Abstract
Beta-lactams, in combination with beta-lactamase inhibitors, are reported to have activity against Mycobacterium tuberculosis bacteria growing in broth, as well as inside the human macrophage. We tested representative beta-lactams belonging to 3 different classes for activity against replicating M. tuberculosis in broth and nonreplicating M. tuberculosis under hypoxia, as well as against streptomycin-starved M. tuberculosis strain 18b (ss18b) in the presence or absence of clavulanate. Most of the combinations showed bactericidal activity against replicating M. tuberculosis, with up to 200-fold improvement in potency in the presence of clavulanate. None of the combinations, including those containing meropenem, imipenem, and faropenem, killed M. tuberculosis under hypoxia. However, faropenem- and meropenem-containing combinations killed strain ss18b moderately. We tested the bactericidal activities of meropenem-clavulanate and amoxicillin-clavulanate combinations in the acute and chronic aerosol infection models of tuberculosis in BALB/c mice. Based on pharmacokinetic/pharmacodynamic indexes reported for beta-lactams against other bacterial pathogens, a cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (%TMIC) of 20 to 40% was achieved in mice using a suitable dosing regimen. Both combinations showed marginal reduction in lung CFU compared to the late controls in the acute model, whereas both were inactive in the chronic model.
INTRODUCTION
Beta-lactam (BL) agents have been widely used for treating a broad spectrum of bacterial pathogens (1). However, there are very few reports on successful clinical use of beta-lactam antibiotics for the treatment of tuberculosis (TB). Mycobacterium tuberculosis is reported to produce a chromosomally encoded beta-lactamase enzyme (2, 3, 4) that degrades the beta-lactam agent, and therefore, use of BL along with a beta-lactamase inhibitor (BLI) like clavulanate is essential for killing M. tuberculosis. Many of the BL-BLI agents are reported to have a poor plasma half-life and poor oral bioavailability (1, 5, 6). Limitations of oral dosing and the requirement for frequent dosing for longer durations may have limited the use of BL-BLI agents for TB treatment. However, recent reports on successful treatment of patients with multidrug-resistant and extremely drug-resistant (MDR/XDR) TB by including meropenem (7, 8) in the second-line combination therapy have raised interest in understanding the efficacy of the meropenem-clavulanate combination in the mouse TB model (9). We have extended this interest to include understanding the pharmacokinetic-pharmacodynamic (PK-PD) relationship for meropenem-clavulanate and amoxicillin-clavulanate combinations in the mouse TB model.
Discovery of novel anti-TB agents or therapies is essential to treat MDR/XDR TB, as well as to shorten the current 6-month-long treatment of drug-sensitive TB. Therefore, activity against drug-resistant strains, as well as against both replicating and nonreplicating M. tuberculosis, is a desirable attribute of a novel anti-TB therapy. In vitro activity of the meropenem-clavulanate combination against a large number of drug-sensitive and -resistant clinical isolates of M. tuberculosis has been reported (10). However, the activities of other beta-lactams against replicating M. tuberculosis and comparisons of activities against replicating and nonreplicating M. tuberculosis isolates have not yet been reported.
We describe the activities of various beta-lactams (with or without clavulanate) against replicating M. tuberculosis (H37Rv) and nonreplicating M. tuberculosis in vitro (H37Rv under hypoxic conditions) (11) and under aerobic conditions using streptomycin-starved M. tuberculosis strain 18b (ss18b) (12), as well as the in vivo efficacies of amoxicillin-clavulanate and meropenem-clavulanate combinations in the acute (actively replicating M. tuberculosis) and chronic (slowly growing/nonreplicating M. tuberculosis) mouse models of TB.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and chemicals.
Clavulanate and all the beta-lactam antibiotics were purchased from Sigma Chemicals. Meropenem was supplied by AstraZeneca. Cilastatin was purchased from BetaPharma Co. Ltd., Shanghai, China.
M. tuberculosis H37Rv was grown in 250-ml roller bottles (Corning) as smooth cultures to mid-log phase (optical density at 600 nm [OD600] = 0.5) and stored frozen as 0.5-ml aliquots in screw-cap cryovials (Corning) at −70°C. Representative vials from the frozen lot were thawed and plated for viable counts after 10 days and were found to contain ∼108 CFU/ml. For subsequent experiments, seed lot vials were thawed, and the cells were diluted to get 3 × 105 to 5 × 105 CFU/ml. The media used for growth of M. tuberculosis were Middlebrook 7H9 broth and 7H10 agar (Difco Laboratories) supplemented with 0.2% glycerol, 0.05% Tween 80, and 10% albumin-dextrose-catalase (ADC). For growth of M. tuberculosis 18b cultures, the same media were supplemented with 50 μg/ml streptomycin when required.
M. tuberculosis H37Rv cultures were adapted to hypoxic conditions as described previously (11) with minor modifications. Briefly, M. tuberculosis cells were grown in Dubos Tween broth in McCartney bottles with a magnetic bead using a defined headspace ratio of 0.5. Methylene blue (1.5 μg/ml) was added as a redox indicator to all the bottles. The culture bottles were incubated at 37°C on a magnetic stirrer. The indicator dye completely decolorized in 12 days. The culture with a partial O2 pressure (pO2) of <0.06% after 14 days of incubation was used for testing bactericidal activity against the nonreplicating state of M. tuberculosis H37Rv.
M. tuberculosis strain 18b was grown to an OD600 of 1.0 in the presence of 50 μg/ml of streptomycin in Middlebrook 7H9 broth. The cell pellet obtained after centrifugation was washed three times with phosphate-buffered saline, pH 7.4 (PBS), containing 0.1% Tween 80. The washed culture was resuspended in growth medium without streptomycin at 5-fold-higher cell density and stored frozen at −80°C with 10% glycerol. One milliliter of frozen stock was inoculated into 50 ml broth, without streptomycin, in a roller bottle. After 14 days of incubation, this culture was used for testing bactericidal activity against the nonreplicating M. tuberculosis ss18b (12).
Determination of antibiotic susceptibility.
The MICs of antibiotics against M. tuberculosis H37Rv were determined by the resazurin-based microplate assay (13). Minimal bactericidal concentrations (MBCs) were determined against replicating M. tuberculosis H37Rv, as well as nonreplicating M. tuberculosis, in a 96-well plate format after 7 days of incubation with the antibiotics. For determination of MBCs against M. tuberculosis H37Rv under hypoxia, 200 μl of hypoxic culture (107 CFU/ml) was dispensed into compound-containing plates, and the plates were packed in gas-permeable polyethylene bags during incubation at 37°C in the hypoxia hood for 6 days. An anaerobic indicator strip was placed inside the chamber to confirm lack of oxygen during the entire process. The assay plates were transferred from the hypoxia hood to the CO2 incubator and further incubated at 37°C for a day to let the cells recover from hypoxic stress. Suitable dilutions of culture aliquots from wells containing replicating M. tuberculosis H37Rv and M. tuberculosis H37Rv under hypoxia exposed to various concentrations of BL-BLI agents greater than the MIC were plated on 7H10 agar plates. In the case of M. tuberculosis ss18b, plating was done on 7H10 agar containing 50 μg/ml streptomycin. CFU were counted after incubating the plates at 37°C for 21 to 28 days. Isoniazid and clofazimine were used as controls in all the assays. Clofazimine had MBCs of 0.3 μg/ml against M. tuberculosis ss18b and 2.6 μg/ml against M. tuberculosis H37Rv under hypoxia, while isoniazid did not have an MBC against the nonreplicating M. tuberculosis.
Combination MICs to determine synergy.
Serial double dilutions of amoxicillin and clavulanate were prepared in rows (B to G) and columns (2 to 11) of a 96-well microplate, respectively. M. tuberculosis H37Rv culture (200 μl at 105 cells/ml) was dispensed into all the wells except those in column 1, which was used as a “no-growth” control. Column 12 did not have antibiotics and was used as a “growth” control. The assay plates were incubated at 37°C for 6 days, and growth was monitored using resazurin dye, as mentioned above. The fractional inhibitory concentration (FIC) was calculated as the ratio of the MIC in combination to the MIC of a single agent. The concentrations at which the sum of the amoxicillin FIC (FICamoxicillin) and the clavulanate FIC (FICclavulanate) was <0.5 were considered to exhibit synergy.
PK-PD studies in mice.
All animal experimentation protocols were approved by the Institutional Animal Ethics Committee registered with the Government of India (registration no. 5/1999/CPCSEA). Six- to 8-week-old BALB/c mice, purchased from RCC Laboratories, Hyderabad, India, were used in PK-PD studies. Animals randomly assigned to the cage were allowed 2 weeks of acclimatization before starting the experiment. Food and water were given ad libitum.
PK was analyzed in healthy, as well as infected, mice. PK data from healthy mice were used for designing the dosing regimen for the efficacy study. PK data from infected mice were used for the PK-PD analysis. One mouse per time point was sampled according to the fast PK protocol (14). Blood samples from the infected mice were processed in the biosafety level 3 (BSL3) laboratory. Meropenem trihydrate, sodium clavulanate, and cilastatin were dissolved together in PBS at final concentrations of 60, 15, and 20 mg/ml, respectively. Amoxicillin and sodium clavulanate were dissolved together in PBS at final concentrations of 40 and 10 mg/ml, respectively. Compound solution or appropriate vehicle in control mice was administered subcutaneously at a 5-ml/kg of body weight dose volume. Blood samples, collected by puncturing the saphenous vein at 5 min, 15 min, 30 min, 1 h, 2 h, 3 h, and 4 h after the first dose (8 a.m.), were centrifuged at 2,000 × g for 5 min to isolate plasma. Bioanalysis was performed using liquid chromatography-tandem mass spectrometry (LC–MS-MS) after precipitating plasma proteins with 90% (vol/vol) acetonitrile. Analytes were separated on a Gemini C18 column (50 by 4.6 mm; particle size, 5 μm; Phenomenex) using isocratic elution with acetonitrile-10 mM ammonium acetate-0.1% formic acid in water (2:6:2 [vol/vol/vol]) at a flow rate of 400 μl/min. A Shimadzu UFLC (Shimadzu Corporation, Kyoto, Japan) was used for chromatography, and an API-3000 triple-quadrupole mass spectrometer (Applied Biosystems) was used for multiple-reaction-monitoring (MRM)-based quantitation. Amoxicillin (366.1 > 349.1 and 366.1 > 114.1) and meropenem (384.2 > 141.1 and 384.2 > 114) were detected in positive ionization mode, while clavulanate (198.2 > 136 and 198.2 > 108) was detected in negative ionization mode. The lower limits of quantitation for clavulanate, amoxicillin, and meropenem were 0.078 μg/ml, 0.078 μg/ml, and 0.313 μg/ml, respectively. The area under the concentration versus time curve PK profile (AUC) was calculated by noncompartmental analysis (WinNonLin 5.2.1; Pharsight Inc.).
Meropenem-clavulanate and amoxicillin-clavulanate combinations were tested for in vivo efficacy in the acute, as well as chronic, TB aerosol infection model in mouse. BALB/c mice were infected using an aerosol chamber (15) with ∼100 and 10,000 bacilli per mouse in the chronic and acute models, respectively. Infected mice were housed in individually ventilated cages (Allentown Technologies) in the BSL3 facility. The groups treated with vehicle or compound contained 3 mice each, and an additional 3 mice, used as early controls, were sacrificed just after infection. Isoniazid, used as a reference drug (at a dose of 3 mg/kg in the acute model and 30 mg/kg in the chronic model), was formulated in 0.5% (wt/vol) hydroxypropyl methylcellulose (HPMC) containing 0.1% Tween 80, whereas test compounds were prepared in the same formulation used for the PK study. Treatment was initiated after 3 days and 4 weeks of infection in the acute and chronic models, respectively, to test efficacy against actively growing and slowly growing/nonreplicating bacteria in the respective models. (For the dosing regimen used to treat infected mice, see Table 3.) The mice were sacrificed at the end of 4 weeks (6 days/week) of treatment, and suitable dilutions of their lung homogenates were plated on Middlebrook 7H11 agar medium to determine viable CFU per mouse lung. Dunnet's multiple-comparison test was used to assess differences in lung CFU in treated versus untreated mice.
Table 3.
PK parameters for infected mice in the efficacy studya
| Dose group | Drug PK | Combination drug | Dose (mg/kg) | Regimenb | Cmaxc (μg/ml) | Tmaxd (h) | AUC (h · μg/ml) | Half-life (h) | %TMIC in 24 he |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Amoxicillin | Clavulanate | 200 | BID, Q12 | 43.07 | 1.00 | 82.12 | 0.58 | 33 |
| 1 | Clavulanate | Amoxicillin | 50 | BID, Q12 | 26.39 | 0.08 | 15.67 | 0.21 | 13 |
| 2 | Meropenem | Clavulanate | 300 | TID, Q8 | 218.04 | 0.08 | 89.65 | 0.39 | 25 |
| 2 | Clavulanate | Meropenem | 75 | TID, Q8 | 24.88 | 0.08 | 13.34 | 0.46 | 19 |
The parameters were estimated after the 10th dose.
BID, twice a day; TID, three times a day; Q12, every 12 h; Q8, every 8 h.
Cmax, maximum concentration of drug in plasma.
Tmax, time to maximum concentration of drug in plasma.
Based on in vitro synergy between amoxicillin and clavulanate, a 1-μg/ml concentration was used for %TMIC calculation for clavulanate. Amoxicillin, clavulanate, and meropenem are reported to be 80%, 75%, and 98% free in plasma, respectively (9, 18). Therefore, the %TMIC values were almost the same for free plasma concentrations.
RESULTS
In vitro activity.
In vitro MICs estimated for various beta-lactam and clavulanate combinations against replicating M. tuberculosis are shown in Table 1. Cephalosporins (cephaloridine, cefpodoxime, cefotaxime, and cefixime) and carbapenems (meropenem, imipenem, and faropenem) showed 2- to 16-fold improvements in potency compared to the penicillin class of drugs (penicillin G, piperacillin, ampicillin, and amoxicillin), which showed up to >200-fold reduction in the MIC in the presence of clavulanate.
Table 1.
MICs against replicating M. tuberculosis H37Rv
| Beta-lactam agent | MIC (μg/ml) |
|
|---|---|---|
| Without clavulanate | With clavulanate (5 μg/ml) | |
| Penicillin G | 256 | 1 |
| Piperacillin | >128 | 0.25 |
| Amoxicillin | 64 | 1 |
| Ampicillin | 32 | 2 |
| Faropenem | 4 | 2 |
| Imipenem | 4 | 0.5 |
| Meropenem | 8 | 1 |
| Cefixime | 32 | 16 |
| Cefotaxime | 2 | 1 |
| Cefpodoxime | >128 | 32 |
| Cephaloridine | 4 | 0.25 |
| Isoniazid | 0.06 | 0.06 |
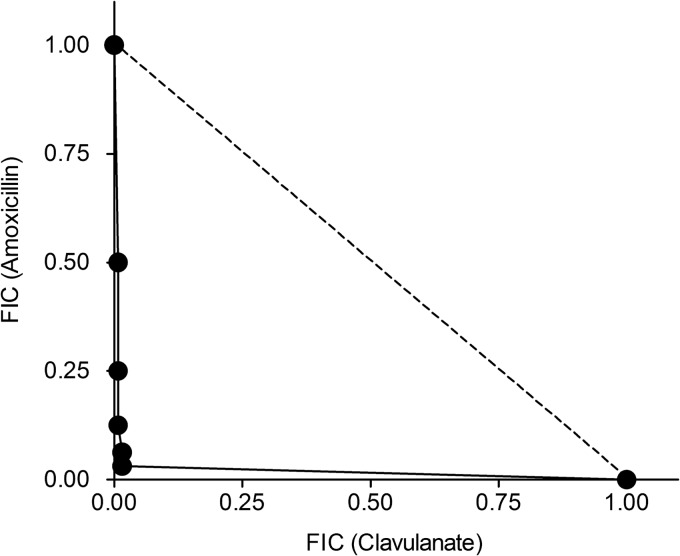
Since the activity of beta-lactams against M. tuberculosis is dependent upon an optimum concentration of beta-lactamase inhibitor, MICs for amoxicillin were estimated using various concentrations of clavulanate. As shown in Fig. 1, the isobologram indicated excellent synergy between amoxicillin and clavulanate. Based on these data, 1 μg/ml of clavulanate was chosen as the target concentration during the efficacy study.
Fig 1.
Isobologram indicating synergy between amoxicillin and clavulanate against replicating M. tuberculosis H37Rv. The dashed line joins the fractional MICs of individual agents. Clavulanate was inactive on its own. Its MIC was assumed to be 80 μg/ml.
MBCs against replicating M. tuberculosis are shown in Table 2. MBCs against replicating M. tuberculosis were within 2-fold of the MIC for all the beta-lactams tested. The same set of beta-lactams was tested for bactericidal activity against nonreplicating M. tuberculosis, M. tuberculosis H37Rv under hypoxia, and M. tuberculosis ss18b under aerobic conditions. MBCs for all 8 beta-lactams, with or without clavulanate, were >16 μg/ml against nonreplicating M. tuberculosis. The MBCs reported here correspond to the minimum concentration required for >2-log-unit reduction in CFU from the starting inoculum. We also looked for drugs giving 1-log-unit reduction during measurement of MBCs. Faropenem and meropenem in combination with clavulanate achieved 1-log-unit reduction in M. tuberculosis ss18b CFU. However, there was no reduction in CFU under hypoxia.
Table 2.
MBCs against replicating M. tuberculosis H37Rv
| Beta-lactam agent | MBCa (μg/ml) |
|
|---|---|---|
| Without clavulanate | With clavulanate (5 μg/ml) | |
| Penicillin G | 256 | 1 |
| Piperacillin | >128 | 0.5 |
| Amoxicillin | 64 | 1 |
| Meropenem | 16 | 2 |
| Imipenem | >64 | 4 |
| Faropenem | 4 | 2 |
| Cephaloridine | 8 | 0.25 |
| Cefotaxime | 2 | 1 |
| Isoniazid | 0.25 | 0.25 |
The MBC is defined as the minimum concentration required for >2-log-unit reduction in CFU from the starting inoculum.
In vivo activity.
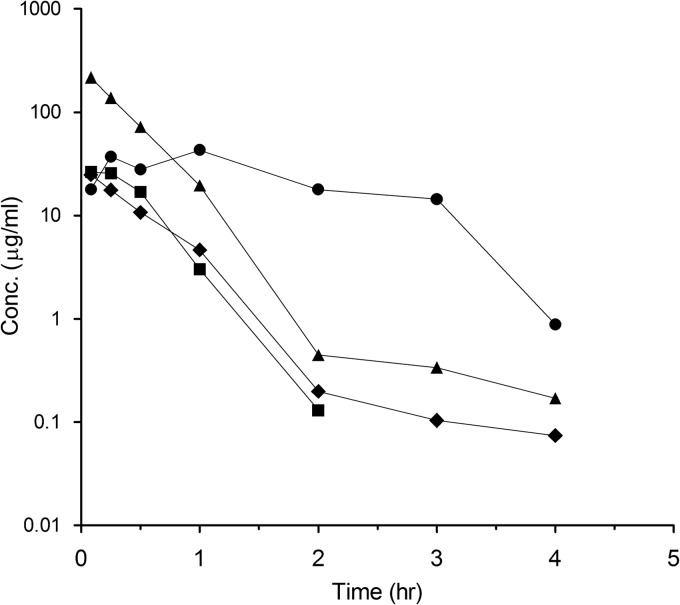
Infected mouse plasma PK profiles for amoxicillin, meropenem, and clavulanate are shown in Fig. 2, and the PK parameters, along with the cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (%TMIC), are shown in Table 3. The amoxicillin-clavulanate formulation was a suspension, whereas meropenem-clavulanate was a clear solution when dosed. The PK profile for amoxicillin may suggest a kind of slow release due to the suspension formulation. Since meropenem is known to be a weak substrate of renal dihydropeptidase (DHP1) in certain species (9), cilastatin was coadministered, along with meropenem and clavulanate, at a dose of 100 mg/kg three times daily. However, cilastatin did not improve meropenem exposure in the infected/healthy mice (data not shown). A three-times-a-day regimen was required for meropenem to achieve 25% TMIC in mouse plasma, whereas twice-daily administration of amoxicillin was sufficient to achieve 33% TMIC (Table 3). The clavulanate concentrations in both dose groups were >1 μg/ml for 19 or 12.5% of the time in 24 h.
Fig 2.
Pharmacokinetic profile for amoxicillin (200 mg/kg) (●) plus clavulanate (50 mg/kg) (■) and meropenem (300 mg/kg) (▲) plus clavulanate (75 mg/kg) (⧫) in infected mice.
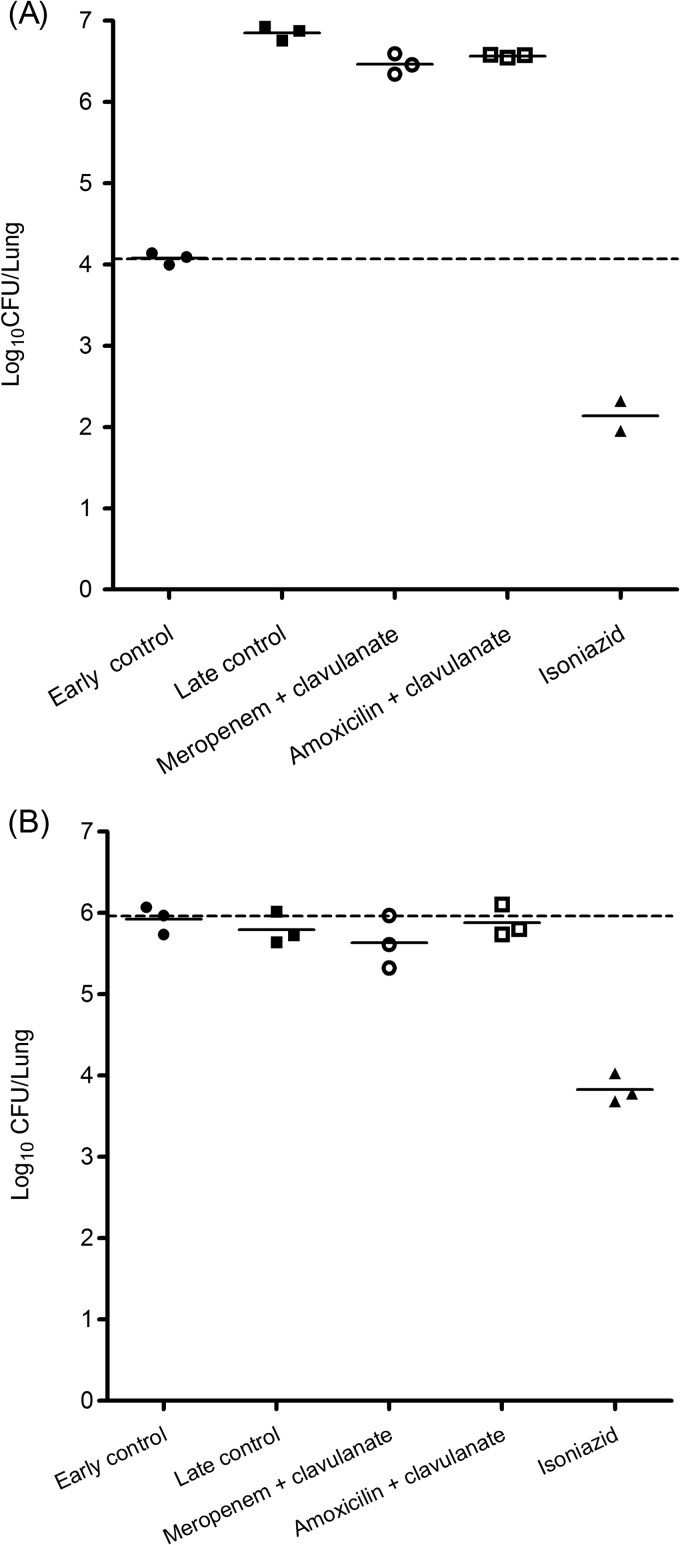
Amoxicillin-clavulanate, as well as meropenem-clavulanate, showed marginal reduction in M. tuberculosis growth compared to the late control in the acute model (Fig. 3A). Both combinations did not show any efficacy in the chronic model (Fig. 3B).
Fig 3.
Efficacies of meropenem-clavulanate and amoxicillin-clavulanate in the acute (A) and chronic (B) mouse models for TB. The bacterial load at the beginning of treatment (early control) is indicated by the dashed lines. Solid horizontal lines indicate the mean values.
DISCUSSION
M. tuberculosis is intrinsically resistant to the beta-lactam class of antibiotics, which has been attributed to the actions of efflux pumps and beta-lactamases (2, 3, 4, 16). We have shown in vitro activity of 3 different classes of beta-lactams, penicillins, cephalosporins, and carbapenems, in combination with clavulanate, against replicating, as well as nonreplicating, M. tuberculosis. Cephalosporin-clavulanate or carbapenem-clavulanate showed lower synergy against replicating M. tuberculosis than the penicillin-clavulanate combination. This could be due to differences in the catalytic efficiencies of M. tuberculosis beta-lactamase in hydrolyzing various classes of beta-lactams (2). Cephalosporins and carbapenems were reported to have 10- to 100-fold lower Kcat/Km ratios for M. tuberculosis beta-lactamase than the penicillin class.
Carbapenems, like meropenem and faropenem, showed moderate bactericidal activity against M. tuberculosis ss18b, as reported previously (12, 17). However, none of the combinations with clavulanate were active against M. tuberculosis under hypoxia. This discrepancy in activity against nonreplicating M. tuberculosis in 2 different in vitro models could not be explained.
We tested 2 combinations for activity against replicating and nonreplicating M. tuberculosis in the mouse TB model to see if the in vitro activity could be translated in vivo. The required %TMIC for in vivo efficacy of carbapenems and penicillins against multiple bacterial pathogens has been reported to be 20 to 40% (18). Therefore, the dosing regimen for the mouse efficacy study was chosen to achieve 20 to 40% TMIC in mouse plasma over a period of 24 h. Lack of efficacy in both the acute and chronic mouse models may suggest that the %TMIC requirement could be significantly higher for M. tuberculosis. A similar magnitude of effect was also reported previously using a twice-daily regimen for the meropenem-clavulanate combination (9). However, the efficacy did not improve even after using a 3-times-daily regimen in this study.
Considering an average doubling time of 0.5 to 1.0 h for bacterial pathogens like Staphylococcus aureus, Pseudomonas aeruginosa, or Streptococcus pneumoniae, a 20 to 40% TMIC of beta-lactams may cover 5 or 6 generations over 24 h. The generation time for M. tuberculosis growing in the infected mouse lung is reported to vary between 24 h during rapid growth in the acute model to 1,676 h during the slowly growing/nonreplicating phase in the chronic model (19, 20). Since the bactericidal effect of beta-lactams is exerted during cell division, poor activity of these beta-lactams against M. tuberculosis in vivo could be due to an insufficient duration of exposure during its growth cycle.
Recently, the meropenem-clavulanate combination was shown to be efficacious in patients with XDR TB (8) using a 3-times-daily (2 g each time) dosing regimen. Based on the reported human PK for meropenem (Merrem product information; AstraZeneca, 27 February 2007), such a dosing regimen is expected to achieve 85% TMIC in human plasma in 24 h. Since meropenem is 98% free in plasma (9), the %TMIC is almost the same for the free-meropenem concentration. Therefore, lack of significant efficacy in the acute mouse model could be due to insufficient %TMIC in mouse plasma. On the other hand, lack of efficacy in the chronic model could be due to insufficient %TMIC, as well as poor in vitro activity against nonreplicating M. tuberculosis.
Beta-lactams are known to inhibit the formation of 3-4 cross-links in the peptidoglycan of the bacterial cell wall through inhibition of d,d-transpeptidases. In addition to 3-4 cross-links, formation of 3-3 cross-links catalyzed by l,d-transpeptidases has been reported during stationary phase in M. tuberculosis (21). Another report (22) described the essentiality of l,d-transpeptidase during stationary phase or the slowly growing/nonreplicating phase in M. tuberculosis and efficacy of amoxicillin-clavulanate against an LDP2 mutant in the mouse chronic model. A once-daily dose of 200 mg/kg amoxicillin and 50 mg/kg clavulanate showed a 2-log-unit reduction in the lung CFU in mice infected with the LDP2 mutant. However, the same regimen did not show efficacy in mice infected with wild-type M. tuberculosis. Since LDP2 was reported to be mainly essential in the stationary phase, we expected better efficacy with an improved (twice daily instead of once daily) dosing regimen of the amoxicillin-clavulanate combination against replicating M. tuberculosis in the acute model. Therefore, the poor efficacy of the amoxicillin-clavulanate combination in the acute model in our study may suggest that LDP2 could be complementing for the activity of d,d-transpeptidases that are inhibited by beta-lactams during active replication of M. tuberculosis.
We conclude that the efficacy of the meropenem-clavulanate or amoxicillin-clavulanate combination in the mouse TB model was negligible, probably due to inadequate %TMIC in the mouse. The efficacy of these agents in XDR TB patients could be due to the 3-fold-higher %TMIC achieved in human plasma than in mouse plasma. Thus, demonstrating efficacy of novel BL-BLI combinations in the mouse model could be a challenging task due to their poor half-life in mice combined with the requirement for %TMIC as a PK-PD index. Therefore, in addition to the mouse model, in vitro models, like hollow-fiber reactors with humanized PK (23), could be used for screening novel anti-TB BL-BLI combinations.
Footnotes
Published ahead of print 18 March 2013
REFERENCES
- 1. Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, Noreddin AM, Karlowsky JA. 2007. Comparative review of the carbapenems. Drugs 67:1027–1052 [DOI] [PubMed] [Google Scholar]
- 2. Hugonnet JE, Blanchard JS. 2007. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry 46:11998–12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nampoothiri KM, Rubex R, Patel AK, Narayanan SS, Krishna S, Das SM, Pandey A. 2008. Molecular cloning, overexpression and biochemical characterization of hypothetical beta-lactamases of Mycobacterium tuberculosis H37Rv. J. Appl. Microbiol. 105:59–67 [DOI] [PubMed] [Google Scholar]
- 4. Tremblay LW, Hugonnet JE, Blanchard JS. 2008. Structure of the covalent adduct formed between Mycobacterium tuberculosis beta-lactamase and clavulanate. Biochemistry 47:5312–5316 [DOI] [PubMed] [Google Scholar]
- 5. Harrison MP, Moss SR, Featherstone A, Fowkes AG, Sanders AM, Case DE. 1989. The disposition and metabolism of meropenem in laboratory animals and man. J. Antimicrob. Chemother. 24(Suppl. A):265–277 [DOI] [PubMed] [Google Scholar]
- 6. Novelli A, Mazzei T, Meli E, Conti S, Fallani S, Periti P. 1996. Clinical pharmacokinetics of meropenem after the first and tenth intramuscular administration. J. Antimicrob. Chemother. 37:775–781 [DOI] [PubMed] [Google Scholar]
- 7. Dauby N, Muylle I, Mouchet F, Sergysels R, Payen MC. 2011. Meropenem/clavulanate and linezolid treatment for extensively drug-resistant tuberculosis. Pediatr. Infect. Dis. J. 30:812–813 [DOI] [PubMed] [Google Scholar]
- 8. Payen MC, De Wit S, Martin C, Sergysels R, Muylle I, Van Laethem Y, Clumeck N. 2012. Clinical use of the meropenem-clavulanate combination for extensively drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 16:558–560 [DOI] [PubMed] [Google Scholar]
- 9. England K, Boshoff HI, Arora K, Weiner D, Dayao E, Schimel D, Via LE, Barry CE. 2012. Meropenem-clavulanic acid shows activity against Mycobacterium tuberculosis in vivo. Antimicrob. Agents Chemother. 56:3384–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sala C, Dhar N, Hartkoorn RC, Zhang M, Ha YH, Schneider P, Cole ST. 2010. Simple model for testing drugs against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 54:4150–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddy J, Madishetti S, Vachaspati PR. 2012. Fast mouse PK (Fast PK): a rapid screening method to increase pharmacokinetic throughput in pre-clinical drug discovery. Eur. J. Pharm. Sci. 47:444–450 [DOI] [PubMed] [Google Scholar]
- 15. Balasubramanian V, Solapure S, Gaonkar S, Mahesh Kumar KN, Shandil RK, Deshpande A, Kumar N, Vishwas KG, Panduga V, Reddy J, Ganguly S, Louie A, Drusano GL. 2012. Effect of coadministration of moxifloxacin and rifampin on Mycobacterium tuberculosis in a murine aerosol infection model. Antimicrob. Agents Chemother. 56:3054–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dinesh N, Sharma S, Balganesh M. 2013. Involvement of efflux pumps in the resistance to peptidoglycan synthesis inhibitors in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 57:1941–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang M, Sala C, Hartkoorn RC, Dhar N, Mendoza-Losana A, Cole ST. 2012. SS18b: a drug discovery tool for latent tuberculosis. Antimicrob. Agents Chemother. 56:5782–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501 [DOI] [PubMed] [Google Scholar]
- 19. Munoz-Elias EJ, Timm J, Botha T, Chan WT, Gomez JE, McKinney JD. 2005. Replication dynamics of Mycobacterium tuberculosis in chronically infected mice. Infect. Immun. 73:546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. 2009. A replication clock for Mycobacterium tuberculosis. Nat. Med. 15:211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubee V, Triboulet S, Mainardi JL, Etheve-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet JE, Arthur M. 2012. Inactivation of Mycobacterium tuberculosis L,D-transpeptidase LdtMt1 by carbapenems and cephalosporins. Antimicrob. Agents Chemother. 56:4189–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta R, Lavollay M, Mainardi J-L, Arthur M, Bishai WR, Lamichhane G. 2010. The Mycobacterium tuberculosis gene, ldtMt2, encodes a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat. Med. 16:466–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642–1651 [DOI] [PubMed] [Google Scholar]