Abstract
Pseudoclavibacter spp. are Gram-positive, aerobic, catalase-positive, coryneform bacteria belonging to the family of Microbacteriaceae. Identification of these species with conventional biochemical assays is difficult. This case report of a Pseudoclavibacter bifida bacteremia occurring in an immunocompromised host diagnosed with an acute exacerbation of chronic obstructive pulmonary disease, with a lethal outcome, confirms that this organism may be a human pathogen.
CASE REPORT
An 86-year-old male patient suffering from dyspnea, with severe respiratory distress and fever, was admitted to our hospital. In 2006, the patient was diagnosed with class I chronic obstructive pulmonary disease (COPD), for which he was receiving inhaled glucocorticoids and long-acting bronchodilators. COPD exacerbation with a left lobular pneumonia led to hospitalization in July 2011. Treatment with amoxicillin-clavulanic acid was initiated and switched to piperacillin-tazobactam due to respiratory insufficiency. Bronchial aspirates and blood cultures remained negative. Normalization of the lung function parameters and improvement in his general condition led to discharge from the hospital. Other relevant medical history comprised arrhythmia, renal failure, and diabetes mellitus type II.
In September 2011, he presented with dyspnea and fever (body temperature, 38.4°C). No other significant symptoms could be elicited. The patient was hemodynamically stable. Hematological investigations revealed a white blood cell count of 33.4 × 103 cells/μl, with 96% neutrophils (reference range, 46 to 64%), a hemoglobin level of 10.3 g/dl (reference range, 12.6 to 17.4 g/dl), a hematocrit of 31.0% (reference range, 39.0 to 50.0%), and a platelet count of 245 × 103/μl (reference range, 150 × 103 to 450 × 103/μl). The C-reactive protein level increased up to 29.5 mg/dl (normal, <1.0 mg/dl) 3 days after admission (initial value at admission, 20.1 mg/dl), and the serum creatinine level was 3.12 mg/dl (reference range, 0.70 to 1.30 mg/dl). The levels of D-dimers (1,906 ng/ml [reference range, <500 ng/nl]) and digoxin (3.35 μg/liter [reference range, 0.80 to 2.00 μg/liter]) were increased. An arterial blood gas examination revealed decreased pO2 and pCO2 levels of 60 mm Hg (reference range, 75 to 100 mm Hg) and 29.1 mm Hg (reference range, 30 to 48 mm Hg), respectively. A bedside chest X ray showed infiltrates in the left and right lobes, suggestive of bilateral pneumonia (Fig. 1).
Fig 1.
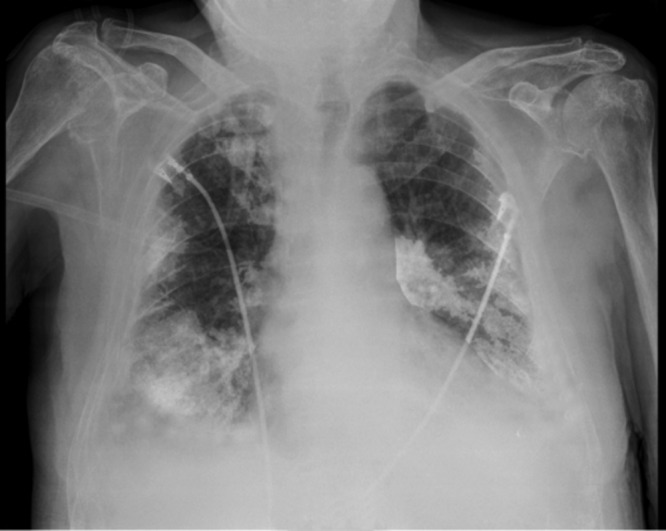
X-ray radiograph of the patient on the day of admission showing alveolar infiltrates in the right upper lobe and lower lobe and in the left middle lobe.
Before intravenous antibiotic treatment with ceftriaxone (2 g every 24 h) was initiated, two aerobic and two anaerobic blood culture bottles (Bactec; Becton, Dickinson, Sparks, MD) were collected at two different fever spikes. One pair was drawn through a catheter; another pair was drawn by peripheral venipuncture. After a mean incubation time of 52.4 h at 35°C, branched, rod-shaped, whitish-grayish, nonfermentative Gram-positive bacteria were observed in both aerobic blood culture bottles. Further bacteriological investigation showed nonmotile, alkaline phosphatase-positive, catalase-positive, and oxidase-negative rods. The isolate grew on blood and chocolate agar after 2 days at 37°C in air supplemented with 5% CO2. The strain was tested using the commercially available API Coryne test (version 3.0; bioMérieux, Marcy l'Etoile, France), which produced a presumptive identification of Corynebacterium spp., with a poor probability (55.7%) of correct identification. For further identification, matrix-assisted laser desorption–ionization time of flight mass spectrometry (MALDI-TOF MS; Microflex, Brüker Daltonik, Bremen, Germany) by the direct-transfer method in combination with the MALDI Biotyper database was used. However, the isolate revealed no clear match with any of the species, with the best identification score being 1,318 for Arthrobacter castelli. Due to this poor and unreliable result, phenotypical identification by Phoenix (software version 6.12A/V5/15A; Becton, Dickinson) was performed. Insufficient growth in the control well still resulted in an undetermined identification.
In vitro susceptibility testing, performed using Etest (bioMérieux) on Mueller-Hinton II agar with 5% sheep blood (Becton, Dickinson) incubated at 37°C in air supplemented with 5% CO2 for 24 h, revealed the following MICs: penicillin G, 0.5 mg/liter; cefotaxime, 0.25 mg/liter; ceftriaxone, 0.5 mg/liter; clindamycin 1.5 mg/liter; ciprofloxacin, 0.19 mg/liter; amikacin, 0.25 mg/liter; and vancomycin, 1.0 mg/liter. Despite an initial episode of recovery, the situation deteriorated a few days later. A few hours prior to death, the patient passed through a fever spike again.
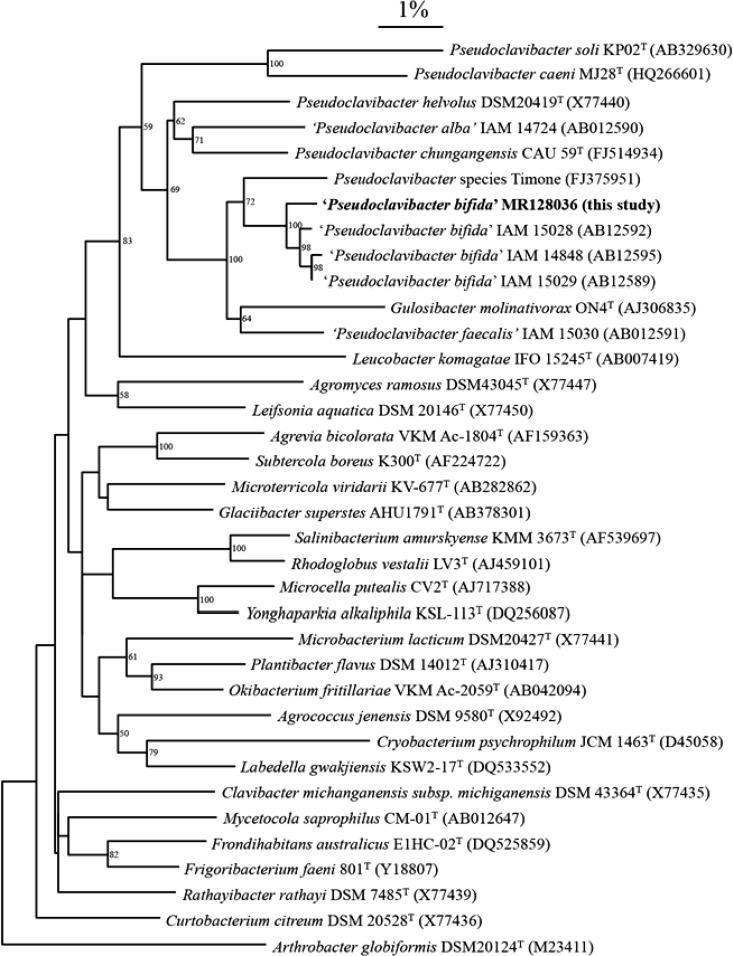
Because of the inconsistent phenotypical identification and the clinical importance of the sample, further identification was performed at the molecular level. DNA was extracted from fresh colonies grown on blood agar (homemade Columbia agar supplemented with 5% horse blood) using the QIAamp DNA minikit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. The bacterial 16S rRNA gene was amplified using the following primers: 5′-AGA GTT TGA TCC TGG CTC AG-3′ (forward; Escherichia coli positions 8 to 27) and 5′-TAC CTT GTT ACG ACT TCG TCC CA-3′ (reverse; E. coli positions 1504 to 1485) (1, 2). Amplification products of approximately 1,350 nucleotides (nt) were checked by agarose gel electrophoresis (2%) and visualized through ethidium bromide staining. Sequencing of the amplicon was carried out using the ABI BigDye Terminator sequencing kit (Life Technologies, Paisley, United Kingdom) with the following forward primers: 5′-AGT TTG ATC CTG GCT CAG-3′ (Escherichia coli 16S rRNA gene sequence positions 8 to 27), 5′-CTC CTA CGG GAG GCA GCA GT-3′ (positions 339 to 358), 5′-CAG CAG CCG CGG TAA TAC-3′ (positions 519 to 536), 5′-AAC TCA AA GAA TTG ACG G-3′ (positions 908 to 926), and 5′-AGT CCC GCA ACG AGC GCA AC-3′ (positions 1093 to 1112). The reverse primer was 5′-TAC CTT GTT ACG ACT TCG TCC CA-3′ (positions 1504 to 1485). Electrophoresis was performed on the ABI 3130XL 16-capillary electrophoresis apparatus (Life Technologies). Analysis of the sequences and gene assembly of the different fragments was done by the Chromas Pro Software (Technelysium, Tewantin, QLD, Australia). The obtained sequence was compared to all known sequences in GenBank by using the Basic Local Alignment Search Tool (BLAST) at the website of the National Center for Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov). A BLAST search for the query sequence of 1,341 nt resulted in the final identification of Zimmermannella bifida, showing 99.5% 16S rRNA gene sequence similarity to that previously reported by Lin et al. (3). Two other independent laboratories, using different primers, confirmed the identification of Zimmermannella bifida by 16S rRNA sequencing. Phylogenetic tree construction of all Pseudoclavibacter species and related organisms (Fig. 2) was done as described before (4). Cluster analysis was performed using GeneBase software (Applied Maths, Sint-Martens-Latem, Belgium) and was based on the neighbor-joining method.
Fig 2.
Neighbor-joining tree showing the relationship between the sequence of the clinical isolate and those of all Pseudoclavibacter bifida strains in GenBank, a related Pseudoclavibacter isolate, all Pseudoclavibacter species, and all type strains of all related genera in the family Microbacteriaceae. Bootstrap values of >50% are shown at the nodes. The bar indicates 1% estimated sequence divergence.
This case report describes, to our knowledge, the first Pseudoclavibacter bifida septicemia in an immunocompromised COPD patient with bilateral pneumonia. These coryneform bacteria are widely distributed in the environment, especially in soil (3). To date, identification to the species level requires special analysis (i.e., 16S rRNA gene sequencing), because phenotypic characteristics are not sufficiently reliable for identification of this microorganism (Table 1).
Table 1.
Characteristics that differentiate P. bifida from other Pseudoclavibacter spp.a
| Trait | Score or result for: |
||||||
|---|---|---|---|---|---|---|---|
| P. bifida | Reference strain: |
||||||
| P. alba | P. caeni | P. chungangensis | Z. faecalis | P. helvolus | P. soli | ||
| Catalase | + | + | + | + | + | + | + |
| Oxidase | − | − | − | − | + | W | − |
| Alkaline phosphatase | + | + | − | + | − | − | + |
| Cystine arylamidase | + | − | W | − | + | − | +/− |
| β-Glucosidase | +/− | +/− | − | +/− | +/− | − | − |
| α-Glucosidase | − | − | − | + | − | − | − |
| Inositol | − | − | + | − | − | − | − |
| Acid production from: | |||||||
| Glycerol | − | W, − | + | + | W, − | +/− | W |
| d-Fructose | − | W, − | + | − | + | − | W |
| d-Mannose | − | − | − | − | + | − | − |
| Rhamnose | +/− | − | + | W | − | ||
| Inositol | − | − | + | − | + | W, + | − |
| Mannitol | − | − | − | + | + | + | − |
| d-Xylose | − | − | + | − | + | − | − |
| Maltose | − | − | − | W | + | − | − |
| Lactose | − | − | + | + | − | +/− | − |
| Growth | Strictly aerobic | Aerobic | Strictly aerobic | Strictly aerobic | Aerobic | Aerobic | Aerobic |
For the first time, Lin et al. described the genus Zimmermannella (family of Microbacteriaceae in the class Actinobacteria), with Z. helvola as the type species and three novel species, Z. alba, Z. bifida, and Z. faecalis, in 2004 (3). However, the genus Pseudoclavibacter was first described by Manaia et al., who reclassified Brevibacterium helvolum as the type strain of the species, renaming it Pseudoclavibacter helvolus. According to the previously published name by Manaia et al., Z. helvola should be named Pseudoclavibacter helvolus and the three species Z. alba, Z. bifida, and Z. faecalis should be renamed P. alba, P. bifida, and P. faecalis (7). In fact, Z. helvolus is an earlier homotypic synonym of P. helvola, and the genus name Zimmermannella is therefore considered to be illegitimate. Based on nomenclature rules, the name Pseudoclavibacter has priority. Until now, no publication of the official conversion of the three other species has appeared. The genus Pseudoclavibacter already consists of four official species (P. caeni, P. chungangensis, P. helvolus, and P. soli), all isolated out of soil material (5–8).
Since 2004, only one case report has described the potential clinical importance of a Pseudoclavibacter-like organism as a cause of cutaneous and subcutaneous infection in a human (9). This isolate (FJ375951) is included in the phylogenetic tree and appears to be closely related to our P. bifida strain (Fig. 2). This, together with clinical data published by Lemaitre et al., suggests that the group of P. bifida strains might be of clinical importance (9). The original isolates of P. bifida and P. alba were also human isolates (human wounds, urine, and blood). In our case, P. bifida was isolated from blood in an immunocompromised COPD patient who was admitted to the hospital due to acute respiratory distress syndrome with dyspnea. Eight days later, the patient died due to bilateral pneumonia.
Laboratory identification of the organism is challenging and impossible using routine laboratory protocols or using the API Coryne test. Fermentation of rhamnose and the presence of β-glucosidase are variably determined (Table 1) (3, 5, 6, 8). P. bifida forms typically whitish-to-yellowish colonies on blood agar and are catalase and alkaline phosphatase positive; oxidase is negative. Optimal growth occurs at 30°C (3).
The use of the Gram-positive identification panel on a Phoenix automated system (Becton, Dickinson) yielded no identification due to insufficient growth in the control well. However, the species is not included in the Epicenter Database. Based on the available test results, a presumptive identification of Corynebacterium spp. was made, although glucose fermentation was not observed. The final identification of P. bifida required analysis by molecular genetic methods relying on PCR, direct DNA sequencing, and GenBank research. An explanation for the rarity of diseases caused by Pseudoclavibacter species is the relatively low pathogenicity of this genus and, more generally, of coryneform bacteria. On the other hand, identification of these bacteria to the species level remains a challenge for most microbiology laboratories, which may explain the low rate of recovery of these strains.
Identifying coryneform bacteria may help physicians distinguishing between infection and colonization. Some coryneform bacteria are known as opportunistic pathogens in (immunocompromised) patients (e.g., Corynebacterium jeikum); others are thought to be “innocent bystanders.” By identifying coryneform bacteria to the species level, a physician can conclude that a specific species may be the cause of infection.
P. bifida infections should be treated according to the antibiogram. We performed susceptibility testing by Etest and found high MICs of clindamycin, confirming the results of Lemaitre at al (9). MICs of other antibiotics tested confirm the results of Mages et al., who have tested the largest cohort of clinically important Arthrobacter-like bacteria so far (10). For ceftriaxone, we found a MIC of 0.5 mg/liter, to which coryneform bacteria are still susceptible according to the CLSI M45-A2 document (11). Although P. bifida is not included in this list, on the basis of this CLSI document, we formulate advice for an antibiotic. Despite optimal antibiotic treatment, the patient's situation deteriorated a few days later. A few hours prior to death, the patient passed through a fever spike again.
In conclusion, the identification of P. bifida as a cause of bacteremia in an immunocompromised host with COPD was possible only with the application of modern techniques of molecular diagnosis, like 16S rRNA gene sequencing. Attempts to identify the microorganism by standard biochemical characterization tests routinely performed in diagnostic laboratories produced misleading results, with a classification of Corynebacterium spp. This case emphasizes Pseudoclavibacter species as a potential pathogen in immunocompromised patients.
Nucleotide sequence accession number.
The GenBank accession number for the partial 16S rRNA gene sequence of P. bifida MR128036 is KC757349.
ACKNOWLEDGMENTS
We are grateful to G. Coppens, H. De Beenhouwer, and J. Frans, who performed conformational and supplementary identification tests.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 27 March 2013
REFERENCES
- 1. Jonckheere S, De Baere T, Schroeyers P, Soetens O, De Bel A, Surmont I. 2012. Prosthetic valve endocarditis caused by Bordetella holmesii, an Acinetobacter lookalike. J. Med. Microbiol. 61:874–877 [DOI] [PubMed] [Google Scholar]
- 2. Baker GC, Smith JJ, Cowan DA. 2003. Review and reanalysis of domain-specific 16S primers. J. Microbiol. Methods 55:541–555 [DOI] [PubMed] [Google Scholar]
- 3. Lin YC, Uemori K, de Briel DA, Arunpairojana V, Yokota A. 2004. Zimmermannella helvola gen. nov., sp. nov., Zimmermannella alba sp. nov., Zimmermannella bifida sp. nov., Zimmermannella faecalis sp. nov. and Leucobacter albus sp. nov., novel members of the family Microbacteriaceae. Int. J. Syst. Evol. Microbiol. 54:1669–1676 [DOI] [PubMed] [Google Scholar]
- 4. Nemec A, De Baere T, Tjernberg I, Vaneechoutte M, van der Reijden T, Dijkshoorn L. 2001. Acinetobacter ursingii sp. nov. and Acinetobacter schindleri sp. nov., isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 51:1891–1899 [DOI] [PubMed] [Google Scholar]
- 5. Srinivasan S, Kim HS, Kim MK, Lee M. 2012. Pseudoclavibacter caeni sp. nov., isolate from sludge of a sewage disposal plant. Int. J. Syst. Evol. Microbiol. 62:786–790 [DOI] [PubMed] [Google Scholar]
- 6. Cho SL, Jung MY, Park MH, Chang YH, Yoon JH, Myung SC, Kim W. 2010. Pseudoclavibacter chungangensis sp. nov., isolated from activated sludge. Int. J. Syst. Evol. Microbiol. 60:1672–1677 [DOI] [PubMed] [Google Scholar]
- 7. Manaia CM, Nogales B, Weiss N, Nunes OC. 2004. Gulosibacter molinativorax gen. nov., sp. nov., a molinate-degrading bacterium, and classification of ‘Brevibacterium helvolum’ DSM 20419, as Pseudoclavibacter helvolus gen. nov. sp. nov. Int. J. Syst. Evol. Microbiol. 54:783–789 [DOI] [PubMed] [Google Scholar]
- 8. Kim MK, Jung HY. 2009. Pseudoclavibacter soli sp. nov., a β-glucosidase producing bacterium. Int. J. Syst. Evol. Microbiol. 59:835–838 [DOI] [PubMed] [Google Scholar]
- 9. Lemaitre F, Stein A, Raoult D, Drancourt M. 2011. Pseudoclavibacter-like subcutaneous infection: a case report. J. Med. Case Rep. 5:468–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mages IS, Frodl R, Bernard KA, Funke G. 2008. Identities of Arthrobacter spp. and Arthrobacter-like bacteria encountered in human clinical specimens. J. Clin. Microbiol. 46:2980–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. CLSI 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline, second edition Document M45-A2. CLSI, Wayne, PA [Google Scholar]