Abstract
Animal fodder is routinely complemented with antibiotics together with other food supplements to improve growth. For instance, sepiolite is currently used as a dietary coadjuvant in animal feed, as it increases animal growth parameters and improves meat and derived final product quality. This type of food additive has so far been considered innocuous for the development and spread of antibiotic resistance. In this study, we demonstrate that sepiolite promotes the direct horizontal transfer of antibiotic resistance plasmids between bacterial species. The conditions needed for plasmid transfer (sepiolite and friction forces) occur in the digestive tracts of farm animals, which routinely receive sepiolite as a food additive. Furthermore, this effect may be aggravated by the use of antibiotics supplied as growth promoters.
INTRODUCTION
There exists widespread concern about the massive amounts of antimicrobials used as growth promoters in livestock fodder and their relationship with the development of antibiotic resistance. Antibiotic use in animal husbandry may result in the selection of resistance determinants that can spread to human pathogens via different ways (1–3). The exposure of bacteria to antibacterial agents results in the selection of preexisting resistant variants that augment the likelihood of pathogen survival. Moreover, many antibiotics increase the mutation and recombination rates of bacteria, raising the probability of evolving resistance to even unrelated antibiotics (4). Finally, antibiotics can promote DNA sequence incorporation from other organisms via horizontal gene transfer (HGT) (5). HGT plays a major role among the mechanisms that control pathogen evolution, allowing bacteria to evade immunological responses, distribute genes that increase virulence, or acquire increased resistance to antibiotics (5). The majority of antibiotic resistances are most probably gained by lateral transfer of resistance genes from other bacterial strains or species (6).
It seems clear that the use of antibiotics in livestock, if not regulated, can be a dangerous source of antibiotic resistance determinants for human pathogens. However, animal feed is routinely complemented not only with antibiotics but also with other food supplements to enhance animal growth. For instance, sepiolite, which was authorized by the European Union in 1990 and registered as a technological additive for animal feed (E-562), is used as a dietary coadjuvant in fodder since it most likely reduces the speed of food passage through the intestinal tract, therefore allowing domestic animals to carry out a more efficient digestion of proteins. This results in an increase in parameters related to animal growth and in an improvement of meat and derived final product quality. Currently, sepiolite is used widely as a feed additive supplied to broiler chickens (7) and pigs (8), among other types of livestock. Furthermore, sepiolite has also been proposed as a remediation agent for contaminated soil (9). Up to the present, the use of this type of food additives has so far been considered innocuous for the development and spread of antibiotic resistance.
Recently, a DNA transformation method based on clay materials was described for bacteria (10, 11). The principle behind this method relies on minerals that, like sepiolite, are able to adsorb DNA and form nanoneedles, which, by the action of friction forces, are able to release and incorporate DNA into bacterial cells (12, 13). The procedure is able to transfer free DNA plasmids between bacterial species (11, 14, 15). Taking together the antibiotic selective pressure exerted on the livestock microbiome and the potential of sepiolite to transform bacterial cells, we studied whether sepiolite could mediate HGT between bacteria without the necessity of providing free DNA plasmids, i.e., could exert a direct transfer between bacteria. Based on the results presented here, we discuss the possibility that sepiolite may induce the transfer of resistance determinants in the digestive tracts of farm animals.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains used as donors in this study were Escherichia coli K-12 strain BW25113 (16), E. coli B strain BL21 (Invitrogen), and Pseudomonas aeruginosa strain PAO1 (17). Recipient strains were E. coli K-12 strain BW25113 dinF::Kan (16, 18), E. coli AB1155 galK::blaTEM1 (19), Salmonella enterica serovar Typhimurium strain SL1344 nagZ::Kan (Kanr) (20), P. aeruginosa strain PAO1 (17), and Mycobacterium smegmatis strain mc2155 (21). Table 1 shows the bacterial strains, plasmids, and antibiotic selection schemes used in the transformation experiments. The nonconjugative plasmids (nonautotransferring) used for the transfer experiments consisted of pCA24N (conferring chloramphenicol resistance [Cmr]) (22), pGEM-T (conferring ampicillin resistance [Apr]) (Promega), pUCP24 (23) and pBBR1MCS5 (24) (both conferring gentamicin resistance [Gmr]), and pVV16 (conferring hygromycin resistance [Hygr]) (25). All plasmids were previously introduced into the donors by electroporation.
Table 1.
Strains, plasmids, and selection schemes used in transformation experiments
| Donor | Receptor | Plasmid (antibiotic marker) | Donor counterselection agent (concn [μg/ml]) |
|---|---|---|---|
| E. coli K-12 BW25113 | E. coli K-12 BW25113 dinF::Kan | pCA24N (Cm) | Kan (50) |
| Salmonella SL1344 nagZ::Kan | pCA24N (Cm) | Kan (50) | |
| P. aeruginosa PAO1 | pUCP24 (Gm) | Kan (50) | |
| M. smegmatis mc2155 | pVV16 (Hyg) | Nal (40) | |
| E. coli B BL21 | E. coli K-12 BW25113 galK::kan | pGEM-T (Amp) | Kan (50) |
| Salmonella SL1344 nagZ::Kan | pGEM-T (Amp) | Kan (50) | |
| P. aeruginosa PAO1 | pBBR1MCS5 (Gm) | Kan (50) | |
| P. aeruginosa PAO1 | E. coli K-12 AB1157 galK::bla | pUCP24 (Gm) | Cb (500) |
In all cases, Luria-Bertani medium (LB; 10 g/liter of tryptone, 5 g/liter of yeast extract, and 5 g/liter of NaCl) was used. Solid LB plates were prepared with 2% agar, as previously described (14). Media were supplemented with antibiotics at the following concentrations: kanamycin (Kan) at 50 μg/ml, chloramphenicol (Cm) at 40 μg/ml, nalidixic acid (Nal) at 40 μg/ml, gentamicin (Gm) at 30 μg/ml, ampicillin (Ap) at 100 μg/ml, carbenicillin (Cb) at 500 μg/ml, and hygromycin (Hyg) at 100 μg/ml.
Plasmid transfer experiments.
About 5 × 108 bacterial cells of donor (containing the appropriate plasmid) and recipient strains from overnight cultures were centrifuged and resuspended in 100 μl of sterilized transformation mixture, consisting of sepiolite (Kremer Pigmente, Spain) suspended in aqueous solution at a final concentration of 100 μg/ml.
Resuspended cells were spread on plates containing fresh LB medium solidified with 2% agar, and petri dishes were predried in a biological safety flow cabinet for 20 min before use. Friction force was provided by streaking bacterial cultures plus sepiolite with sterile glass stir sticks gently pressed onto the medium surface for 1 min, applying as much pressure as possible without breaking the agar gel. Petri dishes were incubated at 37°C for 1 h (2 h in the case of recipient M. smegmatis) to allow for antibiotic gene marker expression. The respective antibiotics to select for transformants (recipients containing the plasmid) and counterselect for donors were added to each plate, in a volume of 250 μl uniformly spread onto the surface of the medium by use of glass beads. Plates were incubated overnight (for 36 h when M. smegmatis was used as a recipient). Every experiment was carried out in quadruplicate and repeated at least twice. Transfer of plasmids was verified by extraction from some transformants of plasmid DNA, followed by restriction analysis. Transfer efficiencies were expressed as numbers of transformants per donor cell.
RESULTS AND DISCUSSION
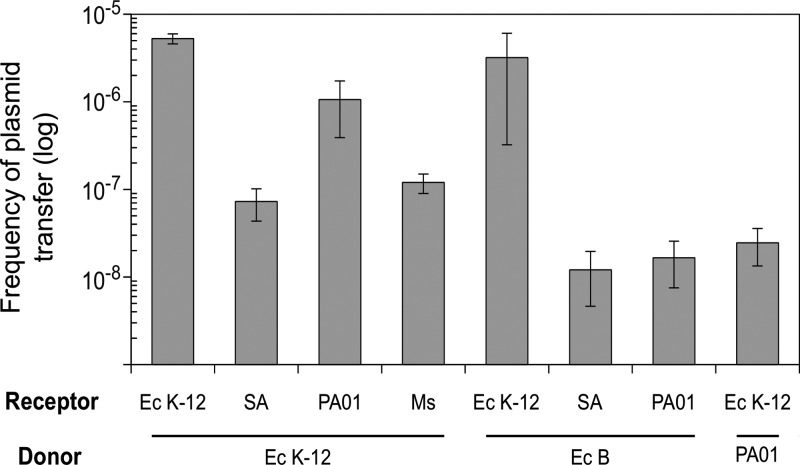
In order to prove the idea that plasmid DNA can be transferred between bacteria without previous plasmid extraction, we designed a simple laboratory experiment: a mixture consisting of E. coli K-12 strain BW25113 harboring plasmid pCA24N (nonautotransferring and conferring Cmr) and E. coli BW25113 dinF::Kan (Kanr) (harboring no plasmids and resistant to kanamycin [with resistance encoded by a chromosomal marker]) was treated with friction forces (i.e., spreading with sterile glass stir sticks gently pressed onto the medium surface, applying as much pressure as possible without breaking the agar gel) in the presence of sepiolite for 1 min. One hour after the application of the friction forces, plates were supplemented with kanamycin (50 μg/ml) to counterselect the donor strain and with chloramphenicol (40 μg/ml) to select for the transformants (recipients containing the plasmid). The results clearly showed that the plasmid was easily transferred to the E. coli recipient strain by the joint action of the friction forces and sepiolite. An average transfer efficiency of 5 × 10−6 transformant per recipient cell was obtained (Fig. 1). Control experiments followed an identical protocol yet omitted sepiolite.
Fig 1.
Efficiencies of direct plasmid transfer. Plasmid transfer was monitored for the following species and strains: E. coli K-12 (Ec K-12), E. coli B (Ec B), S. enterica serovar Typhimurium (SA), M. smegmatis mc2155 (Ms), and P. aeruginosa PAO1 (PA01). Plasmids used consisted of pCA24N and pGEM-T for E. coli and Salmonella, pUCP24 and pBBR1MCS5 for P. aeruginosa PAO1, and pVV16 for M. smegmatis. Data show the means for four experiments. Error bars represent standard deviations.
To test if sepiolite-based plasmid transfer may also occur with regard to other species, we used the same donor strain, BW25113, containing plasmid pCA24N (Cmr), pUCP24 (Gmr) (23), or pVV16 (Hygr) as a donor and S. enterica serovar Typhimurium strain SL1344 nagZ::Kan (Kanr), P. aeruginosa strain PAO1, or M. smegmatis strain mc2155 as the recipient, respectively. Following the same procedure as that described above and selecting transformants with the appropriate antibiotics, we obtained average transfer efficiencies of 5 × 10−8, 5 × 10−7, and 1 × 10−7 transformant per receptor cell for S. enterica, P. aeruginosa, and M. smegmatis, respectively (Fig. 1).
In order to elucidate whether this plasmid transfer was exclusive to E. coli K-12, we also used another E. coli strain of different origin, E. coli B strain BL21, as a donor. Plasmid pGEM-T (Apr) was transferred from the E. coli B strain to E. coli K-12 strain BW25113 dinF::Kan (Kanr) and to S. enterica serovar Typhimurium strain SL1344 nagZ::Kan (Kanr). Plasmid pUCP24 was used to test the transfer from E. coli B strain BL21 to P. aeruginosa strain PAO1. Plasmids were transferred in all cases to the different recipients, at frequencies of 3.2 × 10−6, 1.2 × 10−8, and 1.7 × 10−8 for E. coli K-12 dinF::Kan, S. enterica serovar Typhimurium strain SL1344 nagZ::Kan, and P. aeruginosa strain PAO1, respectively (Fig. 1).
Finally, to test if other species different from E. coli could act as plasmid donors by this procedure, we performed the same experiment using P. aeruginosa strain PAO1 as the pUCP24 plasmid donor and E. coli K-12 strain AB1157 galK::Kan as the receptor. Again, plasmids were successfully transferred at a frequency of 2.5 × 10−8 (Fig. 1).
Most importantly, the control experiments in the absence of sepiolite produced no colonies in any of the cases (efficiencies of ≤2 × 10−9 transformant per receptor cell). Therefore, our data clearly indicate that the transfer of plasmid DNA was due only to the sepiolite-mediated transformation process, ruling out conjugation, competence, or de novo mutations, and thus confirming that sepiolite promotes direct HGT between bacteria.
Recently, Yoshida and Fujiura proposed that seismic movements may promote bacterial transformation in natural clay substrates (26). Our findings demonstrate that this transfer may occur directly from bacterium to bacterium, as friction forces generated in the interface of ground material may equally promote bacterial genetic material release and transformation. We propose here the most probable environment for sepiolite-mediated DNA transfer between bacteria: the digestive tracts of farm animals. All the necessary ingredients that allow for a direct transfer of plasmid DNA between bacteria occur in farm animals: routine administration of antibiotics, sepiolite used as a food additive, pathogenic and commensal bacteria sharing the same niche, and the mechanical friction provided by peristalsis in the rumen or intestines, if not the strong abrasive action of the gizzard in poultry.
The results presented here demonstrate that a direct genetic material exchange among different strains and species is promoted by sepiolite. This should strongly encourage the scientific community working on antibiotic resistance to start studying this phenomenon in vivo, undertaking the appropriate experiments in livestock.
Finally, it has not escaped our attention that this finding provides a new laboratory method suitable for direct plasmid transformation between bacterial strains or species, without having to resort to plasmid isolation and competent cell preparation.
ACKNOWLEDGMENTS
This work was supported by the Instituto de Salud Carlos III, cofinanced by the European Regional Development Fund “A Way to Achieve Europe,” by the Spanish Network for Research in Infectious Diseases (grant REIPI RD06/0008), and by grant PI10/00105 (FIS-ISCIII) and PAR project 241476 of the EU 7th Framework Programme.
We thank Paul Johnston from the Freie Universität in Berlin for his comments on the manuscript.
Footnotes
Published ahead of print 25 March 2013
REFERENCES
- 1. Alcorn T. 2012. Antibiotic use in livestock production in the USA. Lancet Infect. Dis. 12:273–274 [DOI] [PubMed] [Google Scholar]
- 2. Ferber D. 2003. Antibiotic resistance. WHO advises kicking the livestock antibiotic habit. Science 301:1027. [DOI] [PubMed] [Google Scholar]
- 3. Witte W. 2000. Selective pressure by antibiotic use in livestock. Int. J. Antimicrob. Agents 16(Suppl 1):S19–S24 [DOI] [PubMed] [Google Scholar]
- 4. Blazquez J, Couce A, Rodriguez-Beltran J, Rodriguez-Rojas A. 2012. Antimicrobials as promoters of genetic variation. Curr. Opin. Microbiol. 15:561–569 [DOI] [PubMed] [Google Scholar]
- 5. Couce A, Blazquez J. 2009. Side effects of antibiotics on genetic variability. FEMS Microbiol. Rev. 33:531–538 [DOI] [PubMed] [Google Scholar]
- 6. Binnewies TT, Motro Y, Hallin PF, Lund O, Dunn D, La T, Hampson DJ, Bellgard M, Wassenaar TM, Ussery DW. 2006. Ten years of bacterial genome sequencing: comparative-genomics-based discoveries. Funct. Integr. Genomics 6:165–185 [DOI] [PubMed] [Google Scholar]
- 7. Ouhida I, Pérez JF, Piedrafita J, Gasa J. 2000. The effects of sepiolite in broiler chicken diets of high, medium and low viscosity. Productive performance and nutritive value. Anim. Feed Sci. Technol. 85:183–194 [Google Scholar]
- 8. Parisini P, Martelli G, Sardi L, Escribano F. 1999. Protein and energy retention in pigs fed diets containing sepiolite. Anim. Feed Sci. Technol. 79:155–162 [Google Scholar]
- 9. Alvarez-Ayuso E, Garcia-Sanchez A. 2003. Sepiolite as a feasible soil additive for the immobilization of cadmium and zinc. Sci. Total Environ. 305:1–12 [DOI] [PubMed] [Google Scholar]
- 10. Yoshida N, Sato M. 2009. Plasmid uptake by bacteria: a comparison of methods and efficiencies. Appl. Microbiol. Biotechnol. 83:791–798 [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez-Beltran J, Elabed H, Gaddour K, Blazquez J, Rodriguez-Rojas A. 2012. Simple DNA transformation in Pseudomonas based on the Yoshida effect. J. Microbiol. Methods 89:95–98 [DOI] [PubMed] [Google Scholar]
- 12. Kurokawa T, Tominaga T, Katsuyama Y, Kuwabara R, Furukawa H, Osada Y, Gong JP. 2005. Elastic-hydrodynamic transition of gel friction. Langmuir 21:8643–8648 [DOI] [PubMed] [Google Scholar]
- 13. Yoshida N, Ide K. 2008. Plasmid DNA is released from nanosized acicular material surface by low molecular weight oligonucleotides: exogenous plasmid acquisition mechanism for penetration intermediates based on the Yoshida effect. Appl. Microbiol. Biotechnol. 80:813–821 [DOI] [PubMed] [Google Scholar]
- 14. Yoshida N, Nakajima-Kambe T, Matsuki K, Shigeno T. 2007. Novel plasmid transformation method mediated by chrysotile, sliding friction, and elastic body exposure. Anal. Chem. Insights 2:9–15 [PMC free article] [PubMed] [Google Scholar]
- 15. Wilharm G, Lepka D, Faber F, Hofmann J, Kerrinnes T, Skiebe E. 2010. A simple and rapid method of bacterial transformation. J. Microbiol. Methods 80:215–216 [DOI] [PubMed] [Google Scholar]
- 16. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. doi:10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 100:14339–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodriguez-Beltran J, Rodriguez-Rojas A, Guelfo JR, Couce A, Blazquez J. 2012. The Escherichia coli SOS gene dinF protects against oxidative stress and bile salts. PLoS One 7:e34791 doi:10.1371/journal.pone.0034791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegreness M, Shoresh N, Hartl D, Kishony R. 2006. An equivalence principle for the incorporation of favorable mutations in asexual populations. Science 311:1615–1617 [DOI] [PubMed] [Google Scholar]
- 20. Hoiseth SK, Stocker BA. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239 [DOI] [PubMed] [Google Scholar]
- 21. Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR., Jr 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911–1919 [DOI] [PubMed] [Google Scholar]
- 22. Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12:291–299 [DOI] [PubMed] [Google Scholar]
- 23. West SE, Schweizer HP, Dall C, Sample AK, Runyen-Janecky LJ. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81–86 [DOI] [PubMed] [Google Scholar]
- 24. Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, 2nd, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 25. Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, Snapper SB, Barletta RG, Jacobs WR, Jr, Bloom BR. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460 [DOI] [PubMed] [Google Scholar]
- 26. Yoshida N, Fujiura N. 2009. Earthquakes promote bacterial genetic exchange in serpentinite crevices. Astrobiology 9:289–295 [DOI] [PubMed] [Google Scholar]