Abstract
Pathogens resistant to most conventional antibiotics are a harbinger of the need to discover novel antimicrobials and anti-infective agents and develop innovative strategies to combat them. The aim of this study was to assess the in vitro activity of colistin alone or in combination with two bacteriocins, nisin A and pediocin PA-1/AcH, against Salmonella choleraesuis ATCC 14028, Pseudomonas aeruginosa ATCC 27853, Yersinia enterocolitica ATCC 9610, and Escherichia coli ATCC 35150 (O157:H7). The strain most sensitive to colistin was enterohemorrhagic E. coli O157:H7, which was inhibited at a concentration of about 0.12 μg/ml. When nisin A (1.70 μg/ml) or pediocin PA-1/AcH (1.56 μg/ml) was combined with colistin, the concentrations required to inhibit E. coli O157:H7 were 0.01 and 0.03 μg/ml, respectively. The in vitro antigenotoxic effect of colistin was determined by using the comet assay method to measure the level of DNA damage in freshly isolated human peripheral blood leukocytes (PBLs) incubated with colistin for 1 h at 37°C. Changes in the tail extents of PBLs of about 69.29 ± 0.08 μm were observed at a final colistin concentration of about 550 ng/ml. Besides the synergistic effect, the combination of colistin (1 mg/ml) and nisin (2 mg/ml) permitted us to re-evaluate the toxic effect of colistin on Vero (monkey kidney epithelial) cells.
INTRODUCTION
The emergence of multidrug-resistant pathogenic bacteria highlights a matching need for new therapeutic options. Better management and reasonable use of antibiotics are imperatively required in order to reduce the rate of emergence of antibiotic-resistant strains. Currently, clinicians and microbiologists are being forced to re-evaluate the clinical use of colistin, a relatively old polypeptide antibiotic, because there appear to be no promising therapeutic agents in the drug development pipeline (1). Colistin became available for clinical use in the 1960s but was replaced in the 1970s with antibiotics considered less toxic. Nephrotoxicity and neurotoxicity were the major side effects that led to the discontinuation of the routine use of colistin (1, 2). Two forms of colistin are commercially available, colistin sulfate, which is for oral and topical use, and colistimethate sodium (sodium colistin methanesulfonate, colistin sulfomethate sodium), which is for parenteral use or inhalation. The structure of polymyxins consists of a cyclic decapeptide bound to a fatty acid (3). Colistin binds to the anionic part of the lipopolysaccharide (LPS), leading to deep disruption in the bacterial membrane, enhancing its permeability and causing cell lysis (4). A role for LPS as a major receptor of colistin was proposed (5), and the mode of action involved a hydrophobic interaction between the nine-carbon fatty acid side chain of colistin and the fatty acid portion of lipid A (6). Strains of Escherichia coli and Klebsiella pneumoniae have become increasingly resistant to expanded-spectrum cephalosporins (7) but remain globally sensitive to tigecycline and colistin (8). The use of polymyxins to treat infections caused by E. coli O157:H7 has also been reported. Here, we report the synergistic effects observed between colistin and bacteriocins (nisin A and pediocin PA-1/AcH) against Gram-negative bacteria (GNB), including Salmonella choleraesuis ATCC 14028, Pseudomonas aeruginosa ATCC 27853, Yersinia enterocolitica ATCC 9610, and Escherichia coli ATCC 35150 (O157:H7). Besides the synergistic effect, we also established that the cytotoxicity of colistin for animal epithelial cells (Vero cells) can be abolished when mammalian cells are treated simultaneously with colistin and nisin A at concentrations of 1 and 2 mg/ml, respectively. To our knowledge, this is the first report describing the reduction of colistin cytotoxicity by bacteriocins. As part of the continuing debate on the “toxicity” of colistin, we also investigated the impact of this drug on human peripheral blood leukocytes (PBLs) by using the comet assay to assess DNA damage. This study suggests the possibility of better management of colistin and re-evaluation of its toxicity when it is combined with bacteriocins.
MATERIALS AND METHODS
Bacterial strains and growth media.
The target strains used were S. choleraesuis ATCC 14028, P. aeruginosa ATCC 27853, Y. enterocolitica ATCC 9610, and E. coli ATCC 35150 (O157:H7). The indicator strains used were E. coli RR1, Listeria innocua, and Pediococcus acidilactici UL5. All of these strains were grown aerobically for 18 h at 30°C in tryptic soy broth (TSB; Difco Laboratories, Sparks, MD). P. acidilactici UL5 and E. coli RR1 were used as indicator strains to test the activities of nisin A and polymyxin E, respectively, while L. innocua was used as an indicator strain to test the activity of pediocin PA-1/AcH. All of the strains were reactivated from a 20% glycerol stock stored at −80°C. They were subcultured at least three times at 24-h intervals before use in experiments.
Antimicrobial compounds and antimicrobial activity.
The sulfate salt of colistin used in this work was purchased from Sigma-Aldrich (Oakville, ON, Canada). A nisin A stock solution was obtained from Aplin & Barrett Ltd. (Beaminster, United Kingdom) in the form of Nisaplin, which contains 106 IU of nisin A/g. Pediocin PA-1/AcH was purchased from Sigma-Aldrich (Oakville, ON, Canada). Soluble protein concentrations were determined by the method of Lowry et al. with bovine serum albumin as the standard (9). The antimicrobial activities of colistin, pediocin PA-1/AcH, and nisin A were determined by the agar well diffusion method (10). The MIC of each antimicrobial peptide (AMP) alone or in combination was determined by microdilution assay (11) with sterile flat-bottom 96-well polystyrene microplates (Falcon; Becton-Dickinson Labware, Franklin Lakes, NJ). Quality control (QC) was performed by concurrent testing of E. coli ATCC 25922 in accordance with the CLSI methodology (12).
Cell lysis.
Cell lysis was assessed with sterile flat-bottom 96-well microassay plates (Falcon; Becton-Dickinson and Company, Franklin Lakes, NJ) as recently described (13). Briefly, bacterial cells obtained by centrifugation (10,000 × g, 4°C, 10 min) from 10 ml of a 6-h TSB culture were washed and resuspended in 5 ml of 20 mM sodium phosphate buffer (pH 6.5). Wells were loaded with 125 μl of a bacterial suspension of E. coli ATCC 35150 and 125 μl of sodium phosphate buffer containing colistin at 0, 0.12, 0.24, 0.36, or 0.48 μg/ml, and the plates were incubated for 18 h at 30°C. Absorbance at 650 nm was measured with a multidetection microplate reader (Bio-Tek Instruments Inc., Winooski, VT). Cell lysis was calculated as 100 − (At/A0 × 100), where A0 and At were the absorbance of the cell suspension in phosphate buffer without an antimicrobial agent and the absorbance of the suspensions containing an antimicrobial compound(s), respectively, measured at 0, 3, 9, or 18 h of incubation.
Effect of colistin on bacterial growth.
The GNB grown in TSB were incubated for 18 h in sterile flat-bottom 96-well microtiter plates (Falcon; Becton, Dickinson and Company, Franklin Lakes, NJ) in a total volume of 300 μl and inoculated at 1% (vol/vol) into fresh TSB containing colistin at the specific MIC for each strain. Growth was measured as the optical density at 630 nm (OD630; discarded after reading) every 2 h with a Thermomax microplate reader (Zeus Scientific Inc., Raritan, NJ). The inhibitory activity (IA) of colistin was calculated as a percentage as folloes: IA = 100 − 100[OD630(x)/OD630(i)], where x is the culture containing colistin and i is the control culture.
Genotoxicity of colistin.
Fresh blood samples (100 μl) were collected in a lithium-heparin-coated microtube (Sarstedt, 1.3-ml Li Hep PP) after pricking a finger of a healthy volunteer with a blood lancet (ACCU-CHEK, soft touch). Pure colistin base was assigned a potency of 1,000 μg base activity, corresponding to 30,000 IU/mg. One hundred forty-two units per milliliter (4.70 μg/ml) of colistin is the plasma drug level after the administration of a therapeutic dose (1.5 × 106 U) (14). The alkaline comet assay was performed as previously described (15, 16). The degree of damage was evaluated in each individual cell as the tail DNA percentage with the VisCOMET software and mvDELTA frame grabber. Images of 100 randomly selected cells (50 cells from each of two replicate slides) from each sample were analyzed. One scorer was used throughout the study, and all of the slides were scored in a blinded way. Cells with no DNA damage have an intact nucleus without a tail, whereas cells with DNA damage have a comet-like appearance. Cells with no head or a dispersed head were considered to be apoptotic and were not included in the analysis. The antigenotoxicity of a sample was determined by the relative damage index (RDI), which was calculated as follows: RDI = (mean tail length of sample-treated cells − mean tail length of negative control)/(mean tail length of positive control − mean tail length of negative control). Methyl methanesulfonate (MMS) was used as a positive control, and untreated cells were used as a negative control.
Hemolytic activity of colistin.
For hemolytic activity, fresh human red blood cells (RBCs, 20 ml) were centrifuged (4,000 rpm, 10 min), the supernatant was discarded, and the pellets were washed three times with phosphate-buffered saline (PBS, pH 7.4) (17). The final pellet was diluted 1:5 (vol/vol) in PBS. RBC suspensions (2 ml of final volume) were incubated with colistin, pediocin PA-1/AcH, nisin A, or combinations thereof at their respective MICs (Table 1). The resulting mixture was kept under rotary agitation at 37°C for 1 h. After incubation, the cell suspensions were centrifuged (10 min, 4,000 rpm) and the supernatant was carefully collected so as not to disturb the pellet. Hemolysis was monitored by measuring the absorbance at 405 nm of the hemoglobin released in the supernatant with a spectrophotometer (Zeus Scientific Inc.). Hemolysis levels were expressed as percentages, which were calculated as 100 × (As − A0)/At, where A0, As, and At are the absorbances of the erythrocyte suspension in PBS without an antimicrobial agent, with an antimicrobial agent(s), and with 1% (vol/vol) Triton (total lysis), respectively. All experiments were done in duplicate.
Table 1.
MICs of colistin, pediocin, nisin, and combinations thereof for GNB
| GNB | MIC(s), μg/ml (FICI)a |
||||
|---|---|---|---|---|---|
| Colistin | Pediocin | Nisin | Colistin-pediocin | Colistin-nisin | |
| S. choleraesuis ATCC 14028 | 1.21 | <200 | <200 | 0.15/6.25 (0.12) | 0.07/1.56 (0.05) |
| P. aeruginosa ATCC 27853 | 0.52 | <200 | <200 | 0.13/6.25 (0.25) | 0.13/6.25 (0.25) |
| Y. enterocolitica ATCC 9610 | 0.4 | <200 | <200 | 0.05/2.28 (0.12) | 0.075/6.25 (0.19) |
| E. coli ATCC 35150 | 0.12 | <200 | <200 | 0.03/1.56 (0.25) | 0.01/1.17 (0.08) |
FICIA + FICIB = (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone). The FICI was interpreted as follows: synergistic, FICI ≤ 0.5; additive, 0.5 < FICI < 1; indifferent, 1 < FICI < 2; antagonistic, FICI > 2.
Toxicity of colistin for eukaryote cells.
The Vero cell line (ATCC CCL-81) used to assess colistin toxicity was obtained from the European Collection of Cell Cultures (Sigma-Aldrich, L'isle d'Abeau Chesnes, France). The experiment was conducted as follows. A 400-μl volume of Dulbecco's modified Eagle's medium (DMEM) containing 1 × 105 Vero cells was distributed into each well of a 24-well plate and incubated for 24 h at 37°C in a humid atmosphere containing 5% CO2. The next day, cell culture supernatants were removed and replaced with colistin and nisin solutions diluted in DMEM to various concentrations, and the plates were placed back in the incubator. Twenty-four hours later, the cell layers were examined under an inverted microscope to evaluate the extent of cytotoxicity, which was expressed as a percentage. (i) Vero cells were cultured in the presence of colistin or nisin; (ii) colistin at 1 mg/ml was mixed with nisin at various concentrations, and then the mixture was added to Vero cells; and (iii) Vero cells were cultured in the absence or presence of colistin at 1 mg/ml.
Statistical analysis.
Statistical interpretation of the differences between the control and test values was performed by one-way analysis of variance t test. The data were analyzed with the SAS version 9.2 statistical package (SAS Institute Inc., Cary, NC).
RESULTS
Bacterial susceptibility to antimicrobial compounds.
The in vitro inhibition of S. choleraesuis ATCC 14028, P. aeruginosa ATCC 27853, Y. enterocolitica ATCC 9610, and E. coli O157:H7 by colistin, pediocin PA-1/AcH, and nisin is shown in Table 1. The target strains were treated with the antimicrobials alone or in combination in order to see if there was any synergistic effect. Minimal inhibition of the target strains was registered when they were treated with nisin A or pediocin PA-1/AcH alone. Activity against the target strains under these conditions required a high concentration of nisin or pediocin PA-1/AcH, leading to MICs of >200 μg/ml (Table 1). Colistin inhibited all of the target strains, giving MICs ranging from 0.12 to 1.21 μg/ml. The lowest MIC of colistin observed was for E. coli O157:H7, while the highest one observed was for S. choleraesuis ATCC 14028. When colistin was combined with nisin A or pediocin PA-1/AcH, the MIC diminished, indicating a synergistic effect of the combination of these two antimicrobials and the possibility of inhibiting the enterohemorrhagic bacterium E. coli O157:H7. E. coli O157:H7 was inhibited by remarkably low colistin concentrations of about 0.01 and 0.03 μg/ml when it was combined with nisin A (1.17 μg/ml) or pediocin PA-1/AcH (1.56 μg/ml), respectively. QC was conducted with E. coli ATCC 25922 as the reference strain to test the ability of our method to give the correct MIC of colistin. In our study and in accordance with the CLSI method (12), we found a MIC of 2 mg/liter, which is in accordance with the acceptable range published by CLSI (18).
IA of colistin.
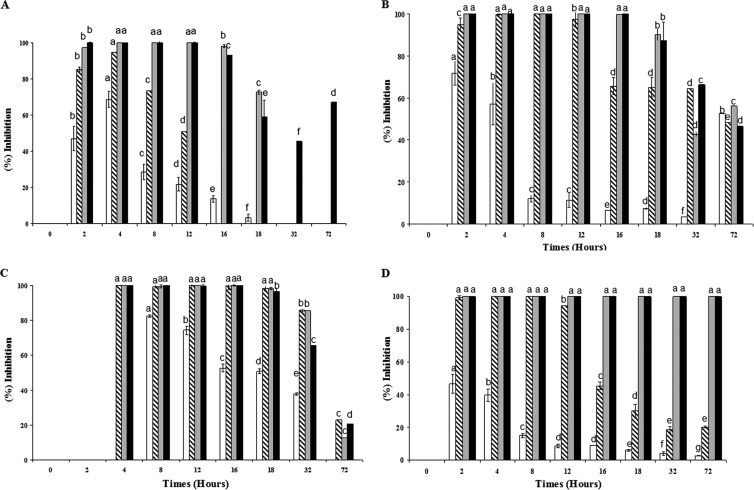
The main findings regarding the effect of colistin against the aforementioned GNB are shown in Fig. 1. The percent inhibition during the growth phase was established in comparison to the control culture. Colistin shows dose-dependent IA against the growth of the above-listed GNB. The inhibitory effects of colistin, at final concentrations of 1.56, 1.20, and 0.36 μg/ml, after 72 h of incubation were, respectively, ∼58.10, 16.70, and 100% for P. aeruginosa ATCC 27853, Y. enterocolitica ATCC 9610, and E. coli O157:H7, confirming the complete inhibition of E. coli O157:H7.
Fig 1.
Inhibition of S. choleraesuis ATCC 14028 (A), P. aeruginosa ATCC 27853 (B), Y. enterocolitica ATCC 9610 (C), and E. coli ATCC 35150 (D) growth by colistin at the respective MICs (white, 1× MIC; gray, 2× MIC; diagonal stripes, 3× MIC; black, 4× MIC) in tryptic soy broth as measured by turbidity (OD630). The MICs of colistin for S. choleraesuis ATCC 14028, P. aeruginosa ATCC 27853, Y. enterocolitica ATCC 9610, and E. coli ATCC 35150 were, respectively, about 1.21, 0.52, 0.4, and 0.12 μg/ml. The IA of colistin was calculated as a percentage as follows: IA = 100 − 100[OD630(x)/OD630(i)], where x is the culture containing colistin and i is the control culture. Means with the same letters are not significantly different (P < 0.05).
Colistin and cell lysis.
The effect of colistin at concentrations at 0.06, 0.12, 0.24, 0.36, and 0.48 μg/ml on E. coli O157:H7 was studied, and the main data are shown in Fig. 2. After 3 h of incubation of E. coli O157:H7 in the presence of colistin at final concentrations of 0.06 and 0.48 μg/ml, the levels of cell lysis measured were 20.60 ± 0.90 and 37.45 ± 1.50%, respectively. When the incubation time was extended to 18 h with colistin at 0.06 and 0.12 μg/ml, the levels of cell lysis measured were about 23.9 ± 0.61 and 34.54 ± 0.50%, showing that the activity is time and dose dependent. In the absence of colistin, lysis of E. coli O157:H7 was about 17.00 ± 0.82%.
Fig 2.
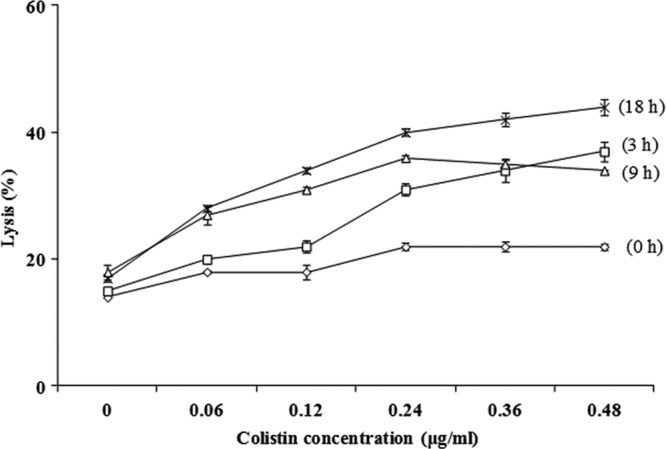
Lysis of E. coli O157:H7 in the presence of colistin at concentration of 0.06, 0.12, 0.24, 0.36, and 0.48 μg/ml for 0 h (♢), 3 h (□), 9 h (△), and 18 h (×) at 30°C. Error bars represent standard deviations calculated from duplicate experiments.
Genotoxicity of colistin.
The typical fluorescence microscopy images (comets) of human PBLs are depicted in Fig. 3A, while those exposed to 1,100 ng/ml of colistin are depicted in Fig. 3B. The comets resulting from the damaged cells (Fig. 3B) have a distinct head with a tail, in contrast to the negative control (Fig. 3A). The mean tail DNA percentage, tail extent, and tail olive moment of PBLs exposed to colistin at final concentrations of about 550, 1,100, and 4,400 ng/ml for 1 h at 37°C are shown in Table 2. The negative control was untreated cells, while the positive control was PBLs treated with MMS. At concentrations of 550, 1,100, and 4,400 ng/ml, colistin was able to induce, in a dose-dependent fashion, tail extensions of about 69.29, 71.44, and 78.24%, respectively, compared to the negative control (Table 2). The comets exposed to colistin concentrations of <550 ng/ml were unaffected, as no modification in the tail was observed.
Fig 3.
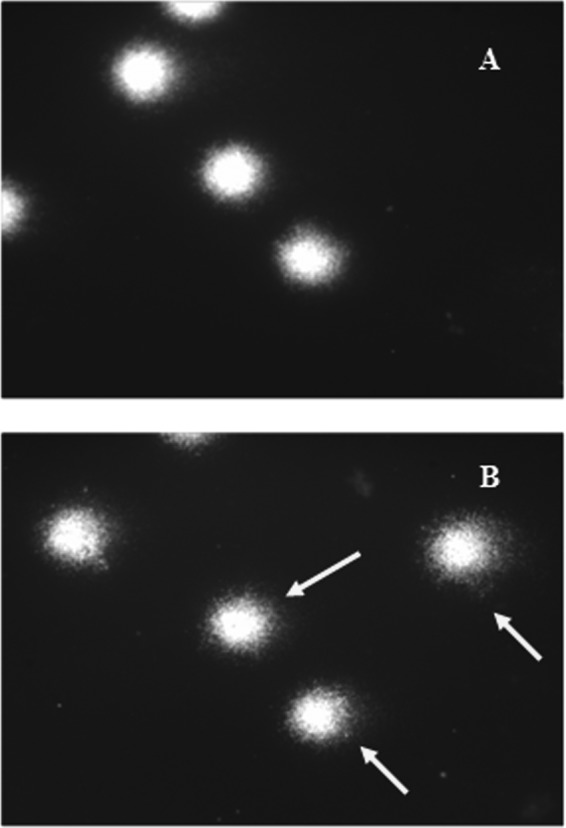
Typical fluorescence microscopy images (comets) of human PBLs left unexposed (A) or exposed to colistin at 1,100 ng/ml (B). The arrows show comet tails indicating DNA damage of leukocytes after treatment with colistin.
Table 2.
Colistin antigenotoxic effects
| Treatment and concn (ng/ml)a | Tail DNA (%)b | Tail extent (μm)c | Tail olive moment (μm)d | RDIe |
|---|---|---|---|---|
| Negative control | 34.54 ± 1.05f | 58.08 ± 6.45 | 1,098.20 ± 18.01 | 0.00 |
| Positive control | 66.38 ± 4.49 | 137.4 ± 29.32 | 3,521.06 ± 490.69 | 1.00 |
| Colistin | ||||
| 550 | 39.08 ± 1.36 | 69.29 ± 0.08 | 1,289.91 ± 53.01 | 0.13 |
| 1,100 | 40.07 ± 5.99 | 71.44 ± 3.45 | 1,351.49 ± 33.52 | 0.17 |
| 4,400 | 45.46 ± 3.96 | 78.24 ± 4.22 | 1,600.21 ± 266.63 | 0.26 |
Each peptide was used at 2 ng/ml.
Tail DNA (%) = 100 − Head DNA (%).
The tail extent is the distance of DNA migration from the body of the nuclear core, and it is used to evaluate the extent of DNA damage.
Olive tail moment = (Tail mean − Head mean) × Tail % DNA/100.
RDI = (mean tail length of treated cells − mean tail length of negative control)/(mean tail length of positive control − mean tail length of negative control). MMS was used as a positive control, and untreated cells were used as a negative control.
Mean value ± standard derivation for 100 randomly selected cells (average ± standard deviation, n = 100).
Hemolytic activity of colistin.
The effects of colistin and nisin alone and in combination on fresh human RBC cultures were studied (Table 3). Under the experimental conditions used, the hemolytic activity was either nonexistent or insignificant (<2%). When the concentration of the antimicrobials was about 4 mg/ml, the hemolytic activities reached 10.66 ± 0.47 and 6.58 ± 1.87%, respectively (data not shown).
Table 3.
AMP hemolytic activities
| AMP, concn (μg/ml) | Mean hemolytic activity (%) ± SD |
|---|---|
| Colistin | |
| 0.01 | 0 |
| 0.03 | 0.44 ± 0.09 |
| 0.12 | 1.83 ± 0.14 |
| Nisin, 1.17 | 0.71 ± 0.07 |
| Pediocin, 1.56 | 0.96 ± 0.14 |
| Colistin-pediocin, 0.03/1.56 | 1.41 ± 0.21 |
| Colistin-nisin, 0.01/1.17 | 0.86 ± 0.09 |
Toxicity of colistin for Vero cells.
Vero cells were incubated in the presence of colistin at concentrations ranging from 0.001 to 1 mg/ml and in the presence of nisin at concentrations ranging from 0.001 to 2 mg/ml. While colistin was toxic at 1 mg/ml (Fig. 4A), nisin did not show any toxicity up to 2 mg/ml (Fig. 4B). Mixtures containing colistin at 1 mg/ml and nisin at different concentrations (0.02 to 2 mg/ml) were prepared, added to Vero cell cultures, and incubated for 24 h at 37°C. The cell layers were examined under an inverted microscope. Under these experimental conditions, nisin at concentrations of 1.5 and 2 mg/ml was able to eliminate the toxicity attributed to colistin (Fig. 4C).
Fig 4.
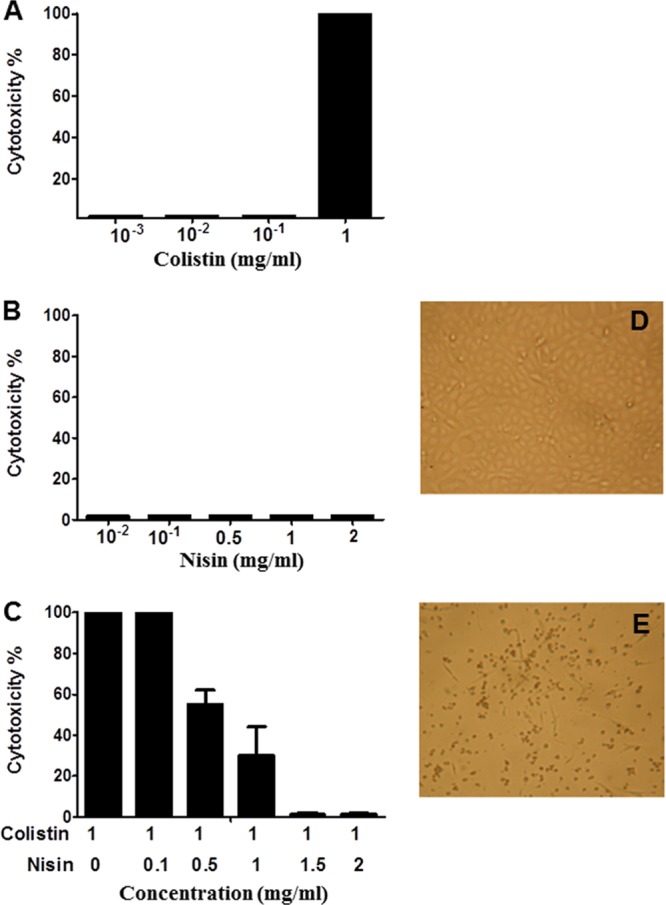
Effects of colistin and nisin on Vero cell cultures. (A) Vero cells cultured in the presence of colistin. (B) Vero cells cultured in the presence of nisin. (C) Vero cells exposed to colistin at 1 mg/ml mixed with nisin at various concentrations. (D and E) Vero cells cultured in the absence or presence of colistin at 1 mg/ml, respectively (magnification, ×48).
DISCUSSION
The antimicrobial compounds used in this work were the antibiotic colistin and two bacteriocins, nisin A and pediocin PA-1/AcH. Bacteriocins are ribosomally synthesized AMPs produced by Gram-positive bacteria and GNB, as well as some archaea (19, 20, 21, 22). Nisin has been used by the food industry for decades as a food preservative. There are four variants of nisin (A, Z, Q, and U). Studies making medical claims for nisin have been published (23, 24, 25). Pediocin PA-1/AcH has been sufficiently characterized to facilitate its application as a food additive, but its clinical potential is basically unexplored. The rapid increase in bacterial resistance to traditional antibiotics has become a major public health problem in the world. Industries, regulatory agencies, and politicians have called for a concerted research effort to tackle this alarming situation. Recently, a panel of internationally recognized experts in the field of antibiotics endorsed a global call to action for a strong and coordinated international program to overcome the paucity of antibiotics (26); this call underscores the seriousness of the situation. Currently, it is believed that only very few new antibiotics are in various developmental stages for the treatment of infections with Gram-negative bacilli, whereas the situation regarding Gram-positive cocci is slightly better, as some potent and novel antibiotics have been made available in recent years. The reintroduction of colistin into the therapeutic circuit has led to numerous publications espousing the merits of this old class of cationic, cyclic polypeptide antibiotic. The combination of colistin with other antimicrobials such as fosfomycin and tigecycline (8), teicoplanin (27), and televancin (28) produced synergistic effects against various pathogens. The in vitro combination of colistin with nisin and/or pediocin PA-1/AcH in the present study revealed synergistic effects against GNB. Colistin and nisin are known to have different targets on bacterial cells, and a hypothesis to explain this synergism let us to think that colistin acts first on the bacterial cell and then nisin reaches other targets. The IA of colistin against the aforementioned GNB was observed in a checkerboard fashion (fractional inhibitory concentration index [FICI]) (Table 1) or in time-kill assays (Fig. 2), and the E. coli O157:H7 strain was the most sensitive to colistin. In our study, the FICI values resulting from the combination of colistin and nisin A or colistin and pediocin PA-1/AcH were under 0.50 (<0.5), pointing out a very significant in vitro synergy. For E. coli O157:H7, the MIC of colistin-pediocin PA-1/AcH decreased from 0.12 to 0.03 μg/ml and that of colistin-nisin decreased from 0.12 to 0.01 μg/ml. This finding suggests that bacteriocins have potential for use in the treatment of GNB infections. Remarkably, the QC conducted in this work led to acceptable MICs, arguing for the acceptability of our MICs. In addition to reducing the amount of colistin needed, this combination permitted us to re-evaluate the cytotoxicity of colistin. Concerning the reduction of toxicity, nisin is likely to impede the access of colistin to target points of Vero cells or these two molecules form dimers and therefore the amount of colistin available is not enough to have any toxic effect.
We recently reported the importance of colistin and bacteriocins in the neutralization of pathogenic bacteria (13). Our contribution to this field consists of putting forward bacteriocins as anti-infective or at least “adjuvant” to the inhibition of pathogenic bacteria. This strategy, if validated, could slow down the massive use of antibiotics that is responsible for spreading resistance in the environment. The toxicity of colistin was the reason why clinicians withdrew this drug. Therefore, it is important, from our point of view, to investigate novel approaches to re-evaluate this toxicity if the drug is to be restored to its original status as a viable option for the treatment of GNB infections. We designed two independent experiments aiming to unravel the hemolytic effect of colistin and to find out more about the genotoxicity of this molecule with the comet assay. The hemolytic activity of colistin and bacteriocins was measured with fresh human RBC cultures. Our data point to a complete absence or a low level (<0.2%) of colistin-induced hemolytic activity. This result was not entirely unexpected because polymyxin has been shown to be a nonhemolytic peptide (29), while bacteriocins have long been known to be safe, as recently outlined in detail (30, 31).
The genotoxicity analysis with the comet assay indicated that colistin causes DNA damage at a concentration of 550 ng/ml (Table 1 and Fig. 4). The comet assay has been used to analyze DNA breakage in mammalian cells (15, 32) and is recognized as a research tool in the carcinogenesis, genotoxicity, radiotherapy, and environmental fields (33, 34, 35, 36, 37). Here, the cytotoxicity of colistin was extended to mammalian cells, specifically, Vero cells, which are a lineage isolated from monkey kidney epithelial cells. Under the experimental conditions used, we observed that colistin at 1 mg/ml was clearly toxic to Vero cells. The formulation based on the use of colistin at 1 mg/ml and nisin at 1.50 or 2.00 mg/ml eliminated the observed toxic effect of colistin and is a noteworthy advance in this field. Overall, the peptides used are not hemolytic, yet colistin is cytotoxic and its cytotoxicity can be reduced by adding nisin at ≥1 mg/ml.
This is the first report showing that a combination of colistin and a bacteriocin (nisin) has a synergistic effect against GNB and offers a possibility to eliminate the toxicity of this drug, as evidenced by the experiments carried out with mammalian cells. However, the full potential of the present findings can be realized only after pharmacokinetic-pharmacodynamic studies confirm the optimal therapeutic doses of colistin-bacteriocin combinations.
ACKNOWLEDGMENT
This research was supported by a grant from Best Environmental Technologies Inc., Edmonton, AB, Canada.
Footnotes
Published ahead of print 9 April 2013
REFERENCES
- 1. Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40:1333–1341 [DOI] [PubMed] [Google Scholar]
- 2. Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A, Forrest A, Bulitta JB, Tsuji BT. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin. Microbiol. Rev. 21:449–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaye KM, Kaye D. 2005. Polymyxins (polymyxin B and colistin), p 435–436 In Mandell GL, Bennett JE, Dolin R. (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 6th ed Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 5. Lopes J, Inniss WE. 1969. Electron microscopy of effect of polymyxin on Escherichia coli lipopolysaccharide. J. Bacteriol. 100:1128–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin NI, Moake MM, Churey JJ, Whittal R, Worobo RW, Vederas JC. 2003. Isolation, structural characterization, and properties of mattacin (polymyxin M), a cyclic peptide antibiotic produced by Paenibacillus kobensis M. J. Biol. Chem. 278:13124–13132 [DOI] [PubMed] [Google Scholar]
- 7. Pitout JD, Thomson KS, Hanson ND, Ehrhardt AF, Moland ES, Sanders CC. 1998. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 42:1350–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corvec S, Furustrand Tafin U, Betrisey B, Borens O, Trampuz A. 2013. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum β-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob. Agents Chemother. 57:1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 10. Wolf CE, Gibbons WR. 1996. Improved method for quantification of the bacteriocin nisin. J. Appl. Bacteriol. 80:453–457 [DOI] [PubMed] [Google Scholar]
- 11. Naghmouchi K, Drider D, Baah J, Teather R. 2010. Nisin A and polymyxin B as synergistic inhibitors of Gram-positive and Gram-negative bacteria. Probiotics Antimicrob. Protiens 2:98–103 [DOI] [PubMed] [Google Scholar]
- 12. Clinical and Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—seventh edition (M7-A7). Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 13. Naghmouchi K, Belguesmia Y, Baah J, Teather R, Drider D. 2011. Antibacterial activity of class I and IIa bacteriocins combined with polymyxin E against resistant variants of Listeria monocytogenes and Escherichia coli. Res. Microbiol. 162:99–107 [DOI] [PubMed] [Google Scholar]
- 14. Jaju M, Ahuja YR. 1983. Cytogenetic effect of colistin on human lymphocytes in vitro: chromosome aberrations, sister chromatid exchanges, mitotic index, cell cycle kinetics, and acrocentric associations. Teratog. Carcinog. Mutagen. 3:515–526 [DOI] [PubMed] [Google Scholar]
- 15. Singh NP, McCoy MT, Tice RR, Schneider EL. 1988. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175:184–191 [DOI] [PubMed] [Google Scholar]
- 16. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35:206–221 [DOI] [PubMed] [Google Scholar]
- 17. Malagoli M. 2007. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebr. Surviv. J. 4:92–94 [Google Scholar]
- 18. Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement (M100-S22). Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 19. Drider D, Rebuffat S. 2011. Prokaryotic antimicrobial peptides: from genes to applications. Springer, New York, NY [Google Scholar]
- 20. Leikoski N, Fewer DP, Sivonen KK. 2009. Widespread occurrence and lateral transfer of the cyanobactin biosynthesis gene cluster in cyanobacteria. Appl. Environ. Microbiol. 75:853–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leikoski N, Fewer DP, Jokela J, Alakoski P, Wahlsten M, Sivonen K. 2012. Analysis of an inactive cyanobactin biosynthetic gene cluster leads to discovery of new natural products from strains of the genus Microcystis. PLoS One 7:e43002 doi:10.1371/journal.pone.0043002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang H, Fewer DP, Sivonen KK. 2011. Genome mining demonstrates the widespread occurrence of gene clusters encoding bacteriocins in cyanobacteria. PLoS One 6:e22384 doi:10.1371/journal.pone.0022384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldstein BP, Wei J, Greenberg K, Novick R. 1998. Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J. Antimicrob. Chemother. 42:277–278 [PubMed] [Google Scholar]
- 24. Giacometti A, Cirioni O, Barchiesi F, Scalise G. 2000. In-vitro activity and killing effect of polycationic peptides on methicillin-resistant Staphylococcus aureus and interactions with clinically used antibiotics. Diagn. Microbiol. Infect. Dis. 38:115–118 [DOI] [PubMed] [Google Scholar]
- 25. Bartoloni A, Mantella A, Goldstein BP, Dei R, Benedetti M, Sbaragli S, Paradisi F. 2004. In-vitro activity of nisin against clinical isolates of Clostridium difficile. J. Chemother. 16:119–121 [DOI] [PubMed] [Google Scholar]
- 26. Carlet J, Jarlier V, Harbarth S, Voss A, Goossens H, Pittet D. 2012. Ready for a world without antibiotics? The pensières antibiotic resistance call to action. Antimicrob. Resist. Infect. Control 14:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wareham DW, Gordon NC, Hornsey M. 2011. In vitro activity of teicoplanin combined with colistin versus multidrug-resistant strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 66:1047–1051 [DOI] [PubMed] [Google Scholar]
- 28. Hornsey M, Longshaw CC, Phee L, Wareham DW. 2012. In vitro activity of telavancin in combination with colistin versus Gram-negative bacterial pathogens. Antimicrob. Agents Chemother. 56:3080–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ried C, Wahl C, Miethke T, Wellnhofer G, Landgraf C, Schneider-Mergener J, Hoess A. 1996. High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-lipopolysaccharide factor. J. Biol. Chem. 271:28120–28127 [DOI] [PubMed] [Google Scholar]
- 30. Belguesmia Y, Madi A, Sperandio D, Merieau A, Feuilloley M, Prévost H, Drider D, Connil N. 2011. Growing insights into the safety of bacteriocins: the case of enterocin S37. Res. Microbiol. 162:159–163 [DOI] [PubMed] [Google Scholar]
- 31. Paiva AD, Oliveira MD, de Paula SO, Baracat-Pereira MC, Breukink E, Mantovani HC. 2012. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol on bacteriocin activity. Microbiology 158:2851–2858 [DOI] [PubMed] [Google Scholar]
- 32. Ostling D, Johanson KJ. 1984. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 123:291–298 [DOI] [PubMed] [Google Scholar]
- 33. Hartmann A, Speit GG. 1995. Genotoxic effect of chemicals in the single cell gel (SCG) test with human blood cells in relation to the induction of sister chromatid exchanges. Mutat. Res. 346:49–56 [DOI] [PubMed] [Google Scholar]
- 34. Collins AR, Duthie SJ, Dobson VL. 1993. Direct enzyme detection of endogenous oxidative base damage in human lymphocytic DNA. Carcinogenesis 14:1733–1735 [DOI] [PubMed] [Google Scholar]
- 35. Ostling D, Johanson KJ. 1987. Bleomycin, in contrast to gamma irradiation, induces extreme variation of DNA strand breakage from cell to cell. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 52:683–691 [DOI] [PubMed] [Google Scholar]
- 36. McKelvey-Martin VJ, Green MHL, Schmezer P, Pool-Zobel BL, De Meo MP, Collins A. 1993. The single cell gel electrophoresis assay (comet assay): a European review. Mutat. Res. 288:47–63 [DOI] [PubMed] [Google Scholar]
- 37. Fairbairn DW, Olive PL, O'Neill KL. 1995. The comet assay: a comprehensive review. Mutat. Res. 339:37–59 [DOI] [PubMed] [Google Scholar]