Abstract
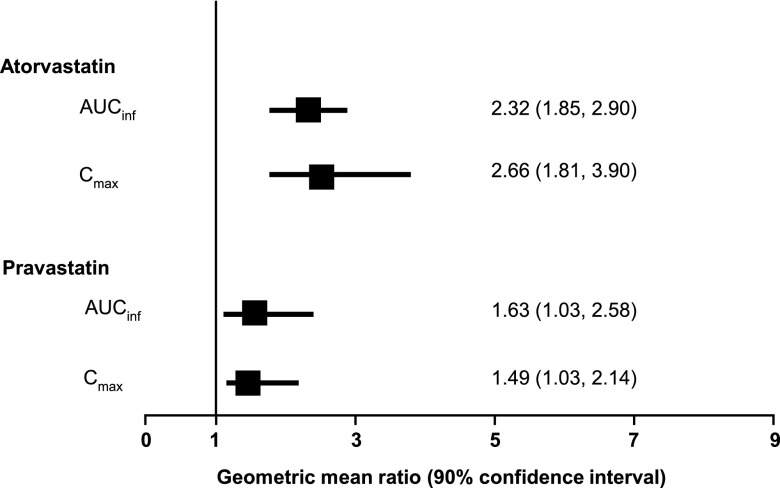
Boceprevir is a potent orally administered inhibitor of hepatitis C virus and a strong, reversible inhibitor of CYP3A4, the primary metabolic pathway for many 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Thus, the aim of the present study was to investigate drug-drug interactions between atorvastatin or pravastatin and boceprevir. We conducted a single-center, open-label, fixed-sequence, one-way-crossover study with 20 healthy adult volunteers. Subjects received single-dose atorvastatin (40 mg) or pravastatin (40 mg) on day 1, followed by boceprevir (800 mg three times daily) for 7 to 10 days. Repeat single doses of atorvastatin or pravastatin were administered in the presence of steady-state boceprevir. Atorvastatin exposure increased in the presence of boceprevir, with atorvastatin area under the concentration-time curve from time zero to infinity after single dosing (AUCinf) increasing 2.3-fold (90% confidence interval [CI], 1.85, 2.90) and maximum observed concentration in plasma (Cmax) 2.7-fold (90% CI, 1.81, 3.90). Pravastatin exposure was slightly increased in the presence of boceprevir, with pravastatin AUCinf increasing 1.63-fold (90% CI, 1.03, 2.58) and Cmax 1.49-fold (90% CI, 1.03, 2.14). Boceprevir exposure was generally unchanged when the drug was coadministered with atorvastatin or pravastatin. All adverse events were mild and consistent with the known safety profile of boceprevir. The observed 130% increase in AUC of atorvastatin supports the use of the lowest possible effective dose of atorvastatin when coadministered with boceprevir, without exceeding a maximum daily dose of 40 mg. The observed 60% increase in pravastatin AUC with boceprevir coadministration supports the initiation of pravastatin treatment at the recommended dose when coadministered with boceprevir, with close clinical monitoring.
INTRODUCTION
Worldwide, there are an estimated 130 to 170 million individuals infected with hepatitis C virus (1). Comorbidities, such as diabetes and obesity, are more common among patients with hepatitis C virus infection than in the general U.S. population, with as many as 25% of patients with hepatitis C virus infection at risk of comorbid disorders of lipid metabolism (2). Thus, patients with hepatitis C virus infection frequently receive concomitant treatments for hyperlipidemia, including the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors atorvastatin and pravastatin. Understanding potential drug interactions between hepatitis C therapies and HMG-CoA reductase inhibitors is important for optimal treatment of this patient population.
Boceprevir is a potent, orally administered ketoamide inhibitor targeting the active site of the hepatitis C virus nonstructural protein 3 (NS3) protease, approved for the treatment of genotype 1 chronic hepatitis C virus infection in adult patients with compensated liver disease (3–6). Addition of boceprevir to a pegylated interferon (peginterferon)-ribavirin backbone is associated with significantly increased rates of sustained virologic response (SVR) (7, 8). It is metabolized by aldoketoreductase and CYP3A4 and is a direct competitive and time-dependent inhibitor of CYP3A4/5, with inhibition constants in the low micromolar range (6), thus having the potential for drug-drug interactions with agents that are metabolized via these common pathways (3, 5). In vitro, boceprevir has been shown to inhibit the hepatic uptake transporter organic anion transporting polypeptide (OATP) 1B1 (50% inhibitory concentration [IC50] of 18 μM), but the clinical significance of this in vitro inhibition is unknown (6). In a recent drug-drug interaction trial with digoxin, boceprevir showed only limited P-glycoprotein (P-gp) inhibitory potential at clinically relevant concentrations (9).
Atorvastatin and pravastatin are two commonly prescribed HMG-CoA reductase inhibitors. Atorvastatin is metabolized extensively by CYP3A4, is a substrate of OATP 1B1, and may be a substrate for P-gp (10). Concomitant use of atorvastatin and strong CYP3A4 inhibitors should be avoided because of an elevated risk of myopathy and rhabdomyolysis associated with increased plasma statin levels (11). Because boceprevir is a strong, reversible CYP3A4 inhibitor, a drug interaction between boceprevir and atorvastatin is feasible. Pravastatin is excreted largely unchanged: it is not metabolized to a significant extent by the CYP450 system, nor is it a substrate for P-gp, but it is a substrate for the OATP 1B1 transporter. Since boceprevir is an OATP 1B1 inhibitor in vitro, drug interactions between boceprevir and pravastatin may occur at the level of OATP 1B1 (12).
The aims of the present study were to determine the effect of steady-state boceprevir on the exposure of atorvastatin and pravastatin and to explore the effects of atorvastatin and pravastatin on the pharmacokinetic (PK) profile of boceprevir.
(These data were presented at the 16th Annual Meeting of HEP DART, 4 to 8 December 2011, Kauai, HI.)
MATERIALS AND METHODS
This was a single-center, open-label, fixed-sequence, one-way-crossover, drug-drug interaction trial conducted with healthy adult volunteers. The study was conducted in accordance with principles of good clinical practice and was approved by the appropriate institutional review boards and regulatory agencies. All subjects provided written informed consent prior to any study-related activities.
Subjects.
Healthy male and female adult volunteers aged 18 to 55 years and with body mass indices (BMI) between 18 and 32 kg/m2 were enrolled. All subjects had clinical laboratory tests and vital signs within normal limits or that were acceptable to the investigator, and all were free of any clinically significant disease. Female subjects who were premenopausal and unsterilized were required to use a medically accepted method of contraception prior to and during the study. Subjects with hepatitis B virus surface antigen, hepatitis C virus antibodies, or HIV were excluded. Subjects with a positive drug screen or a history of alcohol or drug abuse or who smoked >10 cigarettes per day were also excluded.
Study design.
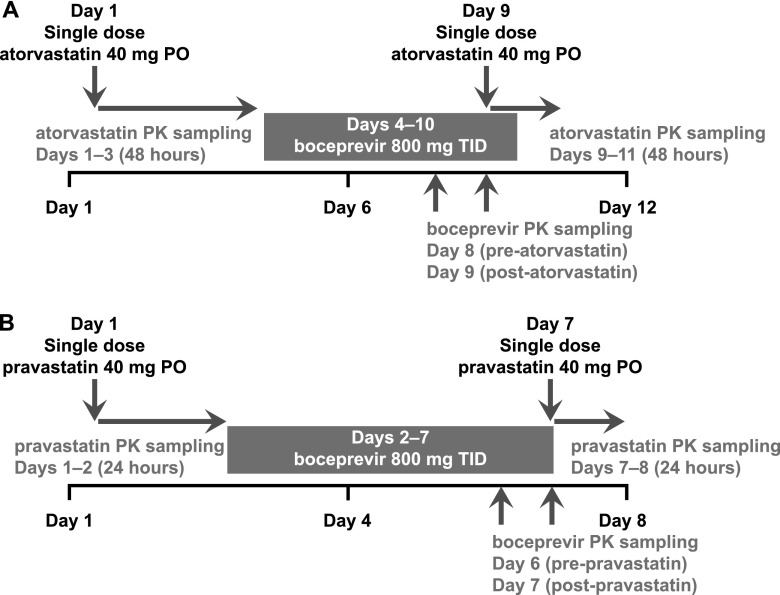
For assessment of drug interactions between boceprevir and atorvastatin, healthy adult volunteers received single-dose atorvastatin (40 mg) following breakfast on day 1 of the study, followed by boceprevir (800 mg three times daily with food) on days 4 to 10 (Fig. 1). On day 9 of the study, all subjects also received a single dose of atorvastatin (40 mg) in addition to their morning dose of boceprevir. Blood samples for assessment of atorvastatin PKs were collected predose and at specified time points up to 48 h postdose on days 1 and 9. Samples for determination of boceprevir PKs were collected on days 8 and 9 prior to the morning dose and for up to 8 h postdose.
Fig 1.
Design of atorvastatin (A) and pravastatin (B) drug-drug interaction studies. PO, orally; TID, three times a day.
For assessment of drug interactions between boceprevir and pravastatin, healthy adult volunteers received a single dose of pravastatin (40 mg) following breakfast on day 1, followed by boceprevir (800 mg three times daily with food) on days 2 to 7 (Fig. 1). On day 7, subjects also received a second single dose of pravastatin (40 mg) in addition to their morning dose of boceprevir. Blood samples for determination of pravastatin PK were collected predose and for up to 24 h postdose on days 1 and 7. Samples for determination of boceprevir PKs were collected on days 6 and 7 prior to the morning dose and for up to 8 h postdose.
In both studies, subjects who failed to comply with dosing, evaluations, or other study-related requirements were discontinued from treatment. Subjects experiencing a serious or life-threatening adverse event (AE) or who became pregnant during the study were also discontinued.
Assessments.
The following primary PK parameters were evaluated: area under the concentration-time curve from time zero to the time of the last measurable sample (AUClast), maximum observed concentration in plasma (Cmax), time to maximum observed concentration in plasma (Tmax), terminal phase half-life (t1/2), area under the concentration-time curve from time zero to infinity after single dosing (AUCinf), area under the concentration-time curve from time zero to 8 h (AUC0–8), apparent total body clearance (CL/F), and minimum observed concentration in plasma (Cmin) (boceprevir only). All subjects also had routine laboratory tests and were monitored for the occurrence of AEs.
Assays and procedures.
Assessment of the concentrations of pravastatin, atorvastatin, and the ortho- and para-hydroxylated metabolites of atorvastatin in plasma was conducted by PharmaNet Canada (Quebec, Canada). The linear range of the assay for atorvastatin was 49.80 to 49,800 pg/ml, the linear range of the assay for the ortho-hydroxylate metabolite was 49.60 to 49,600 pg/ml, and the linear range of the assay for the para-hydroxylated metabolite was 50.40 to 5,040 pg/ml. The linear range of the assay for pravastatin was 0.25 to 98.50 ng/ml. The lower limits of quantitation for pravastatin, atorvastatin, ortho-hydroxylated metabolites, and para-hydroxylated metabolites were 0.25 ng/ml, 49.80 pg/ml, 49.60 pg/ml, and 50.40 pg/ml, respectively.
Assessment of concentrations of boceprevir in plasma was conducted by PPD (Middleton, WI) using a validated high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS-MS) method. The linear range of the assay was 4.80 to 5,200 ng/ml, and the lower limit of quantitation was 4.80 ng/ml.
Statistics.
PK parameters were determined for each subject individually using noncompartmental analysis (WinNonlin, version 5.2). Descriptive statistics and statistical comparisons were carried out on the individually determined PK parameters. The linear trapezoidal rule was used to calculate the AUC. Individual AUCinf was extrapolated from the predicted concentration at the last time point with quantifiable concentrations.
For fitting to determine t1/2, 3 to 5 consecutive time points in the terminal phase were used, including the last time point with quantifiable concentrations but excluding Cmax. The specific time points varied from subject to subject but were in the range of 6 to 24 h postdose. Terminal half-life values were not reported if fewer than 3 consecutive time points in the terminal phase were available (excluding Cmax) or if the regression coefficient was less than 0.9. Concentrations below the lower limit of quantitation were considered undetectable, were assigned a value of 0, and were not used to determine t1/2.
Target enrollment for each study was 10 subjects to ensure that at least 8 patients completed all treatments. Assuming a true geometric mean ratio (GMR) equal to 1 for AUC of atorvastatin and pravastatin when coadministered with boceprevir versus atorvastatin and pravastatin alone, a sample size of 8 conferred >99% probability that the 90% confidence interval (CI) for the GMR fell within the range of 0.50 to 2.00 for AUC.
The GMR for the log-transformed AUC (atorvastatin and pravastatin plus boceprevir versus atorvastatin and pravastatin alone) and the associated 90% CI were calculated using a mixed-effect model extracting the effect due to treatment as a fixed effect and subject as a random effect.
RESULTS
Atorvastatin.
Ten healthy adult volunteers were enrolled and completed the atorvastatin drug-drug interaction study. All subjects were white, of Hispanic or Latino ethnicity, with a mean age of 34.1 years (standard deviation [SD], 8.6 years) and a mean BMI of 26.9 kg/m2 (SD, 3.4 kg/m2). Six subjects were female and 4 subjects were male.
Pharmacokinetics.
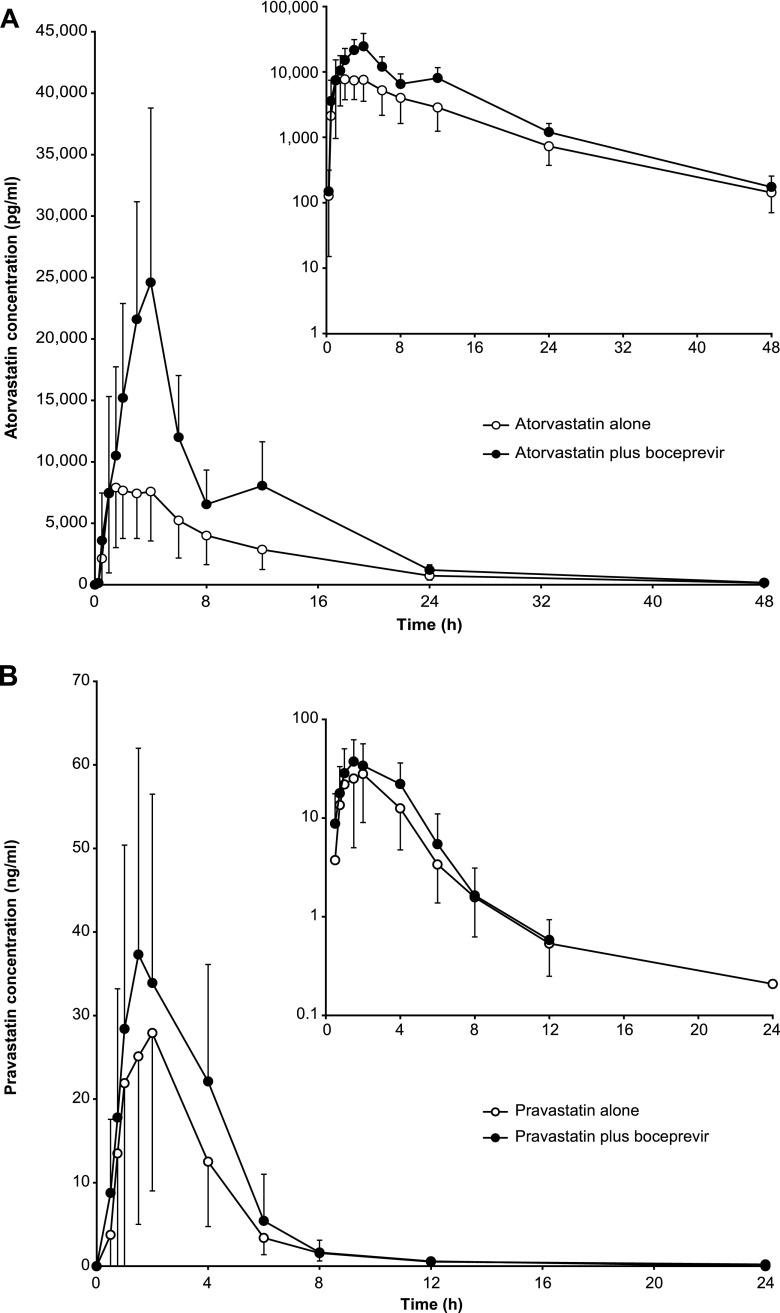
Atorvastatin exposure was increased in the presence of boceprevir. The mean AUCinfs increased from 95.0 to 214 ng · h/ml upon concomitant administration of atorvastatin and boceprevir, while mean Cmaxs increased from 10.3 to 27.7 ng/ml (Fig. 2A and Table 1). The AUCinf and Cmax GMRs for the comparison of atorvastatin plus boceprevir versus atorvastatin alone indicate a 2.3- and 2.7-fold increase, respectively (Table 2). The mean Tmaxs of atorvastatin were 2.25 h when administered alone and 4.00 h in the presence of boceprevir; mean t1/2 values were 8.81 h and 6.73 h, respectively. Mean atorvastatin clearance decreased from 498 to 210 liters/h upon boceprevir coadministration. There was a corresponding reduction in the exposure to the ortho-hydroxy metabolite of atorvastatin with boceprevir coadministration, with a decrease in AUClast from 83.8 ng · h/ml to 35.1 ng · h/ml and Cmax from 7.08 ng/ml to 2.59 ng/ml. In contrast, the AUClast for the para-hydoxy metabolite was largely unchanged in the presence of boceprevir (8.09 ng · h/ml versus 9.49 ng · h/ml), while the Cmax was increased from 0.307 ng/ml to 0.702 ng/ml (Table 1).
Fig 2.
Mean (with standard deviation) plasma concentration-time profiles of atorvastatin (A) and pravastatin (B) when administered alone or with boceprevir.
Table 1.
Pharmacokinetic parameters for atorvastatina
| Form of atorvastatin | Tmax (h) | Cmax (ng/ml) | AUClast (ng · h/ml) | AUCinf (ng · h/ml) | t1/2 (h) | CL/F (liters/h) |
|---|---|---|---|---|---|---|
| Parent form | ||||||
| Atorvastatin alone (day 1, n = 10) | 2.25 (1.00–4.00) | 10.3 (43) | 93.2 (50) | 95.0 (49) | 8.81 (15) | 498 (40) |
| Atorvastatin + boceprevir (day 9, n = 10) | 4.00 (3.00–4.00) | 27.7 (50) | 212 (38) | 214 (38) | 6.73 (11) | 210 (34) |
| ortho-Hydroxy metabolite | ||||||
| Atorvastatin alone (day 1, n = 10) | 4.00 (1.50–6.00) | 7.08 (27) | 83.8 (46) | 86.5 (46) | 9.89 (12) | |
| Atorvastatin + boceprevir (day 9, n = 10) | 4.00 (3.00–8.00) | 2.59 (65) | 35.1 (60) | 40.8b (53) | 7.91b (20) | |
| para-Hydroxy metabolite | ||||||
| Atorvastatin alone (day 1, n = 10) | 8.00 (4.00–12.0) | 0.307 (55) | 8.09 (79) | |||
| Atorvastatin + boceprevir (day 9, n = 10) | 12.0 (3.00–12.0) | 0.702 (53) | 9.49 (64) |
All values are means with coefficients of variations in parentheses, except in the case of Tmaxs, which are medians with ranges in parentheses.
n = 8.
Table 2.
Summary statistics for atorvastatin and boceprevir pharmacokinetic parameters
| Drug | Parameter | Treatment | Geometric meana | Comparison | GMR | 90% CI for GMR |
|---|---|---|---|---|---|---|
| Atorvastatinb | Cmax (ng/ml) | Atorvastatin + boceprevir | 24.7 | Atorvastatin + boceprevir vs atorvastatin alone | 2.66 | 1.81–3.90 |
| Atorvastatin alone | 9.29 | |||||
| AUCinf (ng · h/ml) | Atorvastatin + boceprevir | 201 | Atorvastatin + boceprevir vs atorvastatin alone | 2.32 | 1.85–2.90 | |
| Atorvastatin alone | 86.8 | |||||
| Boceprevirc | Cmax (ng/ml) | Boceprevir + atorvastatin | 1,969 | Boceprevir + atorvastatin vs boceprevir alone | 1.04 | 0.888–1.21 |
| Atorvastatin alone | 1,900 | |||||
| AUC0–8 (ng · h/ml) | Boceprevir + atorvastatin | 6,995 | Boceprevir + atorvastatin vs boceprevir alone | 0.960 | 0.903–1.02 | |
| Atorvastatin alone | 7,290 |
Model-based (least-squares) geometric mean: based on mixed-effect model extracting the effect due to treatment as the fixed effect and subject as the random effect. Ten subjects were included in the study.
Single-dose atorvastatin with and without multiple-dose boceprevir.
Multiple-dose boceprevir with and without single-dose atorvastatin.
Boceprevir exposure was essentially unchanged when the drug was coadministered with atorvastatin, with GMRs of 1.04 and 0.960 for boceprevir Cmax and AUC0–8, respectively (Table 2). The CL/F, t1/2, and Cmin of boceprevir were also similar when boceprevir was administered alone or in combination with atorvastatin (CL/F, 111 liters/h versus 116 liters/h [n = 10]; t1/2, 1.17 h versus 1.28 h [n = 5]; Cmin, 91.3 ng/ml versus 86.6 ng/ml).
Safety.
In total, 15 AEs were reported by 5 subjects; all were mild in intensity, and most occurred within 5 days of starting boceprevir treatment. Of these, 11 AEs were considered treatment related. The most frequently reported AE was mild dysgeusia, which was reported by 3 subjects (30%) receiving boceprevir (Table 3). No subjects discontinued treatment because of an AE, and there were no deaths or serious AEs during the study. There were also no clinically relevant changes in blood chemistry, hematology, blood pressure, pulse rate, oral body temperature, or electrocardiogram.
Table 3.
Drug-related treatment-emergent adverse events in the atorvastatin study
| Adverse event | No. (%) of subjectsb |
|||
|---|---|---|---|---|
| SD ATO (n = 10) | MD BOC (n = 10) | MD BOC, SD ATO (n = 10) | Total (n = 10) | |
| Any drug-relateda adverse event | 1 (10) | 5 (50) | 0 | 5 (50) |
| Gastrointestinal disorders | 0 | 5 (50) | 0 | 5 (50) |
| Abdominal discomfort | 0 | 1 (10) | 0 | 1 (10) |
| Abdominal distension | 0 | 1 (10) | 0 | 1 (10) |
| Constipation | 0 | 3 (30) | 0 | 3 (30) |
| Diarrhea | 0 | 1 (10) | 0 | 1 (10) |
| Flatulence | 0 | 1 (10) | 0 | 1 (10) |
| Gastroesophageal reflux disease | 0 | 1 (10) | 0 | 1 (10) |
| Nervous system disorders | 1 (10) | 3 (30) | 0 | 4 (40) |
| Dizziness | 0 | 1 (10) | 0 | 1 (10) |
| Dysgeusia | 0 | 3 (30) | 0 | 3 (30) |
| Headache | 1 (10) | 0 | 0 | 1 (10) |
| Respiratory, thoracic, mediaspinal disorders | 0 | 1 (10) | 0 | 1 (10) |
| Cough | 0 | 1 (10) | 0 | 1 (10) |
Possibly or probably drug related.
ATO, atorvastatin; BOC, boceprevir; MD, multiple dose; SD, single dose.
Pravastatin.
Ten healthy adult subjects were enrolled, and 9 subjects completed the study. One subject was discontinued due to noncompliance with the protocol. Eight subjects were white and 2 were black or African American (all Hispanic or Latino). The mean age was 39.2 years (SD, 8.8 years), and the mean BMI was 24.7 kg/m2 (SD, 2.6 kg/m2). Six subjects were female and 4 subjects were male.
Pharmacokinetics.
Pravastatin exposure was increased slightly in the presence of boceprevir. The mean pravastatin AUCinf was increased from 99.1 to 145 ng · h/ml upon concomitant administration with boceprevir, while mean Cmax levels increased from 37.4 to 46.9 ng/ml (Fig. 2B and Table 4). The AUCinf and Cmax GMRs for the comparison of pravastatin plus boceprevir versus pravastatin alone indicated 1.63- and 1.49-fold increases, respectively (Table 5). The pravastatin mean elimination half-life was reduced from 4.10 to 1.76 h and the mean CL/F decreased from 673 to 343 liters/h in the presence of boceprevir (Table 5).
Table 4.
Pharmacokinetic parameters for pravastatina
| Drug(s) | Tmax (h) | Cmax (ng/ml) | AUClast (ng · h/ml) | AUCinf (ng · h/ml) | t1/2 (h) | CL/F (liters/h) |
|---|---|---|---|---|---|---|
| Pravastatin alone (day 1, n = 10) | 1.5 (0.500–2.00) | 37.4 (61) | 102 (46) | 99.1b (55) | 4.10b (93) | 673b (92) |
| Pravastatin + boceprevir (day 7, n = 9) | 2.00 (1.00–4.00) | 46.9 (38) | 140 (44) | 145c (49) | 1.76c (22) | 343c (52) |
All values are means with coefficients of variations in parentheses, except in the case of Tmaxs, which are medians with ranges in parentheses.
n = 6.
n = 7.
Table 5.
Summary statistics for pravastatin and boceprevir pharmacokinetic parameters
| Drug | Parameter | Treatment | n | Geometric meana | Comparison | GMR | 90% CI for GMR |
|---|---|---|---|---|---|---|---|
| Pravastatinb | Cmax (ng/ml) | Pravastatin + boceprevir | 9 | 44.7 | Pravastatin + boceprevir vs pravastatin alone | 1.49 | 1.03–2.14 |
| Pravastatin alone | 10 | 30.1 | |||||
| AUCinf (ng · h/ml) | Pravastatin + boceprevir | 7 | 142 | Pravastatin + boceprevir vs pravastatin alone | 1.63 | 1.03–2.58 | |
| Pravastatin alone | 6 | 87.3 | |||||
| Boceprevirc | Cmax (ng/ml) | Boceprevir + pravastatin | 9 | 1,806 | Boceprevir + pravastatin vs boceprevir alone | 0.928 | 0.828–1.04 |
| Pravastatin alone | 9 | 1,947 | |||||
| AUC0–8 (ng · h/ml) | Boceprevir + pravastatin | 9 | 6,555 | Boceprevir + pravastatin vs boceprevir alone | 0.951 | 0.892–1.01 | |
| Pravastatin alone | 9 | 6,889 |
Model-based (least-squares) geometric mean based on mixed-effect model extracting the effect due to treatment as the fixed effect and subject as the random effect.
Single-dose pravastatin with and without multiple-dose boceprevir.
Multiple-dose boceprevir with or without single-dose pravastatin.
The Cmax, AUC, CL/F, and t1/2 of boceprevir were generally unchanged in the presence of pravastatin compared with boceprevir alone, with GMRs between 0.9 and 1.0 for both Cmax and AUC0–8 (Table 5). The CL/F and t1/2 for boceprevir alone and in the presence of pravastatin were 117 liters/h versus 124 liters/h (n = 10) and 1.23 h versus 1.12 h (n = 6), respectively.
Safety.
A total of 14 AEs were reported by 7 subjects; all were considered mild, and most occurred within 5 days of initiating boceprevir. Thirteen of these AEs were considered related to boceprevir therapy, the most common of which were dysgeusia and headache, each reported by 3 patients (30%) (Table 6). No subjects discontinued treatment because of an AE, and there were no deaths or serious AEs during the study. There were also no clinically relevant changes in blood chemistry, hematology, blood pressure, pulse rate, oral body temperature, or electrocardiogram.
Table 6.
Drug-related treatment-emergent adverse events in the pravastatin study
| Adverse event | No. (%) of subjectsb |
|||
|---|---|---|---|---|
| SD PRA (n = 10) | MD BOC (n = 10) | MD BOC, SD PRA (n = 10) | Total (n = 10) | |
| Any drug-relateda adverse event | 0 | 7 (40) | 1 (10) | 7 (70) |
| Gastrointestinal disorders | 0 | 4 (40) | 0 | 4 (40) |
| Abdominal discomfort | 0 | 1 (10) | 0 | 1 (10) |
| Diarrhea | 0 | 2 (20) | 0 | 2 (20) |
| Frequent bowel movements | 0 | 1 (10) | 0 | 1 (10) |
| Gastroesophageal reflux disease | 0 | 1 (10) | 0 | 1 (10) |
| Nausea | 0 | 1 (10) | 0 | 1 (10) |
| Nervous system disorders | 0 | 4 (40) | 1 (10) | 4 (40) |
| Dysgeusia | 0 | 3 (30) | 0 | 3 (30) |
| Headache | 0 | 2 (20) | 1 (10) | 3 (30) |
Possibly or probably drug related.
BOC, boceprevir; MD, multiple dose; PRA, pravastatin; SD, single dose.
DISCUSSION
Data from the present study demonstrate that exposure to atorvastatin is increased but that there is no clinically important effect on pravastatin when these drugs are coadministered with multiple doses of boceprevir (Fig. 3). A 2.3-fold increase in the AUCinf and a 2.7-fold increase in the Cmax of atorvastatin were observed following concomitant administration with boceprevir. For pravastatin, the 1.5-fold increase in Cmax and a 1.6-fold increase in AUCinf observed with boceprevir coadministration are not considered clinically important (13).
Fig 3.
Effect of boceprevir on atorvastatin and pravastatin pharmacokinetics.
Atorvastatin has low bioavailability due to extensive metabolism in the gastric mucosa and first-pass hepatic metabolism (14). It is a substrate for P-gp and OATP 1B1 transporters and undergoes hepatic metabolism via CYP3A4 (10, 14, 15). The increased Cmax and AUC of atorvastatin observed in the present study are consistent with increases in atorvastatin exposure seen with other inhibitors of CYP3A4 metabolism, such as erythromycin and itraconazole, suggesting that inhibition of CYP3A4 metabolism plays a role in the increased exposure of atorvastatin upon boceprevir coadministration (14). However, boceprevir-mediated inhibition of the hepatic uptake transporter OATP 1B1 may also contribute to the increase in atorvastatin exposure.
Hepatic metabolism of atorvastatin by CYP3A4 produces several active metabolites, including the para- and ortho-hydroxylated products (14). These metabolites are thought to be responsible for up to 70% of the overall HMG-CoA reductase inhibitory activity: although the half-life of atorvastatin is approximately 14 h, the half-life of HMG-CoA reductase inhibition is between 20 and 30 h (14). In the present study, the 2.3-fold increase in atorvastatin exposure in the presence of boceprevir was paralleled by a >2-fold decrease in the AUClast of ortho-hydroxy atorvastatin, and there was essentially no change in the AUClast of the para-hydroxy metabolite (8.09 ng · h/ml versus 9.49 ng · h/ml). In clinical terms, the extent to which the increased exposure of atorvastatin might be offset by reduced exposure to the active metabolites remains unclear. Mechanistically, the difference in kinetics between the para- and ortho-hydroxy metabolites may be explained by the observation that CYP2C8, in addition to CYP3A4, can mediate the formation of para-hydroxy atorvastatin but not the ortho-hydroxy metabolite (16).
Approximately 75% of the clinical activity seen with pravastatin is mediated through the parent drug, which is excreted largely unchanged; the major metabolite has substantially less activity than the parent compound (12, 14). We observed an apparent decrease in pravastatin t1/2 and CL/F in the presence of boceprevir. This was driven primarily by 1 to 2 subjects with high t1/2 and CL/F values when receiving pravastatin alone, represented by a large variability in these parameters within this treatment group (Table 4). Since only 6 subjects receiving pravastatin alone had reportable t1/2 and CL/F values, 1 or 2 subjects with high t1/2 and CL/F data could influence the overall mean values, resulting in the apparent decrease in these parameters when the drugs were coadministered.
The 1.6-fold increase in exposure of pravastatin seen with boceprevir coadministration cannot be explained by CYP3A4 inhibition, since pravastatin is not metabolized to a significant extent by the CYP450 enzyme system. More likely, the increase in pravastatin exposure may be due to reduced hepatic pravastatin uptake resulting from boceprevir-mediated inhibition of the OATP 1B1 transporter. Reduced hepatic pravastatin uptake may subsequently affect its excretion into the bile, elimination, and/or further intestinal absorption (14). OATP 1B1 inhibition may also explain the increased exposure of pravastatin when coadministered with cyclosporine (7.9-fold increase in AUC and 22.8-fold increase in Cmax), a known OATP 1B1 inhibitor (14, 15). This hypothesis is also consistent with the decrease in the mean apparent clearance and t1/2 of pravastatin with boceprevir coadministration.
A limitation of the current study is that it was conducted with healthy subjects. Although findings from healthy subjects are generally considered predictive of findings in the patient population for which the drug is intended (17), we cannot be certain that the observed drug interactions between boceprevir and atorvastatin or pravastatin are transferable to patients with hepatitis C virus infection, who may have additional comorbidities or impaired liver function. In clinical practice, boceprevir is administered together with peginterferon and ribavirin. No interactions between peginterferon or ribavirin and statins have been identified.
In conclusion, data from the present study indicate that coadministration of single-dose atorvastatin or single-dose pravastatin with multiple-dose boceprevir is safe and well tolerated in healthy volunteers. The observed 130% increase in the AUC of atorvastatin supports the use of the lowest possible effective dose of atorvastatin when coadministered with boceprevir, without exceeding a maximum daily dose of 40 mg. The observed 60% increase in the pravastatin AUC with boceprevir coadministration supports the initiation of pravastatin treatment at the recommended dose when coadministered with boceprevir, with close clinical monitoring.
ACKNOWLEDGMENTS
Bioanalytical support was provided by Bhavana Kantesaria, statistical support by Jianmin Zhao, and reporting support by Srinivas Annavarapu. All are employees of Merck Sharp & Dohme Corp.
Medical writing and editorial assistance were provided by Tim Ibbotson and Santo D'Angelo of ApotheCom (Yardley, PA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ.
H.-P.F., S.G., F.X., E.O., J.A.W., and J.R.B. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ. E.G.J.H. and M.G.J.A.V.Z. are current or former employees of Merck Sharp & Dohme, The Netherlands.
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ.
Footnotes
Published ahead of print 25 March 2013
REFERENCES
- 1. Lavanchy D. 2011. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 17:107–115 [DOI] [PubMed] [Google Scholar]
- 2. Louie KS, St Laurent S, Forssen UM, Mundy LM, Pimenta JM. 2012. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect. Dis. 12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghosal A, Yuan Y, Tong W, Su A, Gu C, Chowdhury SK, Kishnani NS. 2011. Characterization of human liver enzymes involved in the biotransformation of boceprevir, a HCV protease inhibitor. Drug Metab. Dispos. 39:510–521 [DOI] [PubMed] [Google Scholar]
- 4. Malcolm BA, Liu R, Lahser F, Agrawal S, Belanger B, Butkiewicz N, Chase R, Gheyas F, Hart A, Hesk D, Ingravallo P, Jiang C, Kong R, Lu J, Pichardo J, Prongay A, Skelton A, Tong X, Venkatraman S, Xia E, Girijavallabhan V, Njoroge FG. 2006. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob. Agents Chemother. 50:1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merck & Co. , Inc 2011. Victrelis™ (boceprevir) capsules. Merck & Co., Inc., Whitehouse Station, NJ [Google Scholar]
- 6. Chu X, Cai X, Cui D, Tang C, Ghosal A, Green MD, Kuo Y, Liang Y, Macioleck CM, Palamanda J, Evers R, Prueksaritanont T. 2013. In vitro assessment of drug-drug interaction potential of boceprevir associated with drug metabolizing enzymes and transporters. Drug Metab. Dispos. [Epub ahead of print]. doi:10.1124/dmd.112.049668 [DOI] [PubMed] [Google Scholar]
- 7. Bacon B, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Burroughs M, Brass CA, Albrecht JK, Esteban R. 2011. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy R, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki J-P. 2011. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364:1195–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jumes P, Feng Xuan H-PF, Youngberg S, Wagner J, Butterton J. 2012. Pharmacokinetic interaction between the HCV protease inhibitor boceprevir and digoxin in healthy adult volunteers, abstr PK_05. Abstr. 7th Int. Wkshp. Clin. Pharmacol. Hepatitis Ther., Cambridge, MA [Google Scholar]
- 10. Lennernäs H. 2003. Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 42:1141–1160 [DOI] [PubMed] [Google Scholar]
- 11. Parke-Davis 2005. Lipitor® (atorvastatin calcium) tablets. Pfizer Inc., New York, NY [Google Scholar]
- 12. Neuvonen PJ, Backman JT, Niemi M. 2008. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin, and pravastatin. Clin. Pharmacokinet. 47:463–474 [DOI] [PubMed] [Google Scholar]
- 13. Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R, Pravastatin in Chronic Liver Disease Study Investigators 2007. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled multicenter trial. Hepatology 46:1453–1463 [DOI] [PubMed] [Google Scholar]
- 14. Williams D, Feely J. 2002. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin. Pharmacokinet. 41:343–370 [DOI] [PubMed] [Google Scholar]
- 15. Shitara Y, Sugiyama Y. 2006. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 112:71–105 [DOI] [PubMed] [Google Scholar]
- 16. Jacobsen W, Kuhn B, Soldner A, Kirchner G, Sewing KF, Kollman PA, Benet LZ, Christians U. 2000. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab. Dispos. 28:1369–1378 [PubMed] [Google Scholar]
- 17. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research 2012. Guidance for industry: drug interaction studies—study design, data, analysis, implications for dosing, and labeling recommendations. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf