Abstract
We have developed a human herpesvirus 8 (HHV-8) 50% tissue culture infective dose (TCID50) assay using the T1H6-DC-SIGN cell line. Infection of T1H6-DC-SIGN cells with HHV-8 induces expression of β-galactosidase, which was used to determine TCID50 levels. Validation of TCID50 values was performed by immunofluorescence assay of HHV-8 infection of immature dendritic cells at various TCID50 doses.
TEXT
Human herpesvirus 8 (HHV-8), also termed Kaposi's sarcoma-associated herpesvirus, is the etiologic agent of all forms of Kaposi's sarcoma as well as pleural effusion lymphomas and several forms of multicentric Castleman's disease (1–3). HHV-8 has been detected in several biological samples, including semen, saliva, blood, and tissues (4–11). The detection of HHV-8 in these samples has relied almost exclusively on PCR-based methods to amplify virion DNA. These approaches do not, however, determine levels of infectious virus. Further, as HHV-8 does not cause a cytopathic effect in cell cultures, standard plaque assays cannot be used to determine the titer of infectious virus. While some studies have relied on immunofluorescence-based assays to detect proteins of infectious virus (12–14), this approach suffers from a lack of quantitation. As a result, there is no accurate method for determining infectious titers of wild-type HHV-8 in biological samples or cell culture, hampering the study of this clinically important virus. The purpose of the present study was to develop a rapid and sensitive method for determining levels of infectious, wild-type HHV-8.
This assay utilizes T1H6 cells, a 293T cell line containing the β-galactosidase gene under the control of the HHV-8 T1.1 promoter (15). The 293T cell line is a human embryonic kidney cell line that contains the E6 gene of adenovirus and SV40 T antigen (16). The HHV-8 T1.1 promoter contains a replication and transcription activator (RTA) response element (RRE). The RTA protein (encoded by the HHV-8 open reading frame 50 [ORF50] gene) is sufficient and necessary for reactivation of latent HHV-8 (17) and is actively transcribed during a primary infection. Binding of the RTA protein to the RRE in the T1.1 promoter results in increased transcription of the β-galactosidase gene. HHV-8 infection of the T1H6 cells results in the production of the RTA protein by the infecting virus, which in turn induces expression of β-galactosidase. It has been shown previously that efficient infection of the T1H6 cell line with HHV-8 requires treatment of the cells with Polybrene to allow for receptor-independent infection (15). As a result, parental T1H6 cells are not suitable for measuring natural HHV-8 infectivity.
We have previously reported that HHV-8 utilizes DC-SIGN as a cellular receptor on immature DC and activated macrophages and B cells (18, 19). To utilize this cellular receptor for titration of infectious HHV-8, T1H6 cells were stably transfected with pcDNA3-DC-SIGN, which expresses DC-SIGN under the control of the cytomegalovirus (CMV) immediate early promoter (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, from S. Pohlman, F. Baribaud, F. Kirchhoff, and R. W. Doms), creating the T1H6-DC-SIGN cell line. Flow analyses using anti-DC-SIGN antibody revealed that a majority of the T1H6-DC-SIGN cells (∼70%) and less than 1.5% of the parental T1H6 cells expressed DC-SIGN on the surface of the cells (data not shown).
To demonstrate that the T1.1/β-galactosidase reporter gene (contained in the T1H6-DC-SIGN cells) responds to expression of the RTA protein, a plasmid containing the ORF50 cDNA under the control of the strong CMV promoter was transfected using Lipofectamine 2000 (Life Technologies, Grand Island, NY) onto T1H6-DC-SIGN cells plated in triplicate, and β-galactosidase activity was measured 48 h posttransfection using the β-Gal chemiluminescence detection kit II (Clontech, Mountain View, CA) according to the manufacturer's instructions. β-Galactosidase activity was expressed in a dose response manner to increasing amounts of ORF50 plasmid DNA (data not shown).
To compare induction of β-galactosidase activity by HHV-8 infection on T1H6-DC-SIGN cells, T1H6-DC-SIGN cells were infected with HHV-8, and β-galactosidase activity was monitored at 24 and 48 h postinfection (hpi). HHV-8 was purified from tetradecanoyl phorbol acetate (TPA)-induced BCBL-1 cells as previously described (18, 19). There was a significant increase (P < 0.05) in β-galactosidase expression in T1H6-DC-SIGN-infected cells at 48 hpi compared to that in uninfected cells. Based on these results, all future experiments used 48 h postplating or postinfection as the experimental endpoint.
To determine HHV-8 TCID50 values, T1H6-DC-SIGN cells were plated in black Corning CellBind 96-well plates (Corning, Tewksbury, MA) at 8 × 104 cells/well in replicates of 6 wells. Tenfold dilutions of HHV-8 samples were prepared, and 15 μl was inoculated into each well. Forty-eight hpi, cells were harvested using 100 μl of CellStripper (CellGro, Tewksbury, MA). The contents of each well were collected, and the cell pellet was harvested by centrifugation for 5 min at 13,000 rpm, 4°C. Each pellet was washed 3 times with 100 μl of ice-cold 1× phosphate-buffered saline (PBS). Pellets were resuspended in 100 μl of cold potassium phosphate-dithiothreitol (DTT) lysis buffer (100 mM potassium phosphate, pH 7.8; 1 mM DTT) to prevent oxidation of β-galactosidase. The solution was subjected to 3 freeze-thaw cycles using dry ice-ethanol and a 37°C water incubator. Cell debris was removed by centrifugation at 13,000 rpm for 10 min at 4°C, and lysates were carefully collected. A total of 15 μl of each lysate was plated in individual wells, in replicates of 6. Detection of β-galactosidase was determined using the β-Gal chemiluminescence detection kit II (Clontech, Mountain View, CA) according to the manufacturer's instructions with the following changes. For each well, 117.6 μl of reaction buffer and 2.4 μl of substrate were added in the dark. The substrate and buffer mixture were mixed with the cell lysates, and the reaction mixture was incubated for 1 h at room temperature in the dark. Chemiluminescence was measured using a BioTek Synergy II chemiluminescence reader (BioTek, Winooski, VT), with the data recorded in 10-s integrals at a sensitivity setting of 250. To distinguish a positive infection from a negative infection for TCID50 measurements, the chemiluminescence readings of the uninfected cell lysates (background average chemiluminescence), plus twice the background standard deviation, was subtracted from each test value. If the resulting value was greater than 0, the well was considered to be infected. If the resulting value was ≤0, the well was deemed uninfected.
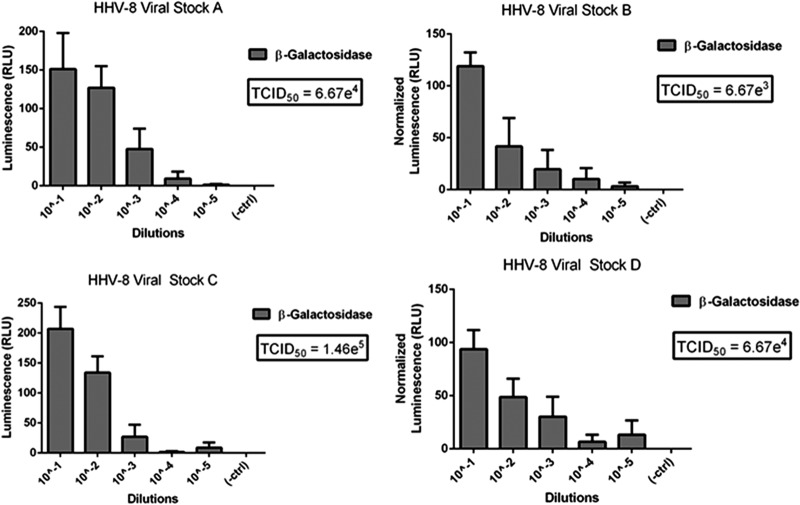
As shown in Fig. 1, β-galactosidase levels demonstrated a dose response with increasing dilutions of each HHV-8 viral preparation. The results from each well (positive or negative infection) were used in the Reed-Muench calculation (20) to determine the HHV-8 TCID50 for each sample. The calculation used was as follows: TCID50/ml = 10(PD − a)/b, where PD = proportional distance, a = log dilution greater than 50% infected, and b = inoculum volume (ml). PD = % of wells infected over 50% − 50%)/(% of wells infected over 50%) − (% of wells infected less than 50%). Figure 1 shows the TCID50 values of four separate HHV-8 viral preparations.
Fig 1.
TCID50 determinations of 4 separate HHV-8 viral preparations. Each graph shows the β-galactosidase levels with decreasing amounts of HHV-8 and the resulting TCID50 values.
HHV-8 TCID50 values were validated using immature monocyte-derived dendritic cells (iMDDC). Peripheral blood mononuclear cells (PBMCs) obtained from healthy blood donors were isolated with lymphocyte separation medium (Mediatech, Manassas, VA). Monocytes were isolated by plastic adherence and treated with 10 ml of IMDM medium (Iscove's modification of Dulbecco's modified Eagle medium; Mediatech, Manassas, VA) containing 10% fetal calf serum (FCS), 1,000 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF), and 1,000 U/ml interleukin 4 (IL-4; Schering Plough, Kenilworth, NJ). Media containing the cytokines were refreshed on day 4 of culture, and the cells (8 × 104/well) were plated on day 5 for experimentation. iMDDC were plated in replicates of 4 in a 96-well plate. The cells were infected with HHV-8 using either 1 or 2 TCID50 amounts. Two different HHV-8 viral preparations were used in these experiments. At 48 hpi, cells were washed, fixed, and stained for expression of viral ORF59, a processivity factor for HHV-8 DNA polymerase (21), using a mouse-anti-ORF59 antibody (ABI, Columbia, MD) followed by goat-anti-mouse IgG-fluorescein isothiocyanate (FITC) (Santa Cruz Biotechnology, Dallas, TX). Images of the infections using both HHV-8 virus preparations were blinded, and the number of infected cells was manually counted by three individuals unrelated to this project. Further, this experiment was performed with iMDDC derived from the monocytes of two separate donors. As shown in Table 1, infection of cells with 1 or 2 TCID50 HHV-8 values resulted in the expected percentage of infected cells using different virus preparations and iMDDC derived from different donors. Since MDDC do not support lytic replication of HHV-8 (19), we confirmed that this TCID50 assay could quantify HHV-8 lytic replication in CD40L–IL-4-activated, peripheral blood B lymphocytes (18) (data not shown).
Table 1.
Validation of TCID50 valuesa
| Monocyte donor | HHV-8 viral preparation | TCID50 level | % infected cells |
|||
|---|---|---|---|---|---|---|
| Count 1 | Count 2 | Count 3 | Mean ± SD | |||
| 1 | 1 | 1 | 60 | 63 | 46 | 56 ± 9 |
| 1 | 2 | 99 | 93 | 85 | 92 ± 7 | |
| 2 | 1 | 52 | 60 | 45 | 52 ± 8 | |
| 2 | 2 | 100 | 94 | 90 | 95 ± 5 | |
| 2 | 1 | 1 | 43 | 61 | 42 | 49 ± 11 |
| 1 | 2 | 100 | 100 | 95 | 98 ± 3 | |
| 2 | 1 | 48 | 59 | 48 | 52 ± 6 | |
| 2 | 2 | 89 | 93 | 94 | 92 ± 3 | |
Validation of TCID50 values. Monocyte-derived immature DC from 2 separate donors were infected with 1 or 2 TCID50 levels of HHV-8 from 2 separate viral preparations. The cells were stained for expression of the HHV-8 ORF59 protein at 48 hpi. Images of the stained cells were blinded, and the percentage of infected cells in each experiment was counted by three separate individuals. Results are presented as the means of the % infected cells ± SD.
The ability to calculate infectious HHV-8 virus titers allows for the determination of specific infectivity, the ratio between TCID50/ml and encapsidated (DNase-treated) viral copies. Specific infectivity demonstrates the ratio of infectious to total viral particles in a particular sample. As shown in Table 2, the specific infectivity of two separate HHV-8 preparations ranged from 3.5 × 10−5 to 1.27 × 10−6, demonstrating that the majority of the HHV-8 virus particles were noninfectious.
Table 2.
Specific activity of HHV-8 viral preparationsa
| HHV-8 sample | TCID50/ml | No. of DNA copies/ml | Specific infectivity |
|---|---|---|---|
| A | 6.11 × 102 | 4.80 × 108 | 1.27 × 10−6 |
| B | 6.11 × 105 | 1.74 × 109 | 3.51 × 10−5 |
Specific activity of HHV-8 viral preparations. The TCID50 values of two separate HHV-8 viral preparations were determined as described in the text. Total virus DNA copies were determined by quantitative PCR. Specific infectivity was calculated by dividing TCID50 values by total DNA copies.
HHV-8 DNA has been detected in a variety of biological samples, including saliva, blood, semen, and various tissues (22–26). These locations are associated with documented transmission of the virus from individual to individual (9, 10, 25, 26). There is currently no method to accurately determine the titer of infectious HHV-8 in these samples or in in vitro-generated samples. The latter is especially important for studies designed to synchronize cell culture infections and for assessment and reproducibility of in vitro experiments. It is also important to be able to determine viral loads in different biological samples in studies designed to study efficiency of transmission. An assay that can measure infectious virus titers can be used to determine neutralizing antibody titers which may be important in studies on HHV-8 immunity and development of Kaposi's sarcoma. Current methods to determine virus titers consist of measuring the amount of encapsidated viral particles by quantitative PCR of DNase-resistant viral DNA in various samples or detecting infected cells by antibody-based techniques, such as immunofluorescence (8, 12, 14). The former suffers from the inability to distinguish infectious from noninfectious particles, while the latter suffers from loss of sensitivity.
The T1H6-DC-SIGN cells allowed the development of a TCID50 assay for the determination of infectious HHV-8 titers in various biological samples. We have determined the infectious titers of several different viral preparations and validated these levels by infecting immature dendritic cells at 1 and 2 TCID50 values. Importantly, our results demonstrate that viral stocks prepared by polyethylene glycol (PEG) precipitation and centrifugation through sucrose cushions results in a significant number of noninfectious virus, as evidenced by specific infectivity values showing 1 infectious virus in 100,000 to 1 million virus particles. As a result, an experiment in which the virus titer is determined by quantitative PCR of DNase-resistant virus particles and the calculated multiplicity of infection (MOI) is 10 may actually be using an MOI of 0.0001 to 0.00001 (based on a specific infectivity of 1 × 10−5 to 1 × 10−6).
The T1H6-DC-SIGN assay represents the first in vitro assay for determining infectious titers of wild-type, nonrecombinant HHV-8. This assay should prove to be very useful for cell culture experiments, including synchronized infections, determination of anti-HHV-8-neutralizing antibody levels, and determining levels of infectious virus in biological samples.
ACKNOWLEDGMENTS
We thank Pawel Kalinski for providing iDCs, Pat Moore and Yuan Chang for providing the ORF50 plasmid, and Naoki Inoue for providing the T1H6 cell line. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pcDNA3-DC-SIGN from S. Pohlman, F. Baribaud, F. Kirchhoff, and R. W. Doms and BCBL-1 from M. McGrath and D. Ganem.
This work was supported by Public Health Service grant CA082053 from the National Cancer Institute and grant U01AI035041 from the National Institute of Allergy and Infectious Disease.
Footnotes
Published ahead of print 3 April 2013
REFERENCES
- 1. Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 2. Moore PS, Chang Y. 2001. Kaposi's sarcoma-associated herpesvirus, p 2803–2833 In Knipe DM, Howley PM. (ed), Fields virology, vol 4 Lippincott Williams and Wilkins, Philadelphia, PA [Google Scholar]
- 3. Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L, Sigaux F. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 [PubMed] [Google Scholar]
- 4. Blackbourn DJ, Ambroziak J, Lennette E, Adams M, Ramachandran B, Levy JA. 1997. Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet 349:609–611 [DOI] [PubMed] [Google Scholar]
- 5. Blackbourn DJ, Lennette ET, Ambroziak J, Mourich DV, Levy JA. 1998. Human herpesvirus 8 detection in nasal secretions and saliva. J. Infect. Dis. 177:213–216 [DOI] [PubMed] [Google Scholar]
- 6. Blackbourn DJ, Levy JA. 1997. Human herpesvirus 8 in semen and prostate. AIDS 11:249–250 [DOI] [PubMed] [Google Scholar]
- 7. Gupta P, Singh MK, Rinaldo C, Ding M, Farzadegan H, Saah A, Hoover D, Moore P, Kingsley L. 1996. Detection of Kaposi's sarcoma herpesvirus DNA in semen of homosexual men with Kaposi's sarcoma. AIDS 10:1596–1598 [DOI] [PubMed] [Google Scholar]
- 8. Koelle DM, Huang ML, Chandran B, Vieira J, Piepkorn M, Corey L. 1997. Frequent detection of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J. Infect. Dis. 176:94–102 [DOI] [PubMed] [Google Scholar]
- 9. Regamey N, Tamm M, Binet I, Thiel G, Erb P, Cathomas G. 1999. Transplantation-associated Kaposi's sarcoma: herpesvirus 8 transmission through renal allografts. Transplant. Proc. 31:922–923 [DOI] [PubMed] [Google Scholar]
- 10. Regamey N, Tamm M, Wernli M, Witschi A, Thiel G, Cathomas G, Erb P. 1998. Transmission of human herpesvirus 8 infection from renal-transplant donors to recipients. N. Engl. J. Med. 339:1358–1363 [DOI] [PubMed] [Google Scholar]
- 11. Vieira J, Huang ML, Koelle DM, Corey L. 1997. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J. Virol. 71:7083–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cai Y, Berger EA. 2011. An immunotoxin targeting the gH glycoprotein of KSHV for selective killing of cells in the lytic phase of infection. Antiviral Res. 90:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Lagunoff M. 2007. The KSHV viral interleukin-6 is not essential for latency or lytic replication in BJAB cells. Virology 359:425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kimball LE, Casper C, Koelle DM, Morrow R, Corey L, Vieira J. 2004. Reduced levels of neutralizing antibodies to Kaposi sarcoma-associated herpesvirus in persons with a history of Kaposi sarcoma. J. Infect. Dis. 189:2016–2022 [DOI] [PubMed] [Google Scholar]
- 15. Inoue N, Winter J, Lal RB, Offermann MK, Koyano S. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 77:8147–8152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, Calos MP. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7:379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lukac DM, Kirshner JR, Ganem D. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348–9361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rappocciolo G, Hensler HR, Jais M, Reinhart TA, Pegu A, Jenkins FJ, Rinaldo CR. 2008. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 82:4793–4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR., Jr 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 176:1741–1749 [DOI] [PubMed] [Google Scholar]
- 20. Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 21. Chan SR, Chandran B. 2000. Characterization of human herpesvirus 8 ORF59 protein (PF-8) and mapping of the processivity and viral DNA polymerase-interacting domains. J. Virol. 74:10920–10929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Sanjose S, Mbisa G, Perez-Alvarez S, Benavente Y, Sukvirach S, Hieu NT, Shin HR, Anh PT, Thomas J, Lazcano E, Matos E, Herrero R, Munoz N, Molano M, Franceschi S, Whitby D. 2009. Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J. Infect. Dis. 199:1449–1456 [DOI] [PubMed] [Google Scholar]
- 23. de Souza VA, Sumita LM, Nascimento MC, Oliveira J, Mascheretti M, Quiroga M, Freire WS, Tateno A, Boulos M, Mayaud P, Pannuti CS. 2007. Human herpesvirus-8 infection and oral shedding in Amerindian and non-Amerindian populations in the Brazilian Amazon region. J. Infect. Dis. 196:844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedman-Kien AE, Saltzman BR. 1990. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi's sarcoma. J. Am. Acad. Dermatol. 22:1237–1250 [DOI] [PubMed] [Google Scholar]
- 25. Guech-Ongey M, Verboom M, Pfeiffer RM, Schulz TF, Ndugwa CM, Owor AM, Bakaki PM, Bhatia K, Figueiredo C, Eiz-Vesper B, Blasczyk R, Mbulaiteye SM. 2010. HLA polymorphisms and detection of Kaposi sarcoma-associated herpesvirus DNA in saliva and peripheral blood among children and their mothers in the Uganda sickle cell anemia KSHV study. Infect. Agents Cancer 5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wojcicki JM. 2003. Traditional behavioural practices, the exchange of saliva and HHV-8 transmission in sub-Saharan African populations. Br. J. Cancer 89:2016–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]