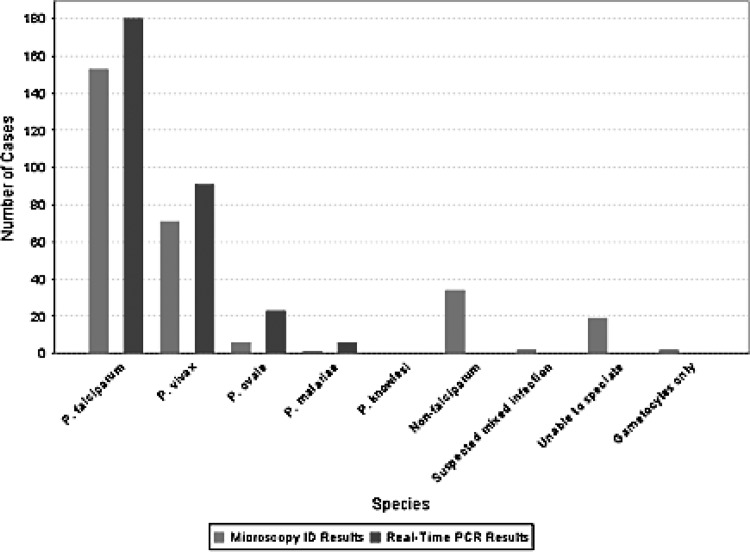
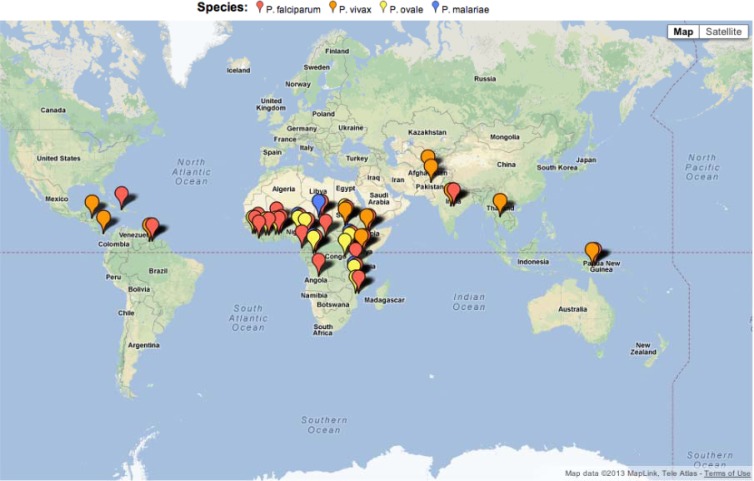
Malaria SOS is a secure web-based application that provides a seamless mechanism to submit case reports using a quick and interactive online form and includes integrated data integrity checks to alleviate data errors. The application provides a facility to interactively query the data and generate various trends, including comparisons of laboratory diagnostic methods. The chart presented in Fig. 1 and the map presented in Fig. 2 were extracted directly from the Malaria SOS application.
Fig 1.
Malaria diagnosis. Results from microscopy (light gray) were compared with those from real-time PCR (dark gray) for identification of Plasmodium species. In all cases where the species could not be defined by microscopy, identification was obtained by real-time PCR.
Fig 2.
Plasmodium species according to country of travel. Map of countries visited by patients with a positive infection by real-time PCR. Colored markers denote the species of infection as follows: red, P. falciparum; orange, P. vivax; yellow, P. ovale; and blue, P. malariae. There were no cases of P. knowlesi infection.
Statistics.
Descriptive epidemiology and clinical characteristics are described using means, medians, and proportions with 95% confidence intervals (CIs). Time abroad, days to presentation, and percent parasitemia were characterized using the range, interquartile range (IQR), median, and 95% confidence interval surrounding the median. All statistics were estimated using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Population.
One clinical specimen from each of 312 suspected cases of malaria was tested by real-time PCR between 25 April 2008 and 31 December 2012. Of these, 297 specimens were positive for malaria by PCR. Of the 297 PCR-positive specimens, 285 were smear positive, 11 were smear negative, and the smear results for 1 specimen were unknown (see Fig. S1 in the supplemental material). One patient was infected twice and is therefore counted as two cases.
General demographics of PCR-positive cases are presented in Table 1. The majority (63%) of cases were between the ages of 19 and 49 years, and 69% were male. Cases were classified either as travelers who were resident in Canada or immigrants who arrived in Canada from abroad. Based on this classification, 65% of cases were travelers. It was not possible to distinguish VFRs from Canadian-born travelers. Most cases acquired malaria in Africa (Sudan, Nigeria, Uganda, and Ghana being the most common destinations) or in South Asia (India and Pakistan) (Table 1). Travel and arrival dates were available for 85% of patients.
Table 1.
Demographic and clinical characteristics of malaria cases
| Characteristic | Result for characteristic | 95% CI |
|---|---|---|
| No. (%) malea | 204 (68.7) | 63.4–74.0 |
| Mean age, yr (range) | 32.3 (1–87) | 30.5–34.1 |
| No. (%) with Alberta as province of residence | 288 (97.3) | 95.5–99.1 |
| No. (%) of immigrants | 98 (35.1) | 29.5–40.7 |
| No. (%) of travelers | 181 (64.9) | 59.3–70.5 |
| Median no. of travel days abroad (range) | 46 (3–1,245) | 40–61 |
| No. (%) of infections acquired by country: | ||
| Ghana | 21 (7.1) | 4.2–10.1 |
| India | 41 (13.9) | 10.0–17.9 |
| Nigeria | 38 (12.9) | 9.1–16.7 |
| Pakistan | 21 (7.1) | 4.2–10.1 |
| Sudan | 43 (14.6) | 10.6–18.6 |
| Uganda | 22 (7.5) | 4.5–10.5 |
| Other | 109 (37.0) | 31.4–42.5 |
| Median no. of days to presentation by group (range): | ||
| Immigrants | 23 (1–2,259) | 19–34 |
| Travelers | 11.5 (0–1,006) | 10–13 |
| Total | 14 (0–2,259) | 12–16 |
| Median % parasitemia by species (range [IQR]): | 0.2 (0.0–9.2 [0.01–0.9]) | 0.1–0.3 |
| P. falciparum | 0.3 (0.0–9.2 [0.01–1.3]) | 0.2–0.5 |
| P. vivax | 0.2 (0.0–3.0 [0.01–0.5]) | 0.1–0.3 |
| P. ovale | 0.1 (0.0–1.4 [0.01–0.1]) | 0.01–0.1 |
| P. malariae | 0.06 (0.01–0.3[0.01–0.3]) | 0.01–0.3 |
Gender was not recorded for 1 patient sample.
Laboratory diagnosis.
By microscopy, the species of Plasmodium was identified in 231 cases; the remainder were either identified as non-falciparum (35 cases), “unable to speciate,” or “suspected mixed infection” (18 cases) (Fig. 1). In one sample, only gametocytes were observed by microscopy and the species was not identified. The percentages of parasitemia from thin film examination ranged from <0.01% to 9.2%, with 30.7% of samples below 0.1% parasitemia. The percentages of parasitemia ranged from 0.0% to 9.2% for P. falciparum infections, compared to the next largest range, 0.0% to 3.0%, for P. vivax (Table 1).
By real-time PCR, 297 samples were positive for Plasmodium (Fig. 1), including 3 relapse infections. Assuming PCR as a “gold standard,” there were 11 false negatives and 3 false positives by microscopy. The majority of infections were caused by P. falciparum (177 single infections), followed by P. vivax (89 infections), Plasmodium ovale (20 infections), and Plasmodium malariae (7 infections). In addition, there were 4 mixed infections: 3 P. falciparum-P. ovale and 1 P. falciparum-P. vivax. Overall, the threshold cycle (CT) values ranged from 16 to 40, but a broader distribution was observed for P. falciparum compared with the other species.
There were 56 samples in which the species could not be identified by microscopy: 53 were positive, while 3 were negative by real-time PCR. The species was resolved by PCR in all 53 samples, including 13 infections caused by P. falciparum (Table 2). Using PCR as the gold standard, the species was misidentified by microscopy in 13 cases: 4 with P. falciparum, 2 with P. vivax, 5 with P. ovale, and 2 with P. malariae. Another 11 samples were tested by PCR that were negative by microscopy; 7 had a positive RDT result, while 4 had clinical suspicion of malaria. All of these were confirmed positive for P. falciparum by real-time PCR.
Table 2.
PCR-positive samples with discordant results by microscopy
| Microscopy result | No. of PCR results |
|||||
|---|---|---|---|---|---|---|
| P. falciparum | P. malariae | P. ovale | P. vivax | Mixed infection | Total | |
| P. falciparum | 0 | 2 | 0 | 1 | 3 | |
| P. malariae | 0 | 0 | 0 | 0 | 0 | |
| P. ovale | 3 | 0 | 2 | 0 | 5 | |
| P. vivax | 1 | 2 | 3 | 0 | 6 | |
| Mixed infection | 0 | 0 | 0 | 0 | 0 | |
| Non-falciparum | 1 | 4 | 10 | 19 | 1 | 35 |
| Species not identified | 11 | 1 | 3 | 3 | 0 | 18 |
| Negative | 10 | 0 | 0 | 0 | 1 | 11 |
| Total | 26 | 7 | 18 | 24 | 3 | 78a |
The percentage of discordant results was 26.4% (n = 78; 95% CI, 21.3% to 31.4%).
Species distribution and travel history.
The species distribution according to countries visited by patients is consistent with prevalence data for each of the four major species of Plasmodium (Fig. 2). The times to presentation differed according to the species of Plasmodium (Table 3). The median time to presentation was significantly shorter in those cases infected with P. falciparum. While the 95% confidence intervals overlapped upon stratification by immigration and travel status, the median time to presentation continued to be significantly longer in cases infected with P. vivax than that in cases infected with P. falciparum.
Table 3.
Median number of days from arrival in Canada to sample collection
| Species | Median no. of days (95% CI) |
||
|---|---|---|---|
| Travelers | Immigrants | Total | |
| P. falciparum | 9 (6–10) | 10.5 (8–21) | 9 (7–11) |
| P. vivax | 93.5 (23–208) | 95 (54–248) | 95 (50–186) |
| P. ovale | 38 (7–171) | 59.5 (8–491) | 38 (17–171) |
| P. malariae | 74 (13–135) | 37 (20–119) | 37 (13–135) |
The median time spent abroad was 46 days (Table 1). Following arrival in Canada as returning travelers or immigrants, patients presented to a health facility with symptoms within a median of 14 days. In addition, travelers presented sooner than immigrants. It should be noted that for the immigrant population, travel history during the interim between arrival in Canada and date of presentation with symptoms was not always known. This could explain the long periods to presentation in certain patients.
DISCUSSION
A new algorithm for malaria diagnosis was implemented at the Alberta ProvLab in 2008, which includes frontline diagnosis by microscopy supported by weekly routine confirmation by real-time PCR. Given the longer turnaround time for PCR, this algorithm relies on rapid diagnosis of P. falciparum by microscopy. This infection is potentially lethal, particularly in travelers with no immunity, and requires immediate treatment. Over the 4-year period, 150/166 cases (90.4%) of P. falciparum were correctly identified by frontline microscopy performed in a variety of health facilities across the province of Alberta, demonstrating proficiency for detection of this species. The greater challenge in malaria diagnosis by microscopy is the discrimination between the non-falciparum species. Although these infections are generally less severe, patients often require different treatment regimens to eliminate the chronic liver stages and prevent relapse. Here, real-time PCR plays an important, complementary role in species identification. Most infections with these species had very low parasitemias, yet all were identified by real-time PCR. These observations are consistent with our previous studies demonstrating higher sensitivity of the real-time PCR assay compared with microscopy in our traveler and refugee populations (19, 20).
Based on our 4-year experience with real-time PCR, this algorithm provides enhanced diagnostics and species identification for malaria. Incorporation of RDTs could also be considered an adjunct to frontline microscopy, but given the proficiency for P. falciparum detection and the reported insensitivity of RDTs for discrimination of the non-falciparum species, RDTs may not significantly impact frontline diagnosis. However, given the high cost of real-time PCR, RDTs may be a more cost-effective alternative for confirmation of P. falciparum infections if the sensitivity is comparable.
The availability of a new online data management application, Malaria SOS, from the Canadian Network for Public Health Intelligence facilitates the integration of laboratory, demographic, and clinical data from all malaria cases in the province. Comparison of results from different diagnostic methods provides quality indicators for each test and can be used to identify specific sites where proficiency training is needed in the province. It can also be used to monitor the performance of the real-time PCR test over time and identify sources of variation within the laboratory or across laboratories using standardized test methods.
Furthermore, the ability to query and graph results from the nonidentifying case data is a powerful tool for surveillance of imported malaria in Canada. Using these functionalities built into the Malaria SOS application, we can identify high-risk groups and monitor the distribution of different species acquired internationally. Consistent with reports from other surveillance networks (6, 10), our higher-risk group includes males ranging in age from 19 to 49 years, although children also represent an important group, accounting for 19.9% of all cases. Most infections were from P. falciparum and were acquired in 4 countries in Africa or in South Asia.
The inclusion of travel dates and time to presentation with symptoms provides an opportunity to characterize the latency of infection, and we report cases in which the infection occurred several years after arrival in Canada. It is therefore important that health professionals consider malaria in the differential diagnosis despite the lengthy periods since malaria exposure. Also of interest is that travelers presented to health facilities earlier than immigrants. This likely reflects more rapid onset of symptoms in patients lacking immunity (21) but may also suggest differences in health-seeking behaviors among these two populations. Although we cannot distinguish VFRs from Canadian-born travelers, the length of time spent abroad is consistent with the travel patterns observed with VFRs. Given these lengthy stays in areas where malaria is endemic, issues related to the cost of antimalarials and adherence to prophylaxis may be important considerations for pretravel prevention strategies.
As many countries where malaria is endemic implement control measures and progress toward elimination phases, the success of these programs will be monitored by quality diagnostic and surveillance systems. Nonimmune travelers can serve as sentinels for the reemergence of malaria and sporadic outbreaks in these countries. Alerting cases through coordinated national and international surveillance networks such as Malaria SOS is one way for countries where malaria is nonendemic to contribute to global malaria control efforts.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Alberta Health Services and Alberta Health.
We thank the CNPHI team for development of the Malaria SOS application and Kimberley Simmonds for assistance with data extraction and comments on the manuscript.
Footnotes
Published ahead of print 3 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00195-13.
REFERENCES
- 1. Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS. 2006. Spectrum of disease and relation to place of exposure among ill returned travelers. N. Engl. J. Med. 354:119–130 [DOI] [PubMed] [Google Scholar]
- 2. Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Schwartz E. 2007. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin. Infect. Dis. 44:1560–1568 [DOI] [PubMed] [Google Scholar]
- 3. Arnaez J, Roa MA, Albert L, Cogollos R, Rubio JM, Villares R, Alarabe A, Cervera A, Lopez-Velez R. 2010. Imported malaria in children: a comparative study between recent immigrants and immigrant travelers (VFRs). J. Travel Med. 17:221–227 [DOI] [PubMed] [Google Scholar]
- 4. Gray TJ, Trauer JM, Fairley M, Krause VL, Markey PG. 2012. Imported malaria in the Northern Territory, Australia—428 consecutive cases. Commun. Dis. Intell. 36:107–113 [DOI] [PubMed] [Google Scholar]
- 5. MacLean JD, Demers AM, Ndao M, Kokoskin E, Ward BJ, Gyorkos TW. 2004. Malaria epidemics and surveillance systems in Canada. Emerg. Infect. Dis. 10:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mali S, Kachur SP, Arguin PM. 2012. Malaria surveillance—United States, 2010. MMWR Surveill. Summ. 61:1–17 [PubMed] [Google Scholar]
- 7. Mizuno Y, Kato Y, Kano S, Takasaki T. 2012. Imported malaria and dengue fever in returned travelers in Japan from 2005 to 2010. Travel Med. Infect. Dis. 10:86–91 [DOI] [PubMed] [Google Scholar]
- 8. Unger HW, McCallum AD, Ukachukwu V, McGoldrick C, Perrow K, Latin G, Norrie G, Morris S, Smith CC, Jones ME. 2011. Imported malaria in Scotland—an overview of surveillance, reporting and trends. Travel Med. Infect. Dis. 9:289–297 [DOI] [PubMed] [Google Scholar]
- 9. Castelli F. 2004. Human mobility and disease: a global challenge. J. Travel Med. 11:1–2 [DOI] [PubMed] [Google Scholar]
- 10. Franco-Paredes C, Santos-Preciado JI. 2006. Problem pathogens: prevention of malaria in travellers. Lancet Infect. Dis. 6:139–149 [DOI] [PubMed] [Google Scholar]
- 11. Hwang J, McClintock S, Kachur SP, Slutsker L, Arguin P. 2009. Comparison of national malaria surveillance system with the national notifiable diseases surveillance system in the United States. J. Public Health Manag. Pract. 15:345–351 [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization 2012. WHO: Centralized Information System for Infectious Diseases (CISID). Malaria. World Health Organization Regional Office for Europe, Copenhagen, Denmark: http://data.euro.who.int/cisid/?TabID=30994 Accessed 12 April 2013 [Google Scholar]
- 13. Public Health Agency of Canada November 2004, posting date Frequently asked questions: malaria. Public Health Agency of Canada, Ottawa, Ontario, Canada: http://www.phac-aspc.gc.ca/media/advisories_avis/mal_faq-eng.php [Google Scholar]
- 14. Herbinger KH, Drerup L, Alberer M, Nothdurft HD, Sonnenburg F, Loscher T. 2012. Spectrum of imported infectious diseases among children and adolescents returning from the tropics and subtropics. J. Travel Med. 19:150–157 [DOI] [PubMed] [Google Scholar]
- 15. Ladhani S, Aibara RJ, Riordan FA, Shingadia D. 2007. Imported malaria in children: a review of clinical studies. Lancet Infect. Dis. 7:349–357 [DOI] [PubMed] [Google Scholar]
- 16. Marx A, Pewsner D, Egger M, Nuesch R, Bucher HC, Genton B, Hatz C, Juni P. 2005. Meta-analysis: accuracy of rapid tests for malaria in travelers returning from endemic areas. Ann. Intern. Med. 142:836–846 [DOI] [PubMed] [Google Scholar]
- 17. Erdman LK, Kain KC. 2008. Molecular diagnostic and surveillance tools for global malaria control. Travel Med. Infect. Dis. 6:82–99 [DOI] [PubMed] [Google Scholar]
- 18. Morassin B, Fabre R, Berry A, Magnaval JF. 2002. One year's experience with the polymerase chain reaction as a routine method for the diagnosis of imported malaria. Am. J. Trop. Med. Hyg. 66:503–508 [DOI] [PubMed] [Google Scholar]
- 19. Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. 2009. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J. Clin. Microbiol. 47:975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matisz CE, Naidu P, Shokoples SE, Grice D, Krinke V, Brown SZ, Kowalewska-Grochowska K, Houston S, Yanow SK. 2011. Post-arrival screening for malaria in asymptomatic refugees using real-time PCR. Am. J. Trop. Med. Hyg. 84:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Askling HH, Bruneel F, Burchard G, Castelli F, Chiodini PL, Grobusch MP, Lopez-Velez R, Paul M, Petersen E, Popescu C, Ramharter M, Schlagenhauf P. 2012. Management of imported malaria in Europe. Malar. J. 11:328 doi:10.1186/1475-2875-11-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.