Abstract
Beta-lactams enhance the killing activity of vancomycin. Due to structural and mechanistic similarities between vancomycin and telavancin, we investigated the activity of telavancin combined with nafcillin and imipenem compared to the known synergistic combination of telavancin and gentamicin. Thirty strains of Staphylococcus aureus, 10 methicillin-susceptible S. aureus (MSSA), 10 methicillin-resistant S. aureus (MRSA), and 10 heterogeneously vancomycin-intermediate S. aureus (hVISA), were tested for synergy by time-kill methodology. Six strains (2 each of MSSA, MRSA, and hVISA) were further evaluated in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model with simulated regimens of 10 mg/kg of body weight of telavancin once daily alone and combined with 2 g nafcillin every 4 h, 500 mg imipenem every 6 h, or 5 mg/kg gentamicin once daily over 72 h. In the synergy test, 67% of strains displayed synergy with the combination of telavancin and gentamicin, 70% with telavancin and nafcillin, and 63% with telavancin and imipenem. In the PK/PD model, the activities of all three combinations against MRSA and hVISA were superior to all individual drugs alone (P ≤ 0.002) and were similar to each other (P ≥ 0.187). The activities of all three combinations against MSSA were generally similar to each other except for one strain where the combination of telavancin and imipenem was superior to all other regimens (P ≤ 0.011). The activity of the combination of telavancin and beta-lactam agents was similar to that of telavancin and gentamicin against S. aureus, including resistant strains. Because beta-lactam combinations are less likely to be nephrotoxic than telavancin plus gentamicin, these beta-lactam combinations may have clinical utility.
INTRODUCTION
Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus (MRSA), remains a major cause of serious infections, with vancomycin continuing as the mainstay of therapy in spite of rising concern over clinical failures of this agent (1–6). In recent years, several novel agents, including telavancin, have been found to be effective against MRSA. Clinically, telavancin has been shown to be effective for the treatment of skin and soft tissue infections as well as hospital-acquired pneumonia (7, 8).
The use of combination antimicrobial therapy is a common occurrence. Multiple guidelines from the Infectious Diseases Society of America (IDSA) advocate for the use of a myriad of combinations antimicrobial therapies for different purposes (9–12). The most commonly utilized agent for synergy against S. aureus is gentamicin. In vitro synergy of gentamicin in combination with many antistaphylococcal agents, including beta-lactams, vancomycin, daptomycin, and telavancin, has been described, usually with positive results (13–16). Gentamicin combinations have even been used in a major clinical study (17). However, gentamicin is not a completely innocuous drug. Along with all aminoglycosides, it comes with serious potential toxicity and risk to the patient, the most common and concerning of which is nephrotoxicity. Previous studies have shown that this toxicity occurs more frequently when gentamicin is given in combination with other, moderately nephrotoxic drugs like vancomycin (18, 19). Indeed, clinical data bear out the fact that initial low-dose gentamicin used for synergy against S. aureus is nephrotoxic; thus, recent IDSA guidelines on the treatment of MRSA generally recommend against the use of gentamicin (12, 20). Telavancin has also been found to be a moderately nephrotoxic drug, showing more renal toxicity than vancomycin in clinical studies (7, 8). This raises serious questions about the safety of the clinical combination of telavancin with gentamicin. For this reason, finding a synergistic combination that has comparable antimicrobial efficacy, but less risk of toxicity, could have clinical value.
One such potential combination is giving telavancin with a beta-lactam agent. Several previous investigations have found synergy between beta-lactams and anti-MRSA agents, including vancomycin, daptomycin, and telavancin, against MRSA (13, 21–23). Beta-lactam drugs are also generally quite safe, with very few side effects, in contrast with other agents such as aminoglycosides. Nafcillin would be an ideal agent for this purpose: it is currently widely used for methicillin-susceptible S. aureus (MSSA) infections, clinicians are comfortable with it, it has a relatively narrow spectrum of activity, it does not require extensive monitoring (in contrast to gentamicin), and it has an excellent safety profile. In addition, telavancin is likely to be used with imipenem or other carbapenems for empirical treatment of hospital-acquired pneumonia; thus, information about the utility of this combination against S. aureus would also be valuable. The objective of this investigation was to characterize the in vitro activity, using time-kill synergy studies and a pharmacokinetic/pharmacodynamic (PK/PD) modeling system, of telavancin combined with nafcillin as well as telavancin combined with imipenem compared to the known synergistic combination of telavancin plus gentamicin.
(A portion of this work was presented at the 22nd European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], London, United Kingdom, 2012 [24], and the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], San Francisco, CA, 2012 [25].)
MATERIALS AND METHODS
Bacterial strains.
Thirty strains of S. aureus, all bloodstream isolates, were used in this investigation for susceptibility and synergy testing: 10 clinical isolates of methicillin-susceptible S. aureus (MSSA) (obtained from Brigham and Women's Hospital, Boston, MA), 10 clinical isolates of methicillin-resistant S. aureus (MRSA) (obtained from Brigham and Women's Hospital, Boston, MA), and 10 clinical strains of heterogeneously vancomycin-intermediate S. aureus (hVISA) (already proven positive by population analysis area under the curve ratio, provided by the Anti-Infective Research Laboratory, Detroit, MI) (26). Six strains (2 MSSA, 2 MRSA, and 2 hVISA) were further evaluated in an in vitro pharmacokinetic/pharmacodynamic model.
Antimicrobial agents.
Telavancin was provided by the manufacturer (Theravance, Inc., South San Francisco, CA). Nafcillin, gentamicin (Sigma-Aldrich, St. Louis, MO), and imipenem (Fisher Scientific, Pittsburgh, PA) were purchased commercially.
Media.
Mueller-Hinton broth (Difco, Detroit, MI) supplemented with 25 mg/liter calcium, 12.5 mg/liter magnesium, and 2% sodium chloride (due to the presence of nafcillin and according to Clinical and Laboratory Standards Institute [CLSI] recommendations) (SMHB) was used for all susceptibility testing, time kills, and PK/PD models (27). Colony counts were determined by using tryptic soy agar (TSA; Difco, Detroit, MI). Mueller-Hinton agar (MHA; Difco, Detroit, MI) was used to test for the emergence of resistance.
Susceptibility testing.
MICs of study antimicrobial agents were determined by broth microdilution at an inoculum of 5 × 105 CFU/ml in SMHB as described above, according to CLSI guidelines, by utilizing dry panels provided by Astellas Pharma (27).
Synergy testing.
The potential for synergy with vancomycin plus nafcillin was determined by time-kill methods in triplicate at a final inoculum of ∼106 CFU/ml. All time-kill experiments were performed at 1/2× the MIC of the respective antibiotic. Aliquots (0.1 ml) were removed at 0, 4, 8, and 24 h; serially diluted in 0.9% sodium chloride; and plated onto TSA plates with a lower limit of detection of 2 log10 CFU/ml. Time-kill curves were constructed by plotting mean colony counts (log10 CFU/ml) versus time. Synergy was defined as a ≥2-log10 CFU/ml increase in killing at 24 h with the combination, in comparison with the killing by the most active single drug. Combinations that resulted in ≥1-log10 bacterial growth in comparison to the least active single agent were considered to represent antagonism. All combinations not meeting the definition of synergism or antagonism were considered indifferent. All samples were incubated at 37°C for 24 h.
In vitro PK/PD infection model.
Six strains of S. aureus, 2 MSSA, 2 MRSA, and 2 hVISA, were chosen to be run in an in vitro PK/PD model consisting of a 125-ml one-compartment glass apparatus with ports for the addition and removal of media, antibiotics, and samples. The model was placed into a water bath at 37°C throughout the simulation, with a magnetic stir bar for mixing. Fresh medium was continuously supplied and removed via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL, USA) set to simulate the half-lives of the antibiotics. A starting inoculum of ∼107 CFU/ml was used for all simulations. This higher inoculum was chosen because hVISA requires a high inoculum to observe the heterogeneous phenotype and to provide more rigorous experimental conditions for the beta-lactam agents, both of which are subject to an inoculum effect on their activity (16, 28). Bolus dosing of free drug concentrations was used to simulate regimens of 10 mg/kg of body weight telavancin every 24 h (targets were a maximum concentration of free, unbound drug in serum [fCmax] of 10.8 μg/ml and a half-life of 8 h) (29), 2 g nafcillin every 4 h (targets were an fCmax of 5.2 μg/ml and a half-life of 1 h) (30, 31), 500 mg imipenem every 6 h (targets were an fCmax of 38.4 μg/ml and a half-life of 1 h) (32), 5 mg/kg gentamicin every 24 h (targets were an fCmax of 13.5 μg/ml and a half-life of 3 h), 10 mg/kg telavancin every 24 h plus 2 g nafcillin every 4 h, 10 mg/kg telavancin every 24 h plus 500 mg imipenem every 6 h, and 10 mg/kg telavancin every 24 h plus 5 mg/kg gentamicin every 24 h. The doses of telavancin and nafcillin are the standard doses used to treat serious staphylococcal infections. The gentamicin dose is a high-peak, extended-interval dose used to maximize the pharmacodynamics of the agent (15). The dose of imipenem is the dose recommended by the Infectious Diseases Society of America for the treatment of nosocomial pneumonia (9). Model simulations involving two drugs with different half-lives were performed by using a previously validated method (33). All models were done in duplicate to ensure reproducibility.
Pharmacodynamic analysis.
Samples (approximately 1 ml each) were drawn from each model at 0, 1, 2, 4, 8, 24, 28, 32, 48, 56, and 72 h and serially diluted in 0.9% sodium chloride. Twenty-microliter spots were then plated onto TSA plates in triplicate for quantification with a lower limit of detection of 2 log10 CFU/ml. Antibiotic carryover was accounted for by using serial dilutions. The total reduction in log10 CFU/ml was determined by plotting time-kill curves of the number of remaining organisms over the 72-h time period.
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained over 72 h for verification of target antibiotic concentrations. Telavancin and nafcillin concentrations were measured by a bioassay using Kocuria rhizophila (formerly Micrococcus luteus) ATCC 9341, as previously described (14, 16). Gentamicin concentrations were measured by using Staphylococcus epidermidis ATCC 27626, as previously described (34). Concentrations of imipenem were measured by a bioassay utilizing Bacillus subtilis ATCC 6633 (35). Due to limitations with this method, only models utilizing a single agent could have pharmacokinetics verified, while combination models were done by using a verified method, as described above (33). Intraday coefficients of variation for all drugs at high, medium, and low standards were ≤11.3% for all bioassays performed. The elimination half-lives (t1/2), areas under the curve (AUCs), peaks (fCmax), and troughs (fCmin) were determined by using WinNonlin PK/PD modeling software (Pharsight, Cary, NC).
Resistance.
Development of resistance was evaluated at multiple time points throughout the 72-h simulations. One-hundred-microliter samples from each time point of simulations using telavancin were plated onto Mueller-Hinton agar plates containing 3× the MIC of telavancin to assess the development of resistance. Plates were then examined for growth after 48 h of incubation at 37°C. The MIC for observed growth was measured by broth microdilution. In addition, growth from quantification plates at 24, 48, and 72 h was subjected to MIC testing by broth microdilution for all simulations using the respective antimicrobial or antimicrobials being used in the particular experiment.
Statistical analysis.
Overall activities of regimens over the 72-h period were compared by calculating the area under the bacterial kill curve (AUBKC) for each regimen by using SigmaPlot software (version 11.1; Systat Software, Inc., San Jose, CA). The AUCs were then compared by using analysis of variance (ANOVA) with Tukey's post hoc test. All statistical comparisons were done with IBM SPSS Statistics (version 19.0; SPSS, Inc., Chicago, IL). A P value of ≤0.05 was considered significant.
RESULTS
In the time-kill studies, 67% (20/30) of strains displayed synergy with telavancin combined with gentamicin, 70% (21/30) with telavancin combined with nafcillin, and 63% (19/30) with telavancin combined with imipenem. For beta-lactam combinations, the percentage displaying synergy was higher against strains resistant to beta-lactams (MRSA and hVISA), with 80% (16/20) of strains showing synergy with telavancin plus nafcillin, versus 50% (5/10) against MSSA, and with 85% (17/20) of strains showing synergy with telavancin combined with imipenem, versus 20% (2/10) against MSSA. This pattern was not observed with the combination of telavancin and gentamicin, with 65% (13/20) of strains displaying synergy against strains resistant to beta-lactams (MRSA and hVISA) and 70% displaying synergy against MSSA (7/10). All remaining combinations displayed indifference. Susceptibility results for all 30 strains are displayed in Table 1.
Table 1.
Full susceptibility data for 30 S. aureus strains testeda
| Drug | MSSA (n = 10) |
MRSA and hVISA (n = 20) |
||||
|---|---|---|---|---|---|---|
| MIC50 (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | MIC range (μg/ml) | |
| TLV | 0.5 | 0.5 | 0.25–0.5 | 0.5 | 0.5 | 0.25–0.5 |
| VAN | 1 | 2 | 1–2 | 1 | 2 | 1–2 |
| NAF | 0.5 | 1 | 0.25–1 | 64 | 256 | 8–256 |
| IMP | 0.03 | 0.06 | 0.015–0.25 | 16 | 64 | 0.25–128 |
| DAP | 0.25 | 0.5 | 0.25–0.5 | 0.5 | 0.5 | 0.25–1 |
| LZD | 2 | 2 | 1–2 | 2 | 2 | 1–4 |
| Q/D | 0.5 | 1 | 0.25–1 | 1 | 1 | 0.25–1 |
| CLI | ≤0.5 | 1 | ≤0.5–≥8 | ≥8 | ≥8 | ≤0.5–≥8 |
| CIP | 0.25 | 4 | 0.125–≥16 | ≥16 | ≥16 | 0.25–≥16 |
| TGC | 0.125 | 0.25 | 0.125–0.25 | 0.25 | 0.5 | 0.06–2 |
| ERY | 0.5 | ≥32 | 0.25–≥32 | ≥32 | ≥32 | 0.5–≥32 |
| GEN | 0.5 | 1 | 0.25–1 | 0.5 | 16 | 0.125–16 |
| SXT | ≤0.5/9.5 | ≤0.5/9.5 | ≤0.5/9.5 | ≤0.5/9.5 | 1/19 | ≤0.5/9.5–≥8/152 |
TLV, telavancin; VAN, vancomycin; TEI, teicoplanin; NAF, nafcillin; IMP, imipenem; DAP, daptomycin; LZD, linezolid; Q/D, quinupristin-dalfopristin; CLI, clindamycin; CIP, ciprofloxacin; TGC, tigecycline; ERY, erythromycin; GEN, gentamicin; SXT, trimethoprim-sulfamethoxazole.
In the PK/PD model, the telavancin and nafcillin doses used are the standard doses used to treat serious staphylococcal infections. The gentamicin dose is a high-peak, extended-interval dose used to maximize the pharmacodynamics of the drug, and the dose of imipenem is a standard dose used to treat serious infections (9, 15). Two strains from each group (MSSA, MRSA, and hVISA) were selected to be run in the PK/PD model. The MSSA strains were randomly selected from the cohort of 10 strains. The MRSA and hVISA strains were selected to represent a range of beta-lactam susceptibilities, focusing particularly on those strains that were less susceptible. This decision was made to determine if the beta-lactam combinations were effective even when the MIC of the particular drug was very high. Susceptibility and synergy testing results for strains used in the PK/PD model are displayed in Table 2. Pharmacokinetic values from the PK/PD models were within 10% of the targets. The observed free peak (fCmax) and half-life (t1/2) values were 9.8 ± 0.4 μg/ml and 8.7 ± 0.2 h for telavancin, 5.1 ± 0.5 μg/ml and 1.1 ± 0.3 h for nafcillin, 14.7 ± 1.1 μg/ml and 3.2 ± 0.4 h for gentamicin, and 40.2 ± 4.5 μg/ml and 0.9 ± 0.3 h for imipenem, respectively.
Table 2.
Susceptibility and synergy testing results for strains used in the PK/PD modela
| Strain | Susceptibility (μg/ml) |
Synergy testing result |
|||||
|---|---|---|---|---|---|---|---|
| TLV | GEN | NAF | IMP | TLV + GEN | TLV + NAF | TLV + IMP | |
| MSSA SNL 4 | 0.5 | 0.5 | 0.5 | 0.25 | Synergy | Indifferent | Indifferent |
| MSSA SNL 9 | 0.5 | 0.5 | 0.5 | 0.015 | Synergy | Synergy | Synergy |
| MRSA SNL 96 | 0.25 | 0.25 | 32 | 32 | Synergy | Synergy | Synergy |
| MRSA SNL 98 | 0.25 | 0.5 | 128 | 64 | Indifferent | Synergy | Synergy |
| hVISA R2729 | 0.5 | 0.25 | 128 | 64 | Synergy | Synergy | Synergy |
| hVISA R3003 | 0.5 | 16 | 256 | 2 | Synergy | Synergy | Indifferent |
Susceptibility results are displayed only for the drugs used in the PK/PD model. Synergy results are derived from the time-kill methodology described in the text. TLV, telavancin; GEN, gentamicin; NAF, nafcillin; IMP, imipenem.
The activities of all three combination regimens against both MRSA and both hVISA strains were superior to those of all individual drugs alone (P ≤ 0.002 for all comparisons) and were all similar to each other (P ≥ 0.187 for all comparisons). As expected, telavancin alone was the only regimen that did not display regrowth over 72 h against MRSA and hVISA, and it was indeed superior to all other individual drugs alone (P ≤ 0.001 for all comparisons). AUBKC values from PK/PD model experiments are displayed in Table 3, and one example graph for MSSA, MRSA, and hVISA is displayed in Fig. 1. No changes in MIC were detected for any MRSA and hVISA strains over the course of the experiment.
Table 3.
Area under the bacterial kill curve values from the PK/PD model
| Strain | Mean area (logCFU · h/ml) under the bacterial kill curve ± SD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Growth control | Telavancin | Gentamicin | Nafcillin | Imipenem | Telavancin + gentamicin | Telavancin + nafcillin | Telavancin + imipenem | |
| MSSA SNL 4 | 610.6 ± 1.3 | 347.6 ± 1.7 | 575.4 ± 9.6 | 288.4 ± 7.8 | 224.9 ± 10.7 | 189 ± 7.4 | 214.4 ± 2.7 | 202.9 ± 9.6 |
| MSSA SNL 9 | 607.0 ± 2.0 | 313.3 ± 10.4 | 575.1 ± 2 | 233.3 ± 1.5 | 225.2 ± 12.3 | 222.2 ± 7.3 | 226.3 ± 12.2 | 165.9 ± 0.1 |
| MRSA SNL 96 | 590.8 ± 0.5 | 312.4 ± 4.2 | 542.9 ± 5.3 | 507.7 ± 8.8 | 418.7 ± 17.3 | 182 ± 15.3 | 204.2 ± 4.2 | 175.4 ± 6.3 |
| MRSA SNL 98 | 568.8 ± 1.7 | 312.4 ± 5.8 | 486.8 ± 4.3 | 515.5 ± 2.3 | 466.6 ± 6.5 | 243.5 ± 12.4 | 229.3 ± 0.6 | 213.8 ± 12.5 |
| hVISA R2729 | 609.6 ± 6.4 | 371.3 ± 14.6 | 561 ± 0.4 | 549.2 ± 4.5 | 507.6 ± 1 | 213.4 ± 6.9 | 210.9 ± 7.5 | 195.6 ± 4.8 |
| hVISA R3003 | 592.1 ± 1.3 | 311.3 ± 17.9 | 589.9 ± 2.3 | 530.1 ± 6.0 | 486.7 ± 3.1 | 209.1 ± 0.5 | 211.4 ± 4.2 | 199.1 ± 4.8 |
Fig 1.
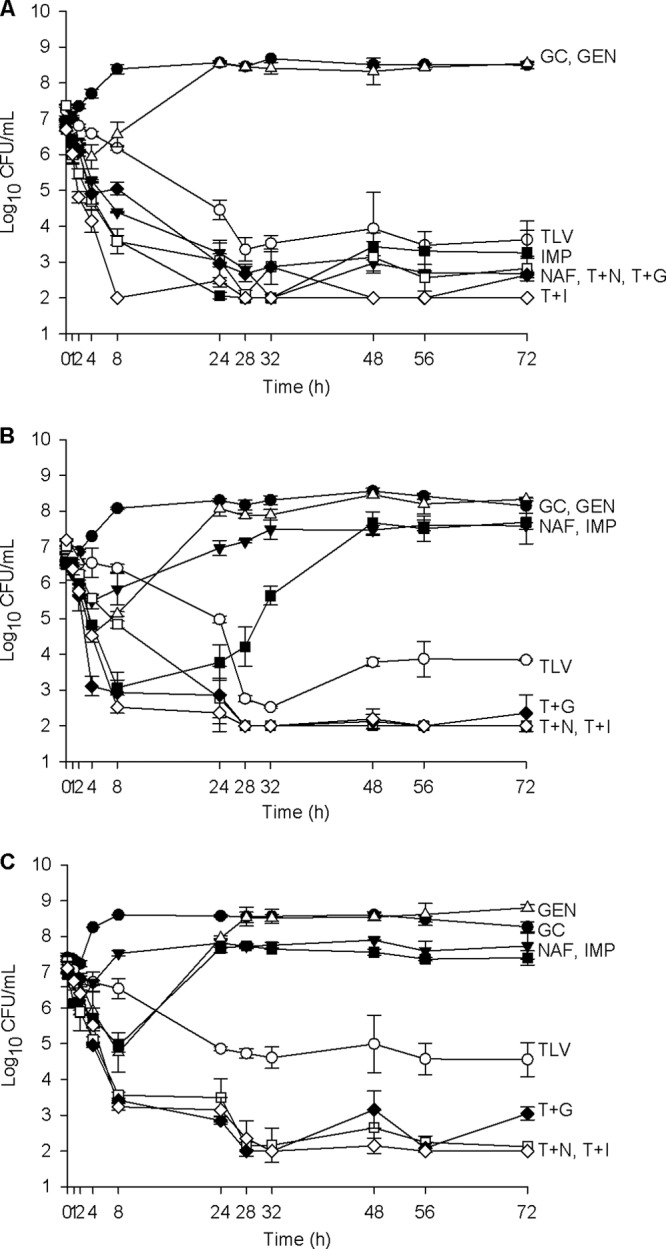
Activities of 10 mg/kg telavancin daily alone and combined with nafcillin, imipenem, and gentamicin against MSSA, MRSA, and hVISA. (A) MSSA SNL 9; (B) MRSA SNL 96; (C) hVISA R2729. Filled circles, growth control (GC) (organism growth with no drug added); open triangles, 5 mg/kg gentamicin (GEN) once daily; open circles, 10 mg/kg telavancin (TLV) once daily; filled inverted triangles, 2 g nafcillin (NAF) every 4 h; filled squares, 500 mg imipenem (IMP) every 6 h; open squares, 10 mg/kg telavancin once daily plus 2 g nafcillin every 4 h (T + N); filled diamonds, 10 mg/kg telavancin once daily plus 5 mg/kg gentamicin once daily (T + G); open diamonds, 10 mg/kg telavancin once daily plus 500 mg imipenem every 6 h (T + I).
Both nafcillin and imipenem were quite active against MSSA, as expected. For one of these strains (SNL 4), all three combinations and imipenem alone were statistically similar (P ≥ 0.092) and were superior to nafcillin (P ≤ 0.004), which was superior to telavancin (P = 0.006). For the other MSSA strain (SNL 9), telavancin combined with imipenem was superior to all regimens (P ≤ 0.011), followed by telavancin combined with gentamicin, telavancin combined with nafcillin, imipenem alone, and nafcillin alone, which were statistically similar to each other (P ≥ 0.958) and superior to telavancin alone (P ≤ 0.001). Gentamicin alone resulted in regrowth of both MSSA strains. No changes in MIC were detected for MSSA.
DISCUSSION
Vancomycin clinical failures represent a potential problem for treatment of staphylococcal infections. Due to these failures, there is a need to find alternative treatments, including novel combinations. Recent studies have demonstrated that the combination of vancomycin and beta-lactam agents improves overall antibacterial activity (21). Due to structural and mechanistic similarities between vancomycin and telavancin, we sought to investigate if the same was true for the combination of telavancin and beta-lactams.
By time-kill analysis, we found that the frequency of synergy between telavancin and both nafcillin and imipenem was similar to that observed between telavancin and gentamicin. The observation of synergy between telavancin and beta-lactams, however, is not new. Lin and colleagues found similarly high rates of synergy with telavancin combinations, particularly the combinations of telavancin and gentamicin (90% synergy), telavancin and ceftriaxone (88%), and telavancin and meropenem (65%) (13). The generally higher rates of synergy which those authors observed may be due to their use of multiple different subinhibitory combinations (4 different combinations of 1/2× and 1/4× MIC), compared to the single one used in this investigation, and their measurement of synergy at 4 different time points (3 h, 6 h, 12 h, and 24 h), compared to just the 24-h time point in this investigation. The notable exception to this is antistaphylococcal penicillin synergy, which was 60% in their investigation with oxacillin, compared to 70% in the present investigation with nafcillin. The reason for this difference is not immediately clear.
The major weakness of synergy data such as these is that, for the most part, they use unrealistic beta-lactam drug concentrations not clinically achievable in humans. In an effort to determine if a similar result could be reproduced with realistic drug concentrations and pharmacokinetics, we ran 6 strains in an in vitro PK/PD model. We found that the activities of all three telavancin combinations against MRSA and hVISA were superior to the activities of all individual agents alone. In addition, both nafcillin and imipenem combined with telavancin produced similar antibacterial activity to that of telavancin combined with gentamicin, even though the concentrations of the beta-lactams were below the MIC of the organism for most or all of the dosing interval. Though perhaps counterintuitive, because the driver of beta-lactam activity is the time during which the drug concentration is above the MIC, the observed enhancement of kill in spite of zero or nearly zero time above the MIC is consistent with previous results with beta-lactams and vancomycin (21).
The relationships to activity against MSSA were not as simple as with MRSA and hVISA. For both strains, all three combinations were superior to telavancin alone and were generally similar to each other, with the exception of one strain in which the combination of telavancin and imipenem was superior to all other regimens. The combinations were also generally similar in overall activity to one or both beta-lactam agents alone. This was largely due to the significantly improved activity of both beta-lactam agents alone, which is expected against MSSA. Similar to both MRSA and both hVISA strains, the beta-lactam combinations were at least as effective, and in one case better than, the combination of telavancin and gentamicin.
Historically, aminoglycosides have been the most commonly used secondary agent for combination therapy for S. aureus infections, but recent data show that this combination's risks, specifically in terms of nephrotoxicity, may outweigh any benefit received (20). Because of these nephrotoxicity risks with aminoglycosides, the finding that beta-lactam agents combined with telavancin are at least as or more bactericidal than telavancin combined with gentamicin, even when the strains are resistant to the beta-lactam agents, is very significant. This is because beta-lactam agents are significantly less likely to result in nephrotoxicity than aminoglycosides and, unlike aminoglycosides, do not seem to show an increase in nephrotoxicity when combined with vancomycin (36). This increase in toxicity when used in combination with vancomycin is concerning due to the possibility of even more renal toxicity when an aminoglycoside is combined with telavancin. A recent meta-analysis of telavancin clinical trials showed that the incidence of nephrotoxicity with telavancin alone is roughly twice that of vancomycin (10% versus 5%), and therefore, finding a less renal-toxic synergistic antimicrobial combination, such as telavancin plus a beta-lactam, could have great benefit (37).
In conclusion, we found that the combinations of telavancin with both nafcillin and imipenem produced generally similar rates of synergy and enhancement of killing in a PK/PD model compared to the known synergistic combination of telavancin and gentamicin. Because these beta-lactam combination regimens should be significantly less nephrotoxic to patients than the combination of telavancin and gentamicin, we believe that these combinations may have clinical utility.
ACKNOWLEDGMENTS
This work was supported by Theravance, Inc., and Astellas Pharma.
S.N.L. has consulted for Astellas Pharma. M.E.S., R.G.G., and M.D.P. have nothing to declare.
Footnotes
Published ahead of print 1 April 2013
REFERENCES
- 1. Lin SH, Liao WH, Lai CC, Liao CH, Tan CK, Wang CY, Huang YT, Hsueh PR. 2010. Risk factors for mortality in patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia in a tertiary care hospital in Taiwan. J. Antimicrob. Chemother. 65:1792–1798 [DOI] [PubMed] [Google Scholar]
- 2. Yoon YK, Kim JY, Park DW, Sohn JW, Kim MJ. 2010. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J. Antimicrob. Chemother. 65:1015–1018 [DOI] [PubMed] [Google Scholar]
- 3. Welsh KJ, Abbott AN, Lewis EM, Gardiner JM, Kruzel MC, Lewis CT, Mohr JF, Wanger A, Armitige LY. 2010. Clinical characteristics, outcomes, and microbiologic features associated with methicillin-resistant Staphylococcus aureus bacteremia in pediatric patients treated with vancomycin. J. Clin. Microbiol. 48:894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bae IG, Federspiel JJ, Miro JM, Woods CW, Park L, Rybak MJ, Rude TH, Bradley S, Bukovski S, de la Maria CG, Kanj SS, Korman TM, Marco F, Murdoch DR, Plesiat P, Rodriguez-Creixems M, Reinbott P, Steed L, Tattevin P, Tripodi MF, Newton KL, Corey GR, Fowler VG., Jr 2009. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J. Infect. Dis. 200:1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maor Y, Hagin M, Belausov N, Keller N, Ben-David D, Rahav G. 2009. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia versus those of methicillin-resistant S. aureus bacteremia. J. Infect. Dis. 199:619–624 [DOI] [PubMed] [Google Scholar]
- 6. Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, Stellrecht K. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, Lentnek A, Ross DP, Fowler VG, Hopkins A, Friedland HD, Barriere SL, Kitt MM, Corey GR. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683–1693 [DOI] [PubMed] [Google Scholar]
- 8. Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, Niederman MS, Kollef MH, Shorr AF, Lee PC, Lentnek AL, Luna CM, Fagon JY, Torres A, Kitt MM, Genter FC, Barriere SL, Friedland HD, Stryjewski ME. 2011. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin. Infect. Dis. 52:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Thoracic Society, Infectious Diseases Society of America 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388–416 [DOI] [PubMed] [Google Scholar]
- 10. Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. 2004. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39:1267–1284 [DOI] [PubMed] [Google Scholar]
- 11. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 52:e56–e93 doi:10.1093/cid/cir073 [DOI] [PubMed] [Google Scholar]
- 12. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak JM, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:e18–e55 doi:10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 13. Lin G, Pankuch GA, Ednie LM, Appelbaum PC. 2010. Antistaphylococcal activities of telavancin tested alone and in combination by time-kill assay. Antimicrob. Agents Chemother. 54:2201–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leonard SN, Vidaillac C, Rybak MJ. 2009. Activity of telavancin against Staphylococcus aureus strains with various vancomycin susceptibilities in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 53:2928–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsuji BT, Rybak MJ. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fowler VG, Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, Levine DP, Chambers HF, Tally FP, Vigliani GA, Cabell CH, Link AS, DeMeyer I, Filler SG, Zervos M, Cook P, Parsonnet J, Bernstein JM, Price CS, Forrest GN, Fatkenheuer G, Gareca M, Rehm SJ, Brodt HR, Tice A, Cosgrove SE. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653–665 [DOI] [PubMed] [Google Scholar]
- 18. Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. 1990. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J. Antimicrob. Chemother. 25:679–687 [DOI] [PubMed] [Google Scholar]
- 19. Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob. Agents Chemother. 43:1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cosgrove SE, Vigliani GA, Fowler VG, Jr, Abrutyn E, Corey GR, Levine DP, Rupp ME, Chambers HF, Karchmer AW, Boucher HW. 2009. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin. Infect. Dis. 48:713–721 [DOI] [PubMed] [Google Scholar]
- 21. Leonard SN. 2012. Synergy between vancomycin and nafcillin against Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. PLoS One 7:e42103 doi:10.1371/journal.pone.0042103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Climo MW, Patron RL, Archer GL. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob. Agents Chemother. 54:3161–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard SN, Gandhi RG, Patel MD.Abstr. 22nd Eur. Congr. Clin. Microbiol. Infect. Dis., abstr P 1889.2012. [Google Scholar]
- 25.Leonard SN, Supple ME.Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother., abstr A-622.2012. [Google Scholar]
- 26. Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sader HS, Jones RN. 2008. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007). J. Clin. Microbiol. 46:2950–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CLSI 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 11th ed CLSI, Wayne, PA [Google Scholar]
- 28. Mizunaga S, Kamiyama T, Fukuda Y, Takahata M, Mitsuyama J. 2005. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 56:91–96 [DOI] [PubMed] [Google Scholar]
- 29. MacGowan AP, Noel AR, Tomaselli S, Elliott HC, Bowker KE. 2011. Pharmacodynamics of telavancin studied in an in vitro pharmacokinetic model of infection. Antimicrob. Agents Chemother. 55:867–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Standiford HC, Jordan MC, Kirby WM. 1970. Clinical pharmacology of carbenicillin compared with other penicillins. J. Infect. Dis. 122(Suppl):S9–S13 [DOI] [PubMed] [Google Scholar]
- 31. Kind AC, Tupasi TE, Standiford HC, Kirby WM. 1970. Mechanisms responsible for plasma levels of nafcillin lower than those of oxacillin. Arch. Intern. Med. 125:685–690 [PubMed] [Google Scholar]
- 32. Jaruratanasirikul S, Raungsri N, Punyo J, Sriwiriyajan S. 2005. Pharmacokinetics of imipenem in healthy volunteers following administration by 2 h or 0.5 h infusion. J. Antimicrob. Chemother. 56:1163–1165 [DOI] [PubMed] [Google Scholar]
- 33. Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl A):125–130 [DOI] [PubMed] [Google Scholar]
- 34. Alcid DV, Seligman SJ. 1973. Simplified assay for gentamicin in the presence of other antibiotics. Antimicrob. Agents Chemother. 3:559–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verpooten GA, Verbist L, Buntinx AP, Entwistle LA, Jones KH, De Broe ME. 1984. The pharmacokinetics of imipenem (thienamycin-formamidine) and the renal dehydropeptidase inhibitor cilastatin sodium in normal subjects and patients with renal failure. Br. J. Clin. Pharmacol. 18:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noel GJ, Bush K, Bagchi P, Ianus J, Strauss RS. 2008. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin. Infect. Dis. 46:647–655 [DOI] [PubMed] [Google Scholar]
- 37. Polyzos KA, Mavros MN, Vardakas KZ, Makris MC, Rafailidis PI, Falagas ME. 2012. Efficacy and safety of telavancin in clinical trials: a systematic review and meta-analysis. PLoS One 7:e41870 doi:10.1371/journal.pone.0041870 [DOI] [PMC free article] [PubMed] [Google Scholar]


