Abstract
Previous studies of the biochemistry and physiology of eosinophils have relied upon cells obtained from patients with eosinophilia (EE). It is unknown whether such cells might have been activated or partially exhausted by the pathological state causing eosinophilia. We examined cell surface charge, membrane transport of deoxyglucose, activation of lyso-somal acid phosphatase, and oxidative metabolism to provide a profile to compare EE with purified normal eosinophils (NE) and normal neutrophils.
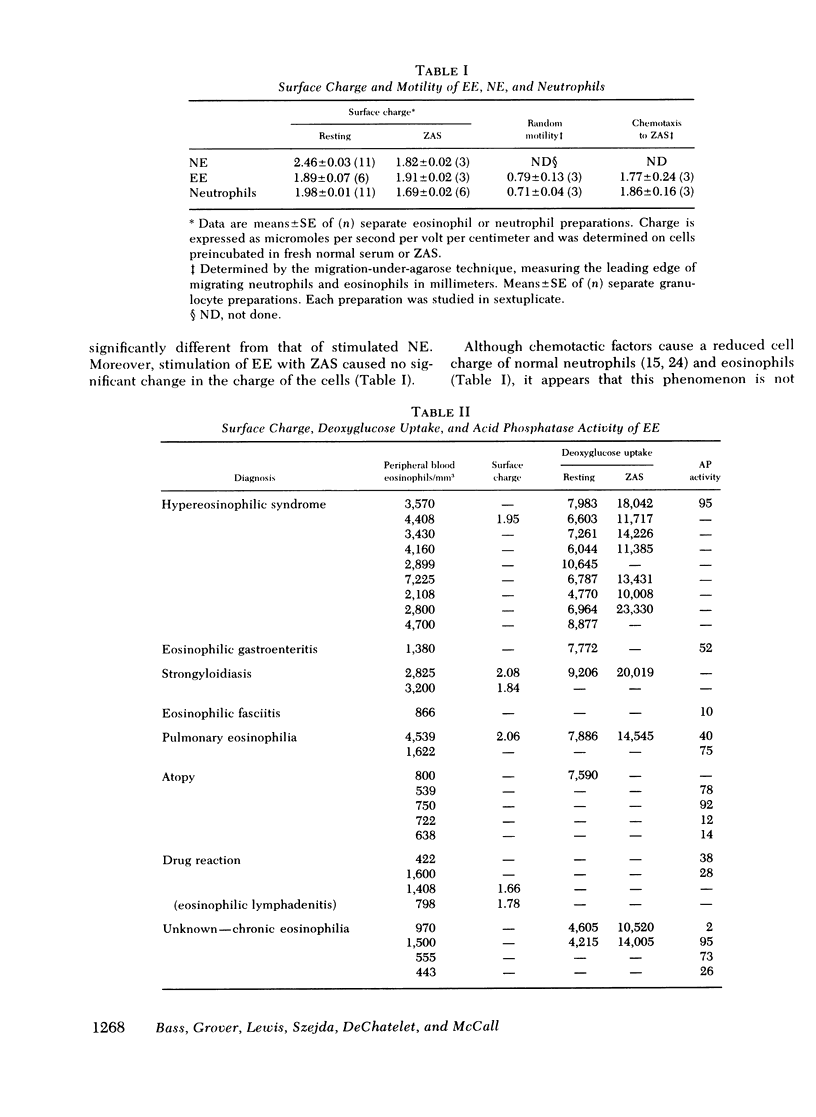
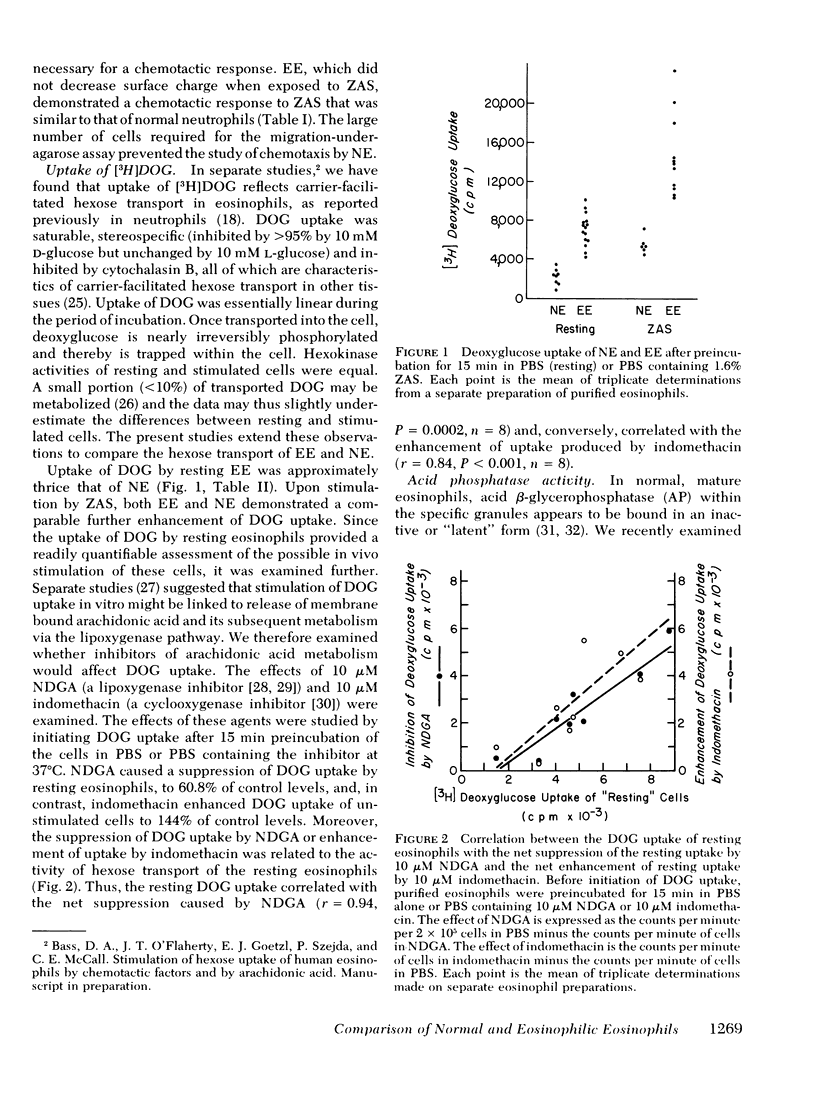
Eosinophils or neutrophils were obtained in >95% purity from normal individuals and patients with eosinophilia of diverse etiologies. Cell surface charge was determined by electrophoretic mobility in micromoles per second per volt per centimeter. Normal eosinophils demonstrated a surface charge of 2.46±0.03. Stimulation of the cells by zymosan-activated serum (ZAS) reduced the surface charge to 1.82±0.02. In contrast, the charge of “resting” EE was already reduced (1.89±0.05) and was not altered by ZAS. Resting and stimulated neutrophils had a charge of 1.98±0.01 and 1.69±0.02, respectively.
Uptake of [3H]2-deoxyglucose has been shown to reflect carrier-facilitated hexose transport in granulocytes. Deoxyglucose uptake by resting NE and NE stimulated by ZAS was 2.40±0.40 and 5.44±0.39 (cpm × 10−3/2 × 105 eosinophils), respectively. Resting and stimulated EE demonstrated deoxyglucose uptake of 7.55±0.58 and 15.3±0.6, respectively.
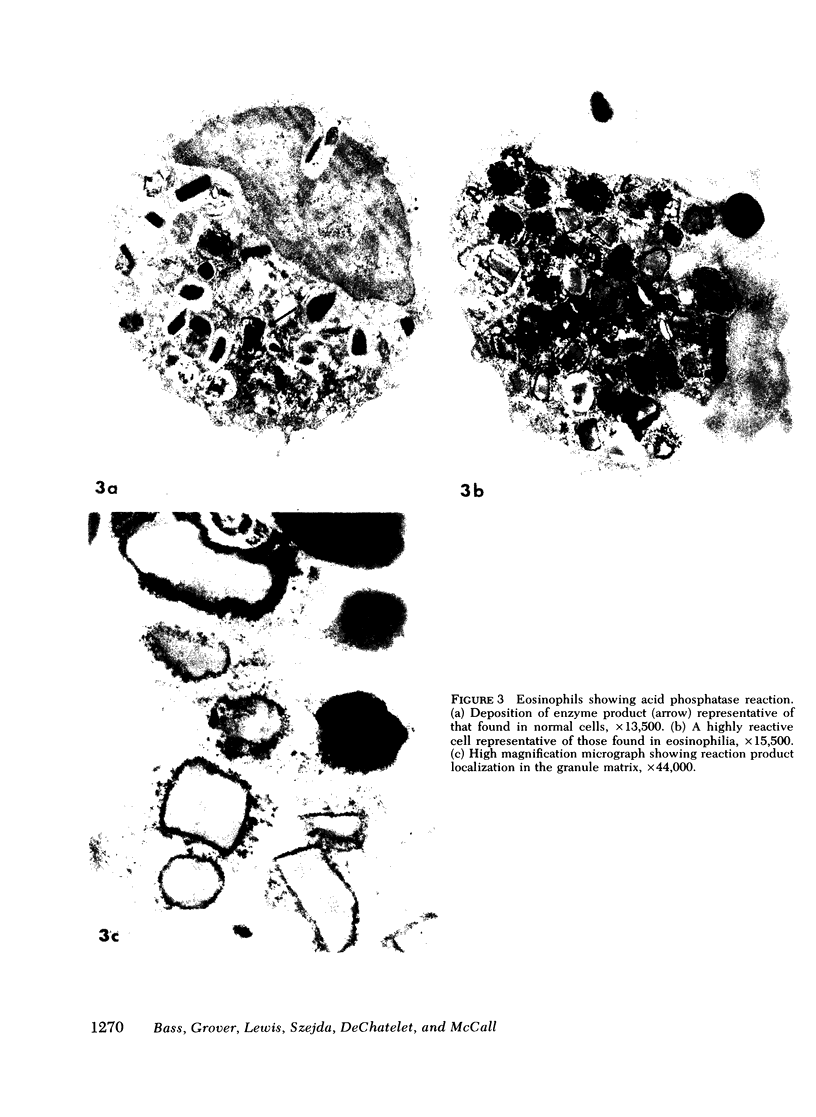
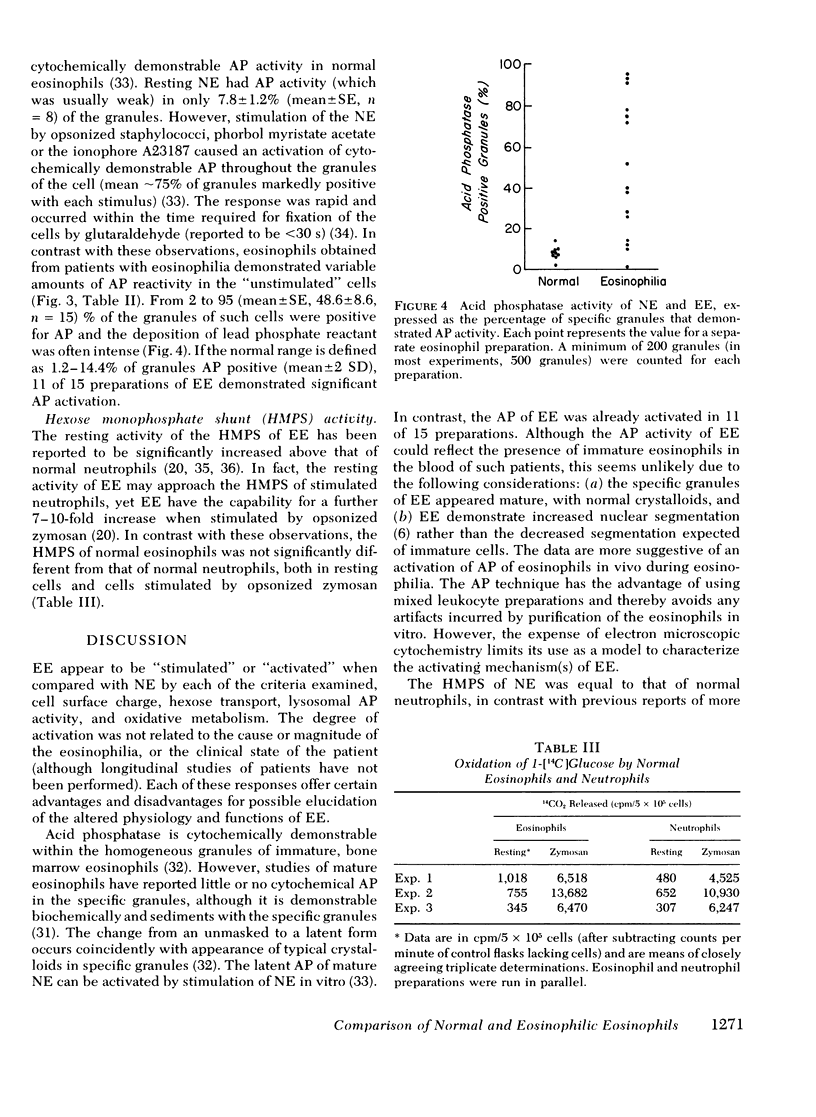
Lysosomal acid phosphatase was determined by an electron microscopic cytochemical technique. In normal eosinophils and neutrophils, lysosomal acid phosphatase in mature cells is held in a latent form. Normal eosinophils demonstrated weakly positive acid phosphatase activity in 7.8±1.2% of the specific granules. Normal eosinophils, stimulated by opsonized staphylococci or the calcium ionophore A23187, develop rapid activation of acid phosphatase in ∼80% of the granules throughout the cells. Resting EE were usually already activated and demonstrated acid phosphatase in 48.6±8.6% of the granules (range, 2-95% granules positive; significant activation was observed in preparations in EE from 11 of 15 patients).
Oxidative metabolism was monitored by measurement of the hexose monophosphate shunt (HMPS) (metabolism of 1-[14C]glucose to 14CO2). Previous studies demonstrated that resting EE have an HMPS activity which is nearly that of stimulated neutrophils, yet EE remain capable of further 7-10-fold increase when stimulated by opsonized zymosan. In contrast, the HMPS of NE (resting and stimulated) was not significantly different from that of neutrophils.
Thus eosinophils obtained from patients with eosinophilia appear significantly activated when compared with normal eosinophils by the criteria of surface charge, activation of lysosomal acid phosphatase, membrane hexose transport, and hexose monophosphate shunt activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar A. R., Kay A. B. Membrane receptors for IgG and complement (C4, C3b and C3d) on human eosinophils and neutrophils and their relation to eosinophilia. J Immunol. 1977 Sep;119(3):976–982. [PubMed] [Google Scholar]
- BOYUM A. SEPARATION OF WHITE BLOOD CELLS. Nature. 1964 Nov 21;204:793–794. doi: 10.1038/204793a0. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Metabolic and bactericidal activities of human eosinophils. Br J Haematol. 1971 Mar;20(3):277–285. doi: 10.1111/j.1365-2141.1971.tb07038.x. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970 Apr;45(1):54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A. Behavior of eosinophil leukocytes in acute inflammation. II. Eosinophil dynamics during acute inflammation. J Clin Invest. 1975 Oct;56(4):870–879. doi: 10.1172/JCI108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A., Dechatelet L. R., McCall C. E. Independent stimulation of motility and the oxidative metabolic burst of human polymorphonuclear leukocytes. J Immunol. 1978 Jul;121(1):172–178. [PubMed] [Google Scholar]
- Bass D. A., Gonwa T. A., Szejda P., Cousart M. S., DeChatelet L. R., McCall C. E. Eosinopenia of acute infection: Production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. 1980 Jun;65(6):1265–1271. doi: 10.1172/JCI109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. A., O'Flaherty J. T., Szejda P., DeChatelet L. R., McCall C. E. Role of arachidonic acid in stimulation of hexose transport by human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5125–5129. doi: 10.1073/pnas.77.9.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Reed P. W. Inhibition of the neutrophil oxidative response to a chemotactic peptide by inhibitors of arachidonic acid oxygenation. Biochem Biophys Res Commun. 1979 Sep 27;90(2):481–487. doi: 10.1016/0006-291x(79)91260-9. [DOI] [PubMed] [Google Scholar]
- Buckley I. K. Studies in fixation for electron microscopy using cultured cells. Lab Invest. 1973 Oct;29(4):398–410. [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Kolterman O. G., Siegel J. A., Olefsky J. M. Insulin-stimulated glucose transport in human adipocytes. Am J Physiol. 1979 Jun;236(6):E621–E625. doi: 10.1152/ajpendo.1979.236.6.E621. [DOI] [PubMed] [Google Scholar]
- Cramer E. B., Gallin J. I. Localization of submembranous cations to the leading end of human neutrophils during chemotaxis. J Cell Biol. 1979 Aug;82(2):369–379. doi: 10.1083/jcb.82.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Campbell T. L., McCall C. E., Shirley P. S., Swendsen C. L. Oxidation of 2-deoxyglucose by human polymorphonuclear leukocytes. Inflammation. 1980 Sep;4(3):249–259. doi: 10.1007/BF00915026. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., McPhail L. C., Huntley C. C., Muss H. B., Bass D. A. Oxidative metabolism of the human eosinophil. Blood. 1977 Sep;50(3):525–535. [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Durocher J. R., Kaplan A. P. Interaction of leukocyte chemotactic factors with the cell surface. I. Chemotactic factor-induced changes in human granulocyte surface charge. J Clin Invest. 1975 May;55(5):967–974. doi: 10.1172/JCI108026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Weller P. F., Sun F. F. The regulation of human eosinophil function by endogenous mono-hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Feb;124(2):926–933. [PubMed] [Google Scholar]
- Grover W. H., Winkler H. H., Normansell D. E. Phagocytic properties of isolated human eosinophils. J Immunol. 1978 Aug;121(2):718–725. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERCHE C. Electrophoresis of Micrococcus pyogenes aureus. Acta Pathol Microbiol Scand Suppl. 1953;98:1–194. [PubMed] [Google Scholar]
- McCall C. E., Bass D. A., Cousart S., DeChatelet L. R. Enhancement of hexose uptake in human polymorphonuclear leukocytes by activated complement component C5a. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5896–5900. doi: 10.1073/pnas.76.11.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Bactericidal and metabolic properties of human eosinophils. Blood. 1972 Jan;39(1):67–80. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Arachidonic acid induced degranulation of rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1979 Mar 15;87(1):292–299. doi: 10.1016/0006-291x(79)91678-4. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Neutrophil aggregation and degranulation. Effect of arachidonic acid. Am J Pathol. 1979 May;95(2):433–444. [PMC free article] [PubMed] [Google Scholar]
- Ottesen E. A., Stanley A. M., Gelfand J. A., Gadek J. E., Frank M. M., Nash T. E., Cheever A. W. Immunoglobulin and complement receptors on human eosinophils and their role in cellular adherence to schistosomules. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):134–141. doi: 10.4269/ajtmh.1977.26.134. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Human eosinophils. Purification and cytotoxic capability of eosinophils from patients with the hypereosinophilic syndrome. Blood. 1978 Mar;51(3):457–473. [PubMed] [Google Scholar]
- Saran R. Cytoplasmic vacuoles of eosinophils in tropical pulmonary eosinophilia. Am Rev Respir Dis. 1973 Nov;108(5):1283–1285. doi: 10.1164/arrd.1973.108.5.1283. [DOI] [PubMed] [Google Scholar]
- Sparrevohn S., Wulff H. R. The nuclear segmentation of eosinophils under normal and pathological conditions. Acta Haematol. 1967;37(2):120–125. doi: 10.1159/000209059. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Tai P. C. Studies on blood eosinophils. II. Patients with Löffler's cardiomyopathy. Clin Exp Immunol. 1976 Jun;24(3):423–434. [PMC free article] [PubMed] [Google Scholar]
- TAPPEL A. L., LUNDBERG W. O., BOYER P. D. Effect of temperature and antioxidants upon the lipoxidase-catalyzed oxidation of sodium linoleate. Arch Biochem Biophys. 1953 Feb;42(2):293–304. doi: 10.1016/0003-9861(53)90359-2. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. Studies on blood eosinophils. I. Patients with a transient eosinophilia. Clin Exp Immunol. 1976 Jun;24(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Vadas M., David J., Butterworth A., Houba V., David L., Pisani N. Comparison of the ability of eosinophils and neutrophils, and of eosinophils from patients with S. mansoni infection and normal individuals, to mediate in vitro damage to schistosomula of S. mansoni. Adv Exp Med Biol. 1979;114:677–682. doi: 10.1007/978-1-4615-9101-6_111. [DOI] [PubMed] [Google Scholar]
- West B. C., Gelb N. A., Rosenthal A. S. Isolation and partial characterization of human eosinophil granules. Comparison to neutrophils. Am J Pathol. 1975 Dec;81(3):575–588. [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. Eosinophil function and disorders. Adv Intern Med. 1974;19:1–25. [PubMed] [Google Scholar]