Abstract
Monoclonal antibodies were prepared against pyruvate kinase (PyKi; ATP: pyruvate phosphotransferase, EC 2.7.1.40) and used to quantitate PyKi content in L2 lung cells and WI-38 fibroblasts cultivated under hypoxic and normoxic conditions. After 96 h of hypoxic cultivation, PyKi activity was significantly increased in both cell types (L2: normoxia [Po2 = 142 torr], 0.11 +/- 0.01 [SD]; hypoxia [Po2 = 14 torr], 0.25 +/- 0.04 U/microgram DNA, P < 0.01). PyKi content increased proportionately in both cell lines (L2: normoxia, 0.44 +/- 0.13; hypoxia, 0.94 +/- 0.13 microgram enzyme protein/microgram DNA). Specific activity was not significantly different after 96 h (L2: normoxia, 261 +/- 11; hypoxia, 261 +/- 14 U/mg enzyme protein). These results indicate that regulation of glycolysis during chronic hypoxia occurs at the level of enzyme content. Chronic O2 depletion leads to either an increased rate of biosynthesis or a decreased rate of biodegradation of PyKi, causing augmented glycolytic capacity. Monoclonal antibodies provide a highly specific, convenient approach to charcterizing enzymes, as well as quantitating cellular enzyme content.
Full text
PDF


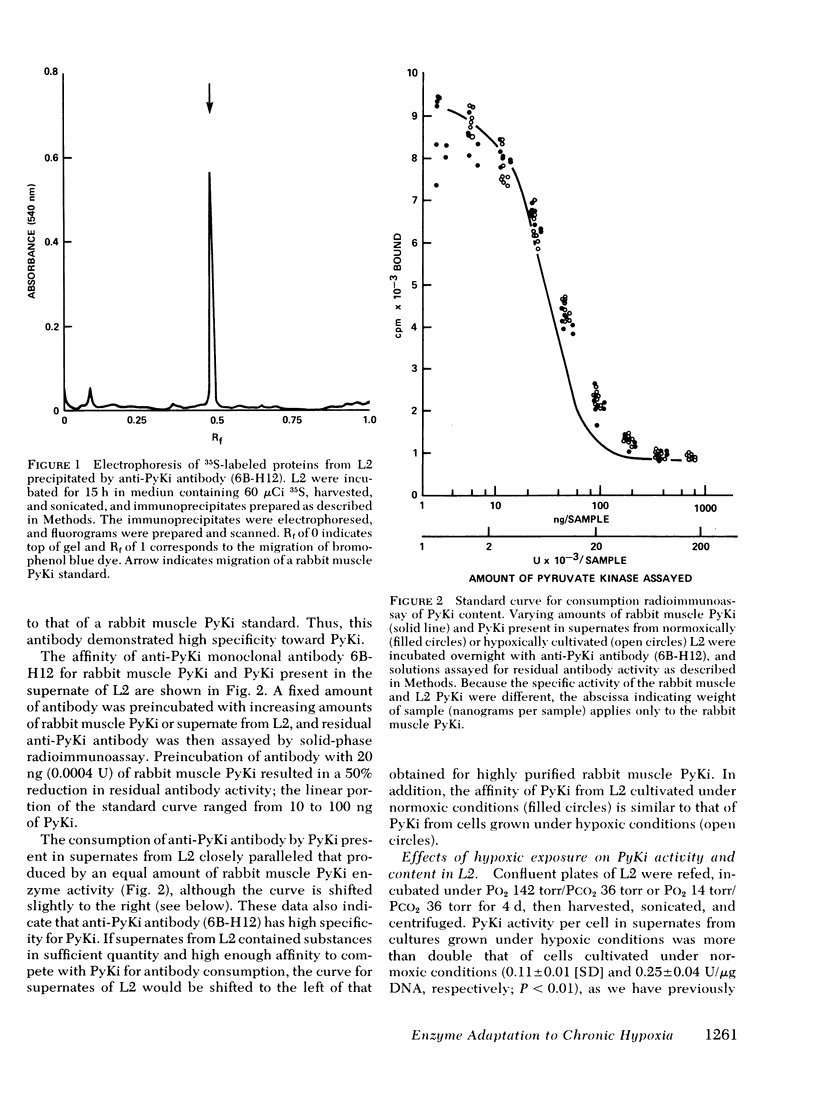
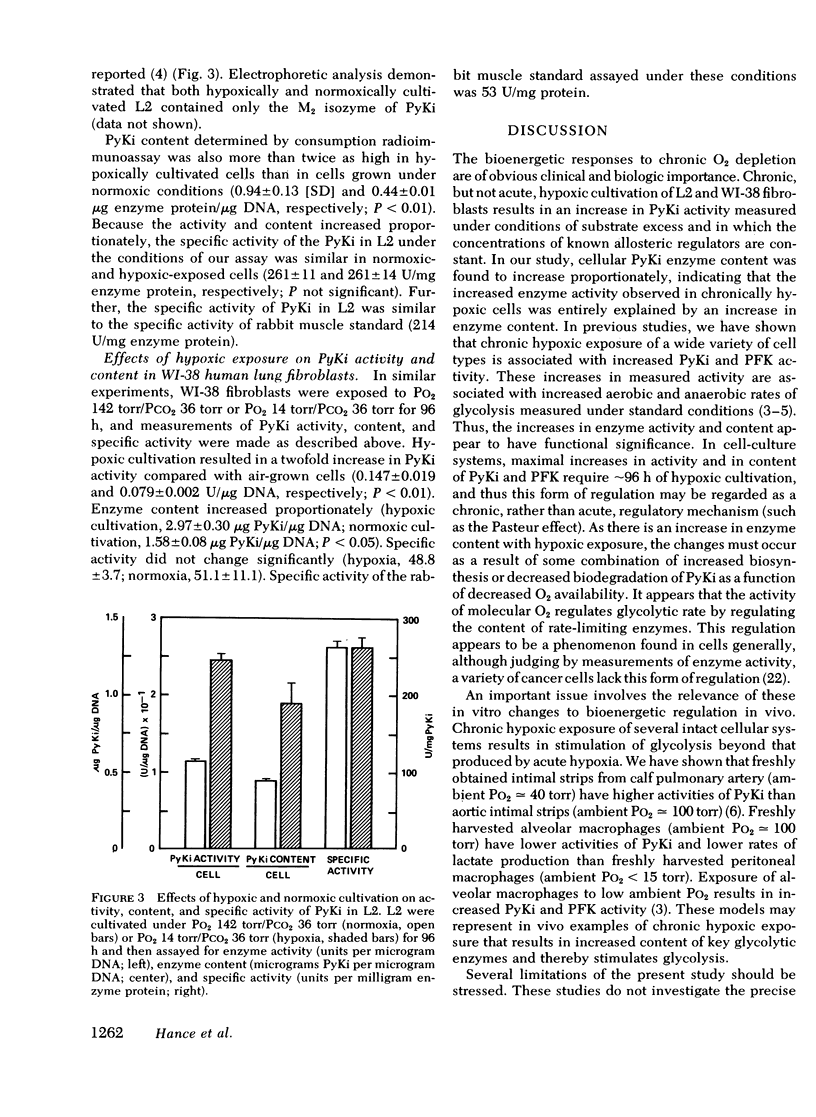


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cardenas J. M., Dyson R. D., Strandholm J. J. Bovine pyruvate kinases. I. Purification and characterization of the skeletal muscle isozyme. J Biol Chem. 1973 Oct 25;248(20):6931–6937. [PubMed] [Google Scholar]
- Cottam G. L., Hollenberg P. F., Coon M. J. Subunit structure of rabbit muscle pyruvate kinase. J Biol Chem. 1969 Mar 25;244(6):1481–1486. [PubMed] [Google Scholar]
- Douglas W. H., Kaighn M. E. Clonal isolation of differentiated rat lung cells. In Vitro. 1974 Sep-Oct;10(3-4):230–237. doi: 10.1007/BF02615237. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Harkins R. N., Black J. A., Rittenberg M. B. M2 isozyme of pyruvate kinase from human kidney as the product of a separate gene: its purification and characterization. Biochemistry. 1977 Aug 23;16(17):3831–3837. doi: 10.1021/bi00636a018. [DOI] [PubMed] [Google Scholar]
- Imamura K., Taniuchi K., Tanaka T. Multimolecular forms of pyruvate kinase. II. Purification of M 2 -type pyruvate kinase from Yoshida ascites hepatoma 130 cells and comparative studies on the enzymological and immunological properties of the three types of pyruvate kinases, L, M 1 , and M 2 . J Biochem. 1972 Oct;72(4):1001–1015. doi: 10.1093/oxfordjournals.jbchem.a129962. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Herzenberg L. A. Localization of murine Ig-1b and Ig-1a (IgG 2a) allotypic determinants detected with monoclonal antibodies. Mol Immunol. 1979 Dec;16(12):1005–1017. doi: 10.1016/0161-5890(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E. From Pasteur to Mitchell: a hundred years of bioenergetics. Fed Proc. 1980 Feb;39(2):210–215. [PubMed] [Google Scholar]
- Ramaiah A. Pasteur effect and phosphofructokinase. Curr Top Cell Regul. 1974;8(0):297–345. doi: 10.1016/b978-0-12-152808-9.50014-6. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Saheki S., Saheki K., Tanaka T. Peptidemapping by limited proteolysis of four pyruvate kinase isozymes. FEBS Lett. 1978 Sep 1;93(1):25–28. doi: 10.1016/0014-5793(78)80796-0. [DOI] [PubMed] [Google Scholar]
- Simon L. M., Robin E. D., Phillips J. R., Acevedo J., Axline S. G., Theodore J. Enzymatic basis for bioenergetic differences of alveolar versus peritoneal macrophages and enzyme regulation by molecular O2. J Clin Invest. 1977 Mar;59(3):443–448. doi: 10.1172/JCI108658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. M., Robin E. D., Raffin T., Theodore J., Douglas W. H. Bioenergetic pattern of isolated type II pneumocytes in air and during hypoxia. J Clin Invest. 1978 May;61(5):1232–1239. doi: 10.1172/JCI109039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susor W. A., Penhoet E., Rutter W. J. Fructose-diphosphate aldolase, pyruvate kinase, and pyridine nucleotide-linked activities after electrophoresis. Methods Enzymol. 1975;41:66–73. doi: 10.1016/s0076-6879(75)41017-5. [DOI] [PubMed] [Google Scholar]


