Abstract
Cache Valley virus was initially isolated from mosquitoes and had been linked to central nervous system-associated diseases. A case of Cache Valley virus infection is described. The virus was cultured from a patient's cerebrospinal fluid and identified with real-time reverse transcription-PCR and sequencing, which also yielded the complete viral coding sequences.
CASE REPORT
In mid-September 2011, a 63-year-old woman presented to an upstate New York hospital with complaints of fever, headache, neck stiffness, and photophobia. One week earlier, she had noticed a macular, nonpruritic lesion on her right forearm, about 3 cm in diameter, with central clearing. Three days prior, as the first lesion was fading, a petechial rash developed on her lower extremities that spread to her torso. She then traveled to Pennsylvania for a weekend, and during this time she developed the mentioned fever and symptoms of meningitis. She returned home and went to the Emergency Department (ED) of the hospital the next morning.
On physical examination, the patient appeared alert and oriented, with a blood pressure of 148-mm/80-mm Hg, a pulse rate of 87 per min, a respiratory rate of 18 per min, an oral temperature of 37.6°C, and an oxygen saturation of 99% on room air. She had a scattered, bilateral, petechial rash on her thighs; meanwhile, the lesions on her back and abdomen were fading. She had moderate neck stiffness. Her neurologic exam was otherwise normal; there was no evidence of encephalitis, cranial nerve abnormalities, or focal findings.
Her medical record included a history of hypertension, hypothyroidism, meningioma, and migraine headaches and of rheumatic fever during childhood. The patient stated that she and her husband frequently camped outdoors. Throughout mid- to late August they camped in Wyoming and Livingston counties in New York state and also embarked on a 5-day camping trip in Dansville, NY, through Vermont and New Hampshire. She lived with her husband, had a cat, and frequently tended to her garden around her home. She had no knowledge of any sick contact.
Her white blood cell count on presentation was 5,700/μl, with a normal differential. Hematocrit was normal at 42%, and platelets were normal at 212,000/μl. Computed tomography of the head without contrast showed mild atrophy without evidence of acute intracranial abnormality. Blood cultures were drawn, and she was sent home on doxycycline. The patient returned to the ED the following day with new complaints of nausea and vomiting in addition to the previously reported symptoms.
The patient was admitted to the hospital with a preliminary diagnosis of aseptic meningitis. A lumbar puncture was performed, and the cerebrospinal fluid (CSF) showed 216 nucleated cells, with 91% lymphocytes, 7% monocytes, 1% basophils, and 1% polymorphonuclear cells. CSF chemistries were normal, with a glucose concentration of 60 mg/dl (56% of the serum level) and protein of 46 mg/dl. No microorganism was seen in the CSF by Gram stain. Magnetic resonance imaging of the brain showed only a small, stable meningioma. A virus culture of the CSF was initiated on several cell lines. PCR testing for enteroviruses in CSF and for herpes simplex virus in blood and CSF was performed. Serologic tests to detect antibodies against Borrelia burgdorferi, Ehrlichia chaffeensis, Anaplasma phagocytophila, and Rickettsia (both typhus and Rocky Mountain spotted fever) in blood were also conducted. All molecular and serologic test results were negative. Bacterial culture of the blood and CSF yielded no growth.
The patient gradually improved and was discharged 4 days after admission to complete a 10 day-course of doxycycline at home. On follow-up 2 months later, she reported transient difficulties in word finding that occurred soon after the hospital discharge and had resolved at the time of the visit. Her headaches, including a migranous aura, were more severe following this hospitalization. She continued seeking medical care for her worsening headaches and memory loss a year later.
Six days after inoculation of the CSF, a questionable cytopathic effect (CPE) was noticed in the Buffalo green monkey kidney (BGMK) cell culture. Other inoculated cell lines included rhesus monkey kidney (RhMK) and human male fetal lung (MRC5); both of these lines failed to produce any CPE. After three passages in BGMK cells, the amplified supernatant was filtered and inoculated, and CPE was successfully produced in human colon adenocarcinoma (CaCo2), human lung carcinoma (A549), MRC5, and an African green monkey kidney cell line (Vero). Characteristics of CPE involved initial cell rounding, followed by clumping and sloughing off of the cell monolayer (Fig. 1). No CPE was observed after the amplified stock was inoculated into RhMK cells, human female fetal lung (WI-38) cells, or human embryonal rhabdomyosarcoma (RD) cells.
Fig 1.
Cytopathic effect of the CVV-infected Vero cells. Vero cells were seeded in a 24-well plate and grown to 90% confluence and were either mock infected (A) or infected at a multiplicity of infection (MOI) of 0.3 (B) or at an MOI of 30 (C) with the MNZ-92011 strain, which was harvested from BGMK cells, amplified, and titrated in Vero cells. Photographs were taken on day 4 postinfection, using a Spot RT-KE monochrome camera connected to a Nikon Diaphot TMD microscope at ×40 magnification and Spot 5.0 Basic software.
Cultured virus from day 2 postinoculation CaCo2 cells was harvested, and nucleic acids were extracted using a QIAcube automated nucleic acid extractor (Qiagen, Germantown, MD). A molecular panel for the detection of encephalitis viruses was performed on the extract (1). Included in this panel was a duplex real-time reverse transcription-PCR (RT-PCR) for the detection of Cache Valley virus (CVV) and the California serogroup viruses (2). The sample tested positive for CVV in this assay. All other PCR results were negative. For confirmation, cDNA was generated from the viral RNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) and a conventional PCR that targets the S segment of the CVV genome (3). Sequence analysis of the 170-nucleotide (nt) amplicon showed 100% identity with several strains of CVV in GenBank.
To better characterize the CVV that infected the patient, the virus genome was subjected to additional sequencing. Briefly, viral RNAs were extracted, reverse transcribed, and PCR amplified with random primers. The PCR products were sequenced using a 454 GS-FLX Titanium platform (454 Life Sciences, Branford, CT), yielding a total of 139,321 reads, of which 815 shared 60.4% to 100% identity to viruses in the family Bunyaviridae. All unknown and Bunyavirus-like reads were then employed to assemble contigs via the supplied Newbler v.2.6 software. A single contig of 905 nt was constructed (>32× coverage) that showed 99% identity (99% coverage; E value, 0.0E + 00) with the S segment of 4 Yucatan strains of CVV (BLASTN version 2.2.27; the GenBank query was performed on 21 August 2012). Five other contigs of length 138 to 2,164 nt were assembled and shared 92.5% to 97% identity with regions of the M segment of various CVV strains. Fourteen contigs of length 110 to 3,014 nt were also generated, sharing 71% to 100% identity with regions of the L segment of Tensaw, Batai, and Ngari viruses as well as of the partially sequenced L segment of several CVV strains. Using targeted-primer RT-PCR followed by TOPO cloning and Sanger sequencing, we closed the gaps between the contigs and confirmed partial sequences of 877 nt for the S segment, of 4,460 nt for the M segment, and of 6,871 nt for the L segment of this CVV.
We next assessed seroconversion in a pair of patient sera collected at the time of admission and at 24 days later. A neutralization assay in 24-well microplates was developed, using the patient strain harvested from BGMK cells and then passaged twice through Vero cells. Briefly, test sera were serially 2-fold diluted in tissue culture medium, mixed with 100 50% tissue culture infective doses (TCID50) of virus, incubated for 1 h at room temperature, and then added in duplicate onto the Vero cell monolayers that were 90% confluent. The cells were incubated in 5% CO2 at 37°C for 5 days, and CPE was monitored by microscopic inspection during the incubation period. Complete protection of the cell monolayers was observed at or below the 1:160 dilution with a positive-control serum, an anti-CVV mouse hyperimmune serum (Centers for Disease Control and Prevention, Fort Collins, CO), and at or below the 1:80 dilution with the patient convalescent-phase serum. In contrast, the acute-phase serum failed to neutralize the virus at all tested dilutions, indicating that the patient was recently infected with CVV.
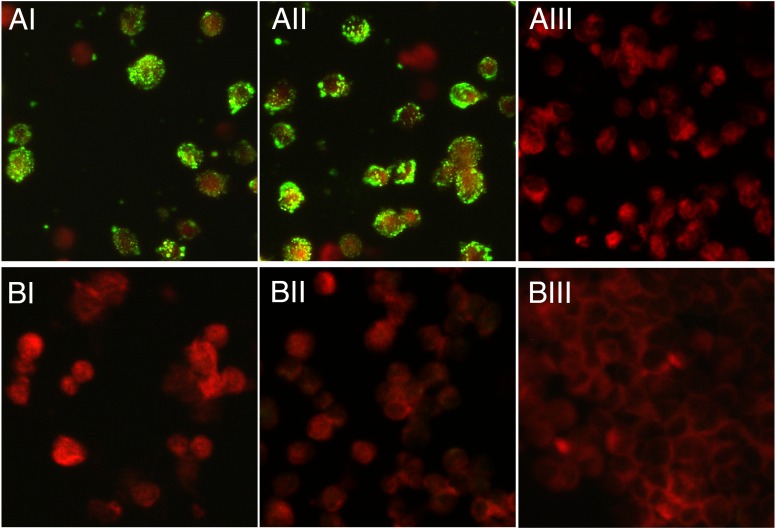
To confirm the neutralization data, an immunofluorescence assay (IFA) was established to detect CVV-specific antibodies. Briefly, the BGMK cell-derived virus was used to inoculate CaCo2 cells, which were harvested at moderate CPE and used to produce slides for the IFA. Cells were trypsinized, washed, and resuspended in tissue culture medium; cell spots were then air dried and fixed in cold acetone for 30 min. Control slides were prepared in the same fashion with mock-infected CaCo2 cells. CVV-infected CaCo2 cells were incubated with the patient's 24-day convalescent-phase serum at dilutions ranging from 1:16 to 1:4,096. To demonstrate specificity, CVV-infected CaCo2 cells were also incubated with an anti-CVV mouse serum, an anti-La Crosse encephalitis virus human serum (Wadsworth Center, Albany, NY), and a negative-control human serum (Focus Diagnostics, Cypress, CA), all at the same dilutions. All described antisera were also tested in parallel at a dilution of 1:16 on control slides. Slides that contained the patient convalescent and anti-CVV mouse sera were reactive, with a titer of 1:1,024 (Fig. 2). Neither the negative-control human serum (data not shown) nor the anti-La Crosse virus human serum was reactive to the CVV-infected cells (<1:16). On control slides of mock-infected cells, no CVV-specific staining pattern was observed, validating the test results for the CVV-infected cells.
Fig 2.
Detection of anti-CVV antibodies in the patient's convalescent-phase serum. The results of an IFA of the patient's 24-day convalescent-phase serum (I), an anti-CVV mouse hyperimmune serum (II), and an anti-La Crosse virus human convalescent-phase serum (III), using Caco2 cells that were mock infected (BI to BIII) or at day 2 postinfection with the MNZ-92011 strain (AI to AIII), are shown. Fluorescein isothiocyanate (FITC)-conjugated dual anti-human/anti-mouse IgG goat antibody (Focus Diagnostics, Cypress, CA) was used as a secondary antibody. Images were taken with a Zeiss AxioCam MRc camera attached to AxioImager A1 microscope at ×400 magnification, using Zeiss AxioVision 3.1 software. All sera were at a dilution of 1:16, with the exception of the anti-CVV mouse serum represented in panels AII and BII, where the serum was diluted 1:1,024.
Cache Valley virus is a negative-sense, single-stranded RNA virus with a tripartite genome. It belongs to the Bunyamwera serogroup in the genus Orthobunyavirus and was named after the location in northern Utah where it was first isolated from mosquitoes (4). The virus has since been found to be widespread in North and Central America, infecting many species of domestic and wild animals such as deer, cattle, sheep, and horses (5, 6, 7, 8). Epidemiologic studies indicate that CVV can be transmitted via a broad range of mosquito species (9), with the white-tailed deer population as a potential natural reservoir (10).
CVV is a concern in animal husbandry due to its ability to cause abortion, stillbirth, and congenital defects of the musculoskeletal and central nervous systems in affected ruminants (11, 12). In humans, CVV disease was first identified in a previously healthy young man from North Carolina following a deer-hunting trip (13). Subsequently, a case of aseptic meningitis due to CVV in an adult male from Wisconsin was reported (14). Other human infections with CVV have been inferred by serologic studies of CVV in areas of endemicity in the Delmarva Peninsula in the United States and in Yucatan, Mexico, with reported infection rates of up to 18% (15, 16).
We describe here a case of CVV disease in a human. Similar to the Wisconsin case, this virus was isolated from the patient's acute-phase CSF. Its positive identification was made via a laboratory-developed real-time RT-PCR, followed by genomic sequencing. We also demonstrated that the anti-CVV antibody titer of a convalescent-phase serum had risen sharply compared to the titer of an acute-phase serum drawn 3 weeks earlier, indicating that the CVV infection had just been acquired. In the absence of other etiology and coupled with previous observations that CVV infection can lead to central nervous system (CNS)-associated diseases, it is reasonable to conclude that CVV was responsible for this patient's illness. Due to the lack of molecular epidemiology of CVV in regions where the patient traveled, however, it was not possible to determine the time and place of her exposure.
Segmented RNA viruses can evolve over time through genomic reassortment. This characteristic has been reported in members of the Bunyamwera serogroup, including CVV (17). Through the effort to identify the CVV strain involved, we obtained a sequence of 877 nt of the virus S segment, containing two open reading frames (ORF) of 233 and 101 amino acids (aa). Their translated sequences possessed 100% identity to the deduced N and NSs proteins, respectively, of 5 CVV mosquito isolates previously collected in the Yucatan area (18). We also sequenced 4,460 nt of the virus M segment, which encodes a hypothetical polyprotein precursor of 1,434 aa. This polyprotein shares at most 94% nucleotide identity and 98% amino acid identity with its homologues in 7 CVV strains isolated in North America (see Fig. S1 and Table S1 in the supplemental material). In addition, we deciphered the majority (6,871 nt) of the virus L segment. It contains an ORF for a protein of 2,228 aa which shows 88% and 83% aa identity with the predicted RNA-dependent RNA polymerase of Tensaw and Batai viruses, respectively. At present, no complete ORF encoding the predicted RNA polymerase of CVV exists in GenBank. This report thus provides full nucleotide sequence coverage for this protein.
In conclusion, we report a confirmed case of meningitis due to CVV infection. Since the discovery of CVV over half a century ago, there have been surprisingly few human cases reported. Given the wide geographic distribution of CVV and its transmission via a large number of mosquito species, we suspect that CVV infection is underreported. A targeted study may be needed to assess the true scope of human infection with this virus. Additionally, inclusion of CVV in routine arbovirus surveillance is suggested. Knowledge of the areas of CVV endemicity might benefit clinicians in the differential diagnoses of CNS-related diseases. And, finally, this study reports complete coding sequences of a CVV, including the L segment-encoded RNA polymerase.
Nucleotide sequence accession numbers.
The sequenced L, M, and S segments of this CVV (MNZ-92011 strain) have been deposited in GenBank under accession numbers KC436106, KC436107, and KC436108, respectively.
Supplementary Material
ACKNOWLEDGMENTS
The anti-CVV mouse hyperimmune serum was a kind gift from Barbara Johnson (CDC, Fort Collins, CO). We thank Christine Mayer for the virus neutralization assay, Rama Ramani for virus culture work, Theresa Church and Valerie Demarest for the IFA, Meghan Fuschino and Michael Popowich for molecular screening, Soumya Mitra and April Davis for microphotography, and the clinical microbiology staff at the Strong Memorial Hospital for their workup on the specimens. Special thanks are given to Joan Zeller, whose tenacity resulted in the initial recognition of the CPE, without which the virus would not have been identified.
This work was supported in part by National Institutes of Health (grant U54 AI057160) to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research.
This study was approved by the Institutional Review Board of participating institutions.
The authors have no conflicts of interest.
Footnotes
Published ahead of print 20 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00252-13.
REFERENCES
- 1. Dupuis M, Hull R, Wang H, Nattanmai S, Glasheen B, Fusco H, Dzigua L, Markey K, Tavakoli NP. 2011. Molecular detection of viral causes of meningitis and encephalitis in New York State. J. Med. Virol. 83:2172–2181 [DOI] [PubMed] [Google Scholar]
- 2. Wang H, Nattanmai S, Kramer LD, Bernard KA, Tavakoli NP. 2009. A duplex real-time reverse transcriptase polymerase chain reaction assay for the detection of California serogroup and Cache Valley viruses. Diagn. Microbiol. Infect. Dis. 65:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuno G, Mitchell CJ, Chang GJ, Smith GC. 1996. Detecting bunyaviruses of the Bunyamwera and California serogroups by a PCR technique. J. Clin. Microbiol. 34:1184–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holden P, Hess AD. 1959. Cache Valley virus, a previously undescribed mosquito-borne agent. Science 130:1187–1188 [DOI] [PubMed] [Google Scholar]
- 5. Neitzel DF, Grimstad PR. 1991. Serological evidence of California group and Cache Valley virus infection in Minnesota white-tailed deer. J. Wildl. Dis. 27:230–237 [DOI] [PubMed] [Google Scholar]
- 6. Sahu SP, Pedersen DD, Ridpath HD, Ostlund EN, Schmitt BJ, Alstad DA. 2002. Serologic survey of cattle in the northeastern and north central United States, Virginia, Alaska, and Hawaii for antibodies to Cache Valley and antigenically related viruses (Bunyamwera serogroup virus). Am. J. Trop. Med. Hyg. 67:119–122 [DOI] [PubMed] [Google Scholar]
- 7. McConnell S, Livingston C, Jr, Calisher CH, Crandell RA. 1987. Isolations of Cache Valley virus in Texas, 1981. Vet. Microbiol. 13:11–18 [DOI] [PubMed] [Google Scholar]
- 8. McLean RG, Calisher CH, Parham GL. 1987. Isolation of Cache Valley virus and detection of antibody for selected arboviruses in Michigan horses in 1980. Am. J. Vet. Res. 48:1039–1041 [PubMed] [Google Scholar]
- 9. Ngo KA, Maffei JG, Dupuis AP, II, Kauffman EB, Backenson PB, Kramer LD. 2006. Isolation of Bunyamwera serogroup viruses (Bunyaviridae, Orthobunyavirus) in New York State. J. Med. Entomol. 43:1004–1009 [DOI] [PubMed] [Google Scholar]
- 10. Blackmore CG, Grimstad PR. 1998. Cache Valley and Potosi viruses (Bunyaviridae) in white-tailed deer (Odocoileus virginianus): experimental infections and antibody prevalence in natural populations. Am. J. Trop. Med. Hyg. 59:704–709 [DOI] [PubMed] [Google Scholar]
- 11. Chung SI, Livingston CW, Jr, Edwards JF, Gauer BB, Collisson EW. 1990. Congenital malformations in sheep resulting from in utero inoculation of Cache Valley virus. Am. J. Vet. Res. 51:1645–1648 [PubMed] [Google Scholar]
- 12. Crandell RA, Livingston CW, Jr, Shelton MJ. 1989. Laboratory investigation of a naturally occurring outbreak of arthrogryposis-hydranencephaly in Texas sheep. J. Vet. Diagn. Invest. 1:62–65 [DOI] [PubMed] [Google Scholar]
- 13. Sexton DJ, Rollin PE, Breitschwerdt EB, Corey GR, Myers SA, Dumais MR, Bowen MD, Goldsmith CS, Zaki SR, Nichol ST, Peters CJ, Ksiazek TG. 1997. Life-threatening Cache Valley virus infection. N. Engl. J. Med. 336:547–549 [DOI] [PubMed] [Google Scholar]
- 14. Campbell GL, Mataczynski JD, Reisdorf ES, Powell JW, Martin DA, Lambert AJ, Haupt TE, Davis JP, Lanciotti RS. 2006. Second human case of Cache Valley virus disease. Emerg. Infect. Dis. 12:854–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buescher EL, Byrne RJ, Clarke GC, Gould DJ, Russell PK, Scheider FG, Yuill TM. 1970. Cache Valley virus in the Del Mar Va Peninsula. I. Virologic and serologic evidence of infection. Am. J. Trop. Med. Hyg. 19:493–502 [DOI] [PubMed] [Google Scholar]
- 16. Blitvich BJ, Saiyasombat R, Talavera-Aguilar LG, Garcia-Rejon JE, Farfan-Ale JA, Machain-Williams C, Loroño-Pino MA. 2012. Orthobunyavirus antibodies in humans, Yucatan Peninsula, Mexico. Emerg. Infect. Dis. 18:1629–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blitvich BJ, Saiyasombat R, Dorman KS, Garcia-Rejon JE, Farfan-Ale JA, Loroño-Pino MA. 2012. Sequence and phylogenetic data indicate that an orthobunyavirus recently detected in the Yucatan Peninsula of Mexico is a novel reassortant of Potosi and Cache Valley viruses. Arch. Virol. 157:1199–1204 [DOI] [PubMed] [Google Scholar]
- 18. Farfan-Ale JA, Loroño-Pino MA, Garcia-Rejon JE, Soto V, Lin M, Staley M, Dorman KS, Bartholomay LC, Hovav E, Blitvich BJ. 2010. Detection of flaviviruses and orthobunyaviruses in mosquitoes in the Yucatan Peninsula of Mexico in 2008. Vector Borne Zoonotic Dis. 10:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.