Abstract
Agaricus brasiliensis (syn. A. subrufescens), a basidiomycete fungus native to the Atlantic forest in Brazil, contains cell walls rich in glucomannan polysaccharides. The β-(1→2)-gluco-β-(1→3)-mannan was isolated from A. brasiliensis mycelium, chemically modified by sulfation, and named MI-S. MI-S has multiple mechanisms of action, including inhibition of herpes simplex virus (HSV) attachment, entry, and cell-to-cell spread (F. T. G. S. Cardozo, C. M. Camelini, A. Mascarello, M. J. Rossi, R. J. Nunes, C. R. Barardi, M. M. de Mendonça, and C. M. O. Simões, Antiviral Res. 92:108–114, 2011). The antiherpetic efficacy of MI-S was assessed in murine ocular, cutaneous, and genital infection models of HSV. Groups of 10 mice were infected with HSV-1 (strain KOS) or HSV-2 (strain 333). MI-S was given either topically or by oral gavage under various pre- and posttreatment regimens, and the severity of disease and viral titers in ocular and vaginal samples were determined. No toxicity was observed in the uninfected groups treated with MI-S. The topical and oral treatments with MI-S were not effective in reducing ocular disease. Topical application of MI-S on skin lesions was also not effective, but cutaneously infected mice treated orally with MI-S had significantly reduced disease scores (P < 0.05) after day 9, suggesting that healing was accelerated. Vaginal administration of MI-S 20 min before viral challenge reduced the mean disease scores on days 5 to 9 (P < 0.05), viral titers on day 1 (P < 0.05), and mortality (P < 0.0001) in comparison to the control groups (untreated and vehicle treated). These results show that MI-S may be useful as an oral agent to reduce the severity of HSV cutaneous and mucosal lesions and, more importantly, as a microbicide to block sexual transmission of HSV-2 genital infections.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) and HSV-2 are responsible for a wide range of diseases, affecting the skin or mucous membranes (cold sores, genital herpes, and gingivostomatitis), the eye (herpetic keratitis), or the central nervous system (necrotizing encephalitis and meningitis). Ocular HSV infections are the leading cause of infectious blindness in developed countries, and neonatal HSV-2 infection has a mortality rate of approximately 30% when antivirals are used (1, 2).
In the United States, 57.7% of the population was seropositive for HSV-1 between 1999 and 2004, and the incidence of HSV-2 infection is approximately 20% for those older than 12 years of age (3). A study performed in Brazil between 1996 and 1997, with 1,090 people from the general population aged from 1 to 40 years, showed seroprevalence of 67.2% and 11.3% for HSV-1 and HSV-2, respectively (4). Another study performed in 2000 showed an HSV-2 seroprevalence of 42.9% in females and 25.9% in males (5).
Genital herpes is a common sexually transmitted infection (STI), and HSV is among the most frequent viral infections in AIDS patients, intensifying their morbidity and mortality. Moreover, HSV genital infection increases the risk of acquiring human immunodeficiency virus (HIV) in an unprotected relationship 3-fold (6–8), indicating that it is clearly a cofactor for the spread of HIV-1. In this sense, agents that would reduce the rate of acquisition of HSV genital infection could have a significant effect on the HIV-1 epidemic.
Several antivirals effective against HSV are approved for clinical use including acyclovir, valacyclovir, penciclovir, famciclovir, and docosanol. Although they are effective, they cannot eliminate latent virus. In addition, breakthrough reactivations can occur in the presence of the drugs. Resistant strains of virus can emerge, particularly in immunosuppressed patients, and toxic side effects can occur in some people (9). Given that once an infection is established it cannot be cleared, one attractive antiviral strategy is to prevent transmission of infection to new hosts. One potential approach to reduce or eliminate transmission is the use of microbicidal preparations prior to the initiation of genital contact.
Microbicides are prophylactic agents that can be applied topically in the vagina or rectum as a single agent or with other components that have the ability to prevent the transmission of STIs. A promising candidate must be efficacious, easy to use, nonirritating, and nontoxic and preferably have a broad spectrum of activity against common pathogens in the genital tract (10, 11). Considerable effort has been applied to the development of microbicides, especially to reduce the risk of sexual acquisition of HIV. Sulfated polysaccharides such as dextrin-2-sulfate, carrageenan, and cellulose sulfate have recently been evaluated in clinical trials (11–13). These compounds are thought to act primarily through inhibition of fusion between the membranes of the pathogen and mucosal cells and/or by binding to the pathogen and preventing attachment to the host receptors (14). However, the clinical trials for carrageenan and cellulose sulfate were halted early due to lack of significant effect on viral transmission (carrageenan) (15) or insufficient statistical power (cellulose sulfate) (16). Since there is no vaccine or microbicide available to prevent HSV infections and current antiherpes drugs cannot eliminate latent virus and may have side effects or induce the emergence of drug-resistant virus strains, the search for new agents capable of preventing and/or treating HSV infections is still needed.
We previously determined that the sulfated derivative of a cell wall glucomannan obtained from Agaricus brasiliensis mycelium (MI-S) had in vitro anti-HSV-1 and 2 activities, primarily by inhibiting attachment and entry of the virus (17). In addition, we have recently determined that MI-S is a sulfated β-(1→2)-gluco-β-(1→3)-mannan (Fig. 1) (18). Considering the economically feasible biotechnological production of A. brasiliensis mycelial biomass and the promising in vitro antiherpetic activity of MI-S, the goal of this study was to characterize the anti-HSV spectrum of activity of MI-S, using different viral strains, as well as to assess its in vivo anti-HSV-1 and anti-HSV-2 activity in murine models of herpes ocular, cutaneous, and genital infections.
Fig 1.
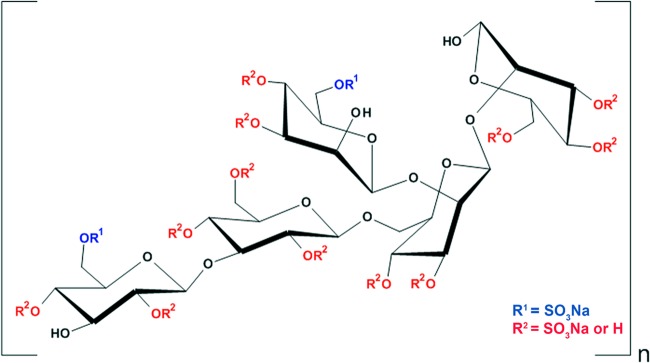
Structure of the sulfated derivative of Agaricus brasiliensis mycelial polysaccharide (MI-S). Determination of the structure was described in reference 18.
MATERIALS AND METHODS
Compound.
The sulfated derivative of Agaricus brasiliensis Wasser (syn. Agaricus subrufescens Peck) mycelial polysaccharide was prepared as previously described (17).
In vitro antiviral activity.
The antiviral spectrum of activity of MI-S against HSV-1 strains CJ311, CJ360, CJ394, OD4 (19, 20), and KOS as well as HSV-2 strain 333 was evaluated by yield reduction assay. Briefly, Vero cells (2.5 × 105 cells/ml) were seeded into 24-well plates and incubated for 24 h at 37°C in a CO2 incubator. Cells were infected at a multiplicity of infection (MOI) of 1 and simultaneously incubated with MI-S at concentrations of 0.73, 0.36, 0.18, 0.09, 0.04, and 0 μM for HSV strains CJ360, CJ394, OD4, and KOS. For strains CJ311 and 333, the following concentrations of MI-S were used in the simultaneous treatment protocol: 0.18, 0.09, 0.04, 0.02, 0.01, and 0 μM. For the postinfection treatment, MI-S concentrations of 1.17, 0.58, 0.29, 0.14, 0.07, and 0 μM were used and added 1 h after the adsorption period. Treated cells were then incubated at 37°C in a CO2 incubator for 24 h. Viral titers were determined by plaque assay as we described previously (21). The 50% effective concentrations (EC50) were determined by regression analysis of concentration-response curves.
In vivo antiviral activity.
In vivo antiviral activity was determined as follows.
Animals.
Groups of 10 female BALB/c mice, 4 to 6 weeks old (Harlan Sprague-Dawley, Indianapolis, IN), were used. The animal studies were approved by the University of Wisconsin—Madison Institutional Animal Care and Use Committee (protocol M00267) and conformed to the NIH guidelines on the care and use of animals in research.
HSV-1 corneal infection.
Under isoflurane anesthesia, the right corneas of mice were scratched three times vertically and three times horizontally with a sterile 30-gauge needle. Mice were infected by application of 5 μl of Dulbecco's modified Eagle medium (DMEM) (2% serum) containing 1.0 × 105 PFU of HSV-1 strain KOS to the scarified cornea. There were 6 groups in this study. Group 1 corresponded to the infected, untreated group. For group 2, the mice were treated topically (eye drop) with 3 μl of MI-S formulated in phosphate-buffered saline (PBS) at 11.69 μM (1 mg/ml). Treatments began within 4 h of infection and continued 5 times per day for 10 days. Group 3 was treated with MI-S, as described for group 2, but the mice were not infected. Mice in group 4 were infected and then treated topically (eye drop) with 3 μl of vehicle (PBS) 5 times per day for 10 days. For group 5, infected mice were given MI-S in PBS by oral gavage (100 μl) at a dose of 1 mg twice per day for 7 days postinfection. For group 6, infected mice were gavaged with PBS twice per day (100 μl) for 7 days (21).
Ocular disease scoring.
The mice were examined microscopically to determine ocular disease severity by using a scoring system as described previously (21). Briefly, blepharitis was scored as follows: 1, puffy eyelids; 2, puffy eyelids with some crusting; 3, eye swollen shut with severe crusting; and 4, eye completely swollen shut and crusted over. Vascularization was scored as follows: 1, <25% of the cornea involved; 2, 25% to 50% corneal involvement; and 3, >50% corneal involvement. Stromal disease was scored as follows: 1, cloudiness, some iris detail visible; 2, iris detail obscured; 3, cornea totally opaque; and 4, corneal perforation. Scoring was done in a masked fashion.
Measurement of ocular viral titers.
On days 1, 3, 5, 7, 9, 11, and 13 after infection, tear samples were harvested from the right eye as follows. The mice were anesthetized with isoflurane, and the infected corneas were flushed with 10 μl of DMEM (2% serum). The rinse was added to 190 μl of DMEM (2% serum) and stored at −80°C until use. Serial 10-fold dilutions were quantified by using a standard plaque assay on Vero cells (21).
HSV-1 cutaneous infection.
The right midflank of each mouse was clipped and depilated with a chemical depilatory (Nair; Church and Dwight, Princeton, NJ). Three days later, the skin was scratched in a grid-like pattern using a sterile 27-gauge needle, and 15 μl of HSV-1 KOS (1 × 105 PFU) in DMEM with 2% serum was applied to the scarified area (22). A cream base, formulated with 70% (wt/wt) of Eucerin (Smith & Nephew Inc., Lachine, Canada), 15% (wt/wt) of white mineral oil (USP; Sigma, St. Louis, MO), and 13% (wt/wt) of water (23), was mixed with MI-S to achieve a final concentration of 2% (wt/wt). There were 8 treatment arms with 10 mice per group. Group 1 corresponded to the infected, untreated group. Group 2 received the topical treatment with 2% MI-S cream, applied topically on the scratched area, beginning within 4 h after infection and continued five times per day, for 8 days. Group 3 was treated with MI-S, as described for group 2, but the mice were not infected. For group 4 (vehicle), infected mice were treated with base cream only, according to the schedule described for group 2. Mice in group 5 were given 100 μl of MI-S in PBS, via oral gavage at a dose of 1 mg twice a day for 7 days postinfection. Mice in group 6 were orally gavaged with PBS only, twice a day for 7 days postinfection. For group 7, mice were orally gavaged with 1 mg of MI-S in PBS (100 μl) twice a day, beginning 3 days before virus inoculation and continuing to 7 days postinoculation. The mice in group 8 were orally gavaged with PBS only, as described for group 7.
HSV-1 cutaneous disease scoring.
The cutaneous herpes infection was scored according to the method of Park et al. (24) with minor modifications, as follows: 0, no visible infection; 1, yellowish swelling visible (prelesions), or healing ulcers; 2, ulcers at inoculation site only with swelling, crust, and/or erythema, or healing ulcers with crusting; 3, spreading ulceration, crusting, some clear ulcers, or healing ulcers beyond the shaved region; 4, zosteriform rash; 5, rash confluent but not yet necrotic or ulcerated; 6, complete rash with necrosis or ulceration, hind limb paralysis, bloating, or death. Scoring was done in a masked fashion.
HSV-2 genital infection.
The model previously described by Bernstein et al. (25), with minor modifications, was used. At days 5 and 1 prior to intravaginal infection, mice were subcutaneously injected with 2 mg of medroxyprogesterone acetate (Greenstone, Peapack, NJ, USA) in the upper back. The mice were then inoculated intravaginally with 10 μl of virus suspension (HSV-2 strain 333, 1 × 105 PFU). There were 8 treatment arms with 10 mice per group. Group 1 mice were infected and left untreated. Group 2 was the postinfection treatment group. The mice were intravaginally administered 25 μl of MI-S solution prepared in 2% methylcellulose (final concentration, 10 mg/ml; 116.93 μM) three times a day, beginning within 4 h of infection and continuing for 8 days. Group 3 was designed to test the toxicity of MI-S. Noninfected animals received 25 μl of MI-S at 10 mg/ml (116.93 μM) in the 2% methylcellulose vehicle intravaginally three times a day, as described for group 2. For group 4, the infected mice were treated as described for group 2, but only vehicle (2% methylcellulose) was administered. Mice in group 5 were orally gavaged with 1 mg of MI-S in PBS (100 μl) twice a day for 7 days postinfection. The mice in group 6 were orally gavaged with PBS only, as described for group 5. For mice in group 7, 25 μl of MI-S prepared in 2% methylcellulose vehicle (final concentration, 20 mg/ml; 233.86 μM) was administered once intravaginally 20 min before virus inoculation. For group 8, 25 μl of the 2% methylcellulose vehicle was administered once intravaginally 20 min before virus inoculation.
Genital disease scoring.
Clinical signs of genital infection were scored on days 1, 3, 5, 6, 7, 8, and 9 postinfection, according to a composite scale from 0 to 5, as described by Gill et al. (26), with minor modifications. The disease was graded as follows: 0, no sign of infection; 1, slight redness of external genitalia; 2, swelling and redness of external genitalia, and/or pus/mucus; 3, severe swelling of external genitalia with pus/mucus and some alopecia in the surrounding area; 4, ulceration of genital tissue, redness, and swelling; 5, increased ulceration, redness, swelling, hind limb paralysis, or death. Mice that became moribund with hind limb paralysis were euthanized. The body weights were determined in all studies at the day before infection (baseline) and at days 3 and 7 postinfection. The mortality rates of mice intravaginally infected with HSV-2 were calculated for 9 days after virus inoculation. Scoring was done in a masked fashion.
Measurement of vaginal viral titers.
Samples to determine viral shedding were obtained prior to the administration of MI-S on days 1, 3, 5, 7, and 9 after infection by vaginal swabbing with a DMEM-moistened Calgiswab (Pur-Wraps). Each swab was placed in 200 μl of DMEM supplemented with 2% serum and stored at −80°C. Serial dilutions of the samples were quantified by plaque assay on Vero cells, as we described previously (21).
Histopathological analysis.
At the time of sacrifice, the genital tissues were excised, fixed in 4% paraformaldehyde in PBS, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Slides were examined under ×100 and ×400 magnification using the Qimage analysis system. The transverse sections of vaginal tissue were examined for epithelial exfoliation, vascular congestion, leukocyte infiltration, mucus, and lamina propria thickness (edema).
Statistical analysis.
Statistical analyses were performed using Graph Pad Prism version 5.01. Differences in disease score data (means ± standard errors of the mean [SEM]) among groups were compared for each day through analysis of variance (ANOVA) followed by the Bonferroni or Student-Newman-Keuls multiple-comparison test. Viral titers were compared by ANOVA and Dunn's multiple-comparison test. The survival curve comparison between treated and control groups was performed by the log rank (Mantel-Cox) test. A P value of <0.05 was considered statistically significant.
RESULTS
In vitro antiviral evaluation.
Previously, we characterized the antiviral activity of MI-S against one HSV-1 and one HSV-2 strain. To determine if MI-S had broad-spectrum activity against herpes simplex virus, we assessed the activity against four ocular HSV-1 isolates (CJ 360, CJ311, CJ394, and OD4 [19, 20]) as well as HSV-1 KOS and HSV-2 333 in a yield reduction assay, adding the MI-S either simultaneously with the virus or 1 h after the adsorption period. The resulting EC50s are shown in Table 1. The EC50s for the HSV-1 strains when MI-S was added simultaneously ranged from 2.35 to 17.27 μg/ml (0.03 to 0.20 μM). When MI-S was added after the adsorption period, the EC50s increased 1.10- to 4.56-fold for all of the HSV-1 strains tested. For HSV-2 333, the EC50 for the postinfection treatment was 2.8-fold higher than when MI-S was added simultaneously. These results are consistent with our previous findings (17) and suggest that MI-S inhibits multiple strains of HSV-1 and HSV-2.
Table 1.
Spectrum of anti-HSV activity of Agaricus brasiliensis sulfated polysaccharide (MI-S)
| Virus strain | EC50a (μg/ml) (μM) |
|
|---|---|---|
| Simultaneous treatment | Postinfection treatment | |
| HSV-1 CJ360 | 15.76 ± 3.84 (0.18 ± 0.04) | 17.79 ± 1.18 (0.21 ± 0.01) |
| HSV-1 CJ311 | 2.35 ± 0.83 (0.03 ± 0.01) | 29.98 ± 2.54 (0.35 ± 0.03) |
| HSV-1 CJ394 | 5.67 ± 0.77 (0.07 ± 0.01) | 25.97 ± 0.93 (0.30 ± 0.01) |
| HSV-1 KOS | 17.27 ± 3.95 (0.20 ± 0.05) | 31.75 ± 3.35 (0.37 ± 0.04) |
| HSV-1 OD4 | 10.41 ± 1.96 (0.12 ± 0.02) | 37.53 ± 5.34 (0.44 ± 0.06) |
| HSV-2 333 | 4.73 ± 0.04 (0.06 ± 0.0004) | 13.32 ± 2.68 (0.16 ± 0.03) |
EC50, 50% inhibitory concentration. Values represent the means ± SD of three independent experiments.
In vivo toxicity assessment.
The in vivo toxicity of MI-S was assessed by including MI-S-treated but noninfected groups for each of the models. No local signals of toxicity, such as edema, erythema, corneal clouding, or swelling, were seen at the administration sites in all of the topical application models. We also did not observe evidence of systemic toxicity such as ruffled fur, behavioral changes, or weight loss in any of the animals given MI-S topically. In a pilot study in which mice were given 2 mg of MI-S by gavage three times per day (6 mg total), we found that the mice developed signs of toxicity that included ruffled fur and lethargy. When the dose of MI-S was lowered to 1 mg twice per day, there were no signs of toxicity. Thus, topical MI-S was well tolerated, but when given orally at high doses, it displayed toxic effects.
Activity against HSV-1 keratitis.
To determine if MI-S was active when topically applied to the cornea, we infected mice with HSV-1 strain KOS and then applied MI-S solution topically 5 times per day for 10 days. As shown in Fig. 2, there was no significant reduction in the severity of blepharitis. Significant differences in the severity of neovascularization and stromal disease between the untreated and MI-S- and PBS-treated groups were noted on some days (Fig. 2), but the MI-S and PBS groups were not significantly different. This indicates that the differences were not due to a specific antiviral effect of MI-S and that flushing the eye frequently can reduce disease severity.
Fig 2.
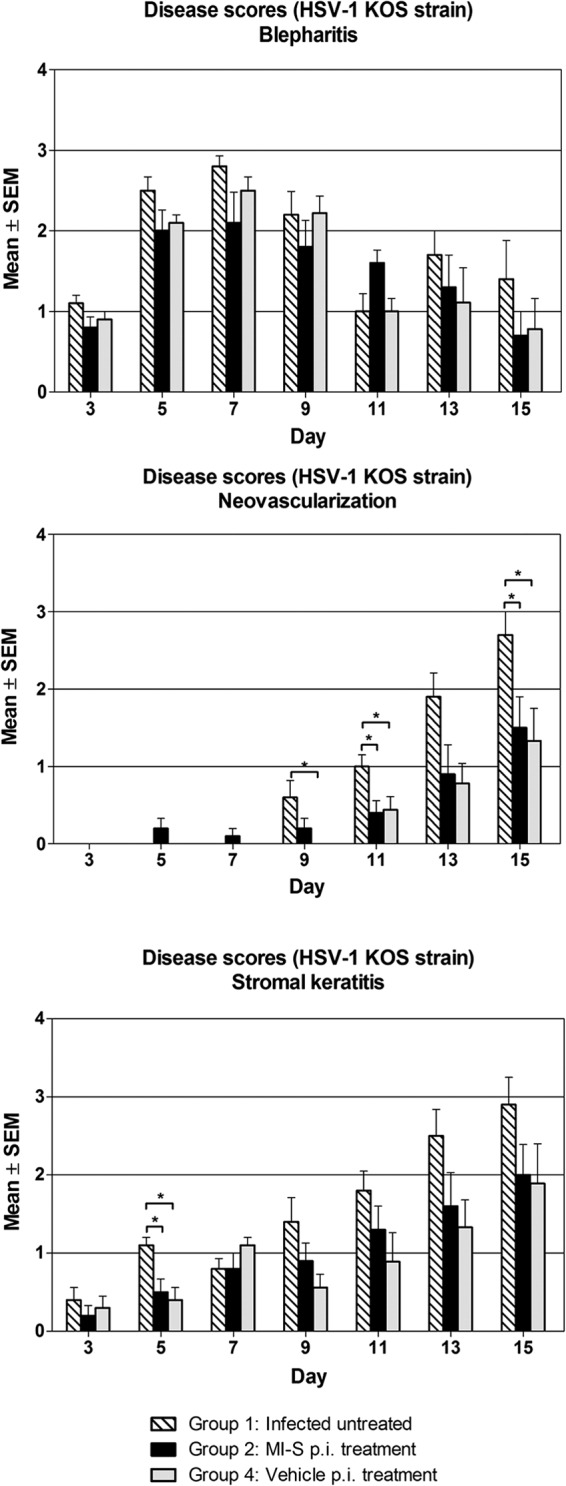
Effect of MI-S topical treatment on HSV-1 mean ocular disease scores. Mice (n = 10) had their right corneas infected and treated as follows: group 1, infected, untreated; group 2, MI-S treatment at 1 mg/ml (11.69 μM), 5 times daily after infection, for 10 days; group 4, vehicle (PBS) treatment, 5 times daily after infection for 10 days. The disease scores were determined as described in Materials and Methods. Values are expressed as means ± SEM from 10 mice in each group. Statistical analysis (one-way ANOVA followed by the Student-Newman-Keuls test) demonstrated significant differences among groups; *, P < 0.05.
On day 7, the MI-S and infected, untreated groups had significantly lower titers, but these groups were not different from each other, suggesting that the lower titers were not due to the antiviral activity of MI-S (Fig. 3). Thus, MI-S was not effective against HSV-1 keratitis, at least at the concentration tested when formulated in PBS. We also did not find significant effects on ocular disease or viral shedding in mice given MI-S orally (data not shown).
Fig 3.
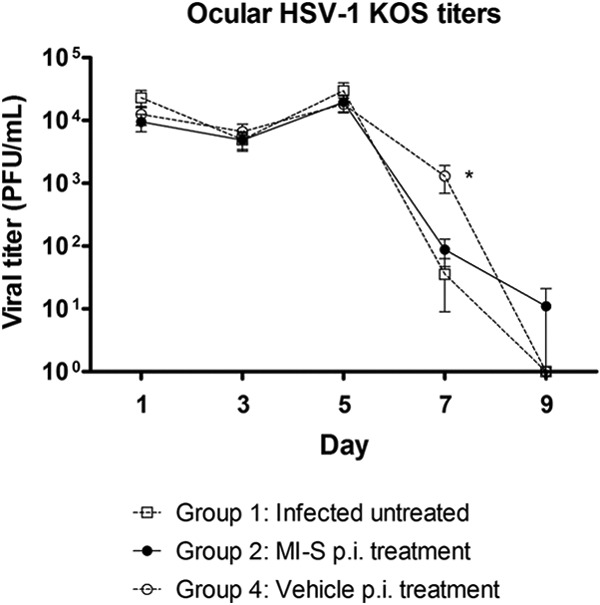
Effect of MI-S topical treatment on ocular viral titers. Mice (n = 10) had their right corneas infected and treated as follows: group 1, infected, untreated; group 2, MI-S treatment at 1 mg/ml (11.69 μM), 5 times daily after infection for 10 days; group 4, vehicle (PBS) treatment, 5 times daily after infection, for 10 days. Tear film samples were collected, and titers were determined as described in Materials and Methods. Values are expressed as means ± SEM from 10 mice in each group. Statistical analysis (one-way ANOVA followed by the Dunn's multiple comparison test) demonstrated significant differences between group 1 and groups 2 and 4, on day 7 postinfection; *, P < 0.05.
Antiviral activity against cutaneous HSV-1 infection.
The activity of MI-S against cutaneous HSV infection was tested by scarifying the skin on the right flank of the mouse, infecting with HSV-1 KOS, and applying 2% MI-S in cream 5 times per day for 8 days to the site of infection. The site of infection was swabbed every other day to measure viral titers. In this model, we found no significant differences in the disease scores between the infected untreated group, the vehicle (cream)-only group, and the group treated with MI-S cream (data not shown).
The results from treating cutaneously infected mice orally with MI-S are shown in Fig. 4A, and Fig. 4B shows representative examples of the skin lesions. Signs of infection were first seen on day 3 or 5 in both groups and continued to increase until day 8, when the scores peaked at approximately 3.0. The skin lesions then began healing and had almost completely healed by day 15 postinfection. When comparing the vehicle (PBS)-only group and the MI-S group, there were no significant differences in the disease scores through day 8. However, beginning at day 9, the disease scores in the MI-S-treated mice were significantly lower than those of the vehicle-only group through day 13, indicating that oral administration of MI-S enhanced healing of cutaneous HSV-1 lesions. The scores were not different on day 15 because the lesions were healed or almost healed in all groups.
Fig 4.
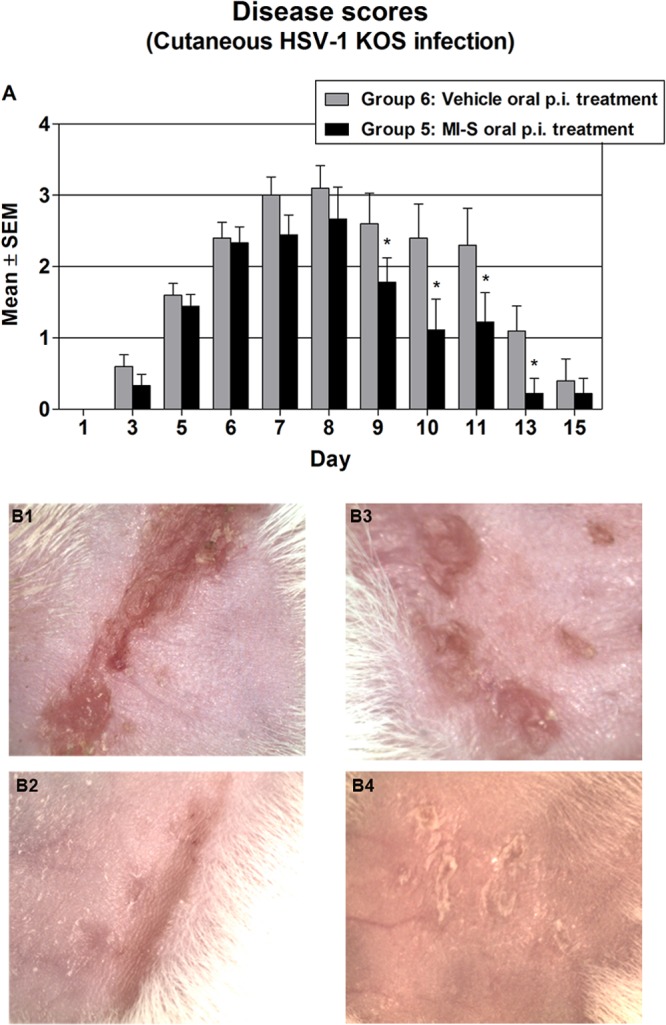
Effect of MI-S postinfection oral treatment on HSV-1 cutaneous disease. (A) Groups of 10 mice were treated after infection by oral gavage (100 μl), as follows: group 6, vehicle treatment, whereby mice were treated with PBS twice a day for 7 days; group 5, MI-S treatment, whereby MI-S solution was administered twice a day (2 mg/day) for 7 days. The severity of disease was scored from day 1 to day 15 as described in Materials and Methods. Values are expressed as means ± SEM from 10 mice in each group. Groups were compared by two-way ANOVA followed by the Bonferroni posttest; *, P < 0.05. (B) Representative examples of cutaneous infection at day 8. (B1) Zosteriform lesion, group 6, score 4; (B2) zosteriform healing lesion, group 5, score 3; (B3) spreading ulceration, group 6, score 3; (B4) healing ulcers, group 5, score 2.
We also tested an oral 3-day pretreatment with 7 days postinfection treatment; however, no differences in cutaneous HSV infection in comparison to the 7-day postinfection oral treatment were found (data not shown). None of the swabs from any of the groups yielded infectious virus, so we were not able to determine if there was any effect on viral replication (data not shown).
Activity against HSV-2 genital infection.
To determine if MI-S was effective against genital herpes, mice were treated intravaginally either once 20 min prior to infection with HSV-2 333 (microbicide model) or 3 times daily for 8 days, commencing within 4 h postinfection. The results are shown in Fig. 5. Signs of infection began to appear on day 5 and then continued to increase in severity through day 9, when the study was halted (see below). Postinfection treatment with MI-S did not significantly reduce the severity of genital herpes on any of the days (P > 0.05). However, there was a significant reduction in disease scores in the animals that received a single intravaginal dose of MI-S 20 min before infection.
Fig 5.
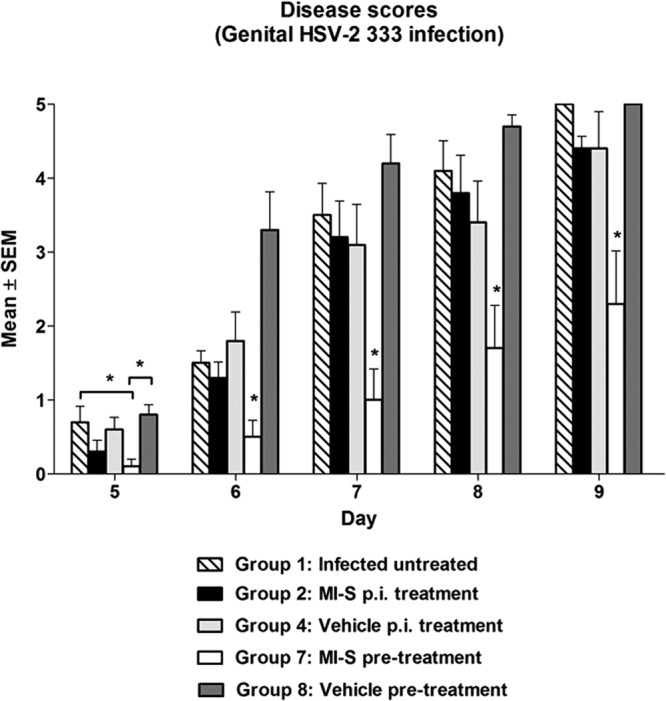
Effect of MI-S on HSV-2 mean genital pathology scores. Mice (n = 10) were intravaginally treated as follows: group 1, infected, untreated; group 2, MI-S treatment at 10 mg/ml (116.93 μM), 3 times daily after infection, for 8 days; group 4, vehicle-only treatment, 3 times daily after infection, for 8 days; group 7, MI-S treatment at 20 mg/ml (233.86 μM), once, 20 min before infection; group 8, vehicle-only treatment, once, 20 min before infection. The lesion scores were determined as described in Materials and Methods. Statistical analysis (one-way ANOVA followed by the Student-Newman-Keuls test) demonstrated significant differences among groups; *, P < 0.05. Group 7 was significantly different from all the others groups at days 6 to 9.
When viral titers were measured, we found that there was a significant reduction (at least 46-fold) in the amount of virus shed in the MI-S-pretreated group, compared to the untreated and vehicle-only-treated groups on day 1 postinfection (Fig. 6A). We also observed significant reduction in viral shedding on day 9 in the MI-S- and vehicle-pretreated mice, in relation to the infected untreated group. In the mice treated after infection (Fig. 6B), a significant reduction in viral shedding was seen only on day 1 postinfection in relation to the infected untreated group. The mortality data for the HSV-2 333-infected mice are shown in Fig. 7. By day 9, when the study was terminated due to high mortality in the untreated groups, only 40% of the mice given MI-S gel 20 min prior to infection had to be sacrificed due to extreme morbidity. In contrast, the infection was lethal to 100% of the mice in the infected, untreated group and the vehicle-pretreated group. The differences in mortality were significant when comparing the MI-S group to the control groups (P < 0.0001; Fig. 7A), while the two control groups were not significantly different from each other (P = 0.1649). Thus, a single preinfection dose of MI-S not only reduced the severity of disease and viral shedding but also protected the mice from lethal infection. Figure 7B shows that MI-S postinfection treatment slightly reduced animal mortality in relation to the other groups. However, a statistically significant reduction was observed only in comparison to the infected, untreated group (P = 0.0459). MI-S oral treatment was not effective against genital infection (data not shown).
Fig 6.
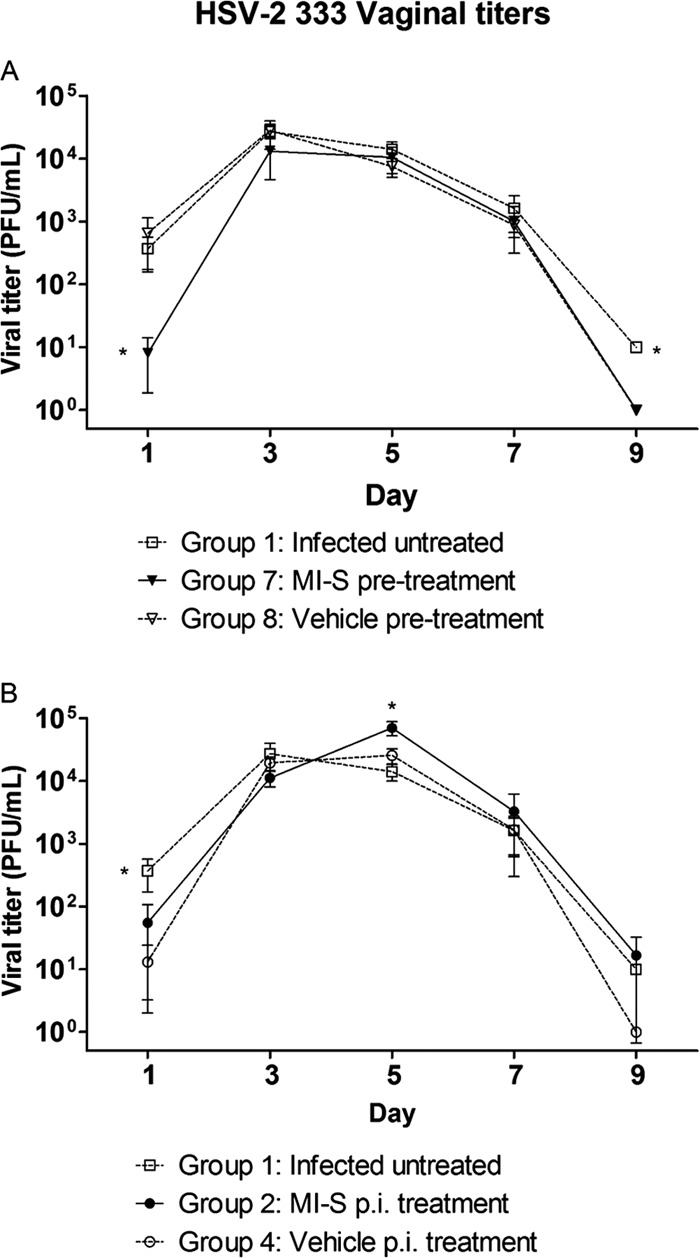
Effect of MI-S topical treatment on viral titers of vaginal swab samples. Mice (n = 10) were intravaginally treated as follows: Group 1, infected, untreated; Group 7, MI-S treatment at 20 mg/ml (233.86 μM), once, 20 min before infection; Group 8, vehicle-only treatment, once, 20 min before infection; Group 2, MI-S treatment at 10 mg/ml (116.93 μM), 3 times daily after infection, for 8 days; Group 4, vehicle treatment, 3 times daily after infection, for 8 days. The viral titers were determined by plaque assay as described in Materials and Methods. (A) Pretreatment; (B) postinfection treatment. Asterisks represent statistically significant differences in comparison to the other groups (P < 0.05) by one-way ANOVA followed by Dunn's multiple comparison test.
Fig 7.
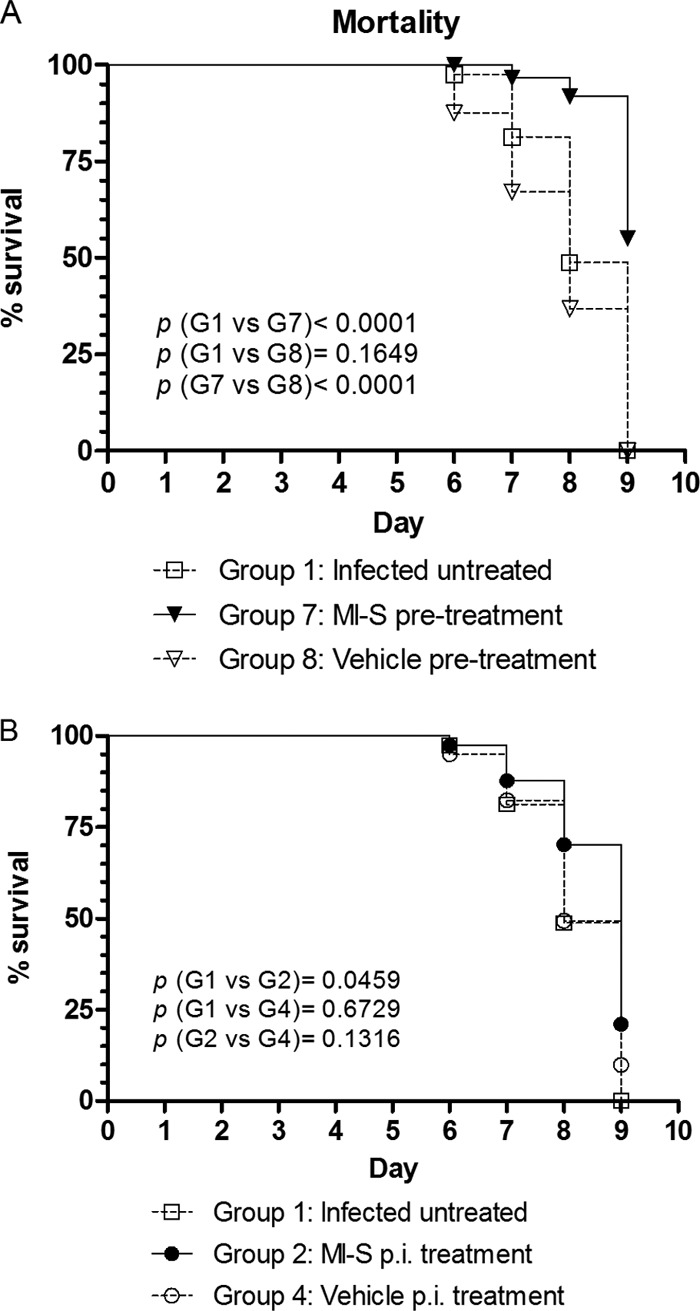
Effect of MI-S treatment on mortality of infected mice after HSV-2 vaginal challenge. Mice (n = 10) were intravaginally treated as follows: Group 1, infected, untreated; Group 7, MI-S treatment at 20 mg/ml (233.86 μM), once, 20 min before infection; Group 8, vehicle-only treatment, once, 20 min before infection; Group 2, MI-S treatment at 10 mg/ml (116.93 μM), 3 times daily after infection, for 8 days; Group 4, vehicle-only treatment, 3 times daily after infection, for 8 days. Survival was monitored for up to 9 days after virus infection. (A) Pretreatment; (B) postinfection treatment. The data for the different groups were compared by the log rank (Mantel-Cox) test; P values of <0.05 were considered statistically significant.
Histopathology of genital tissues.
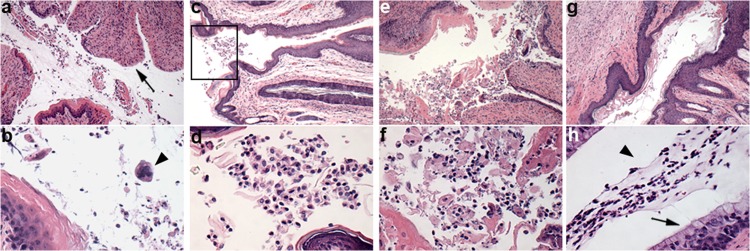
Vaginal tissues were collected from mice sacrificed on day 9, fixed, sectioned, and stained with hematoxylin and eosin for analysis. Representative results are shown in Fig. 8. The induction of diestrus by medroxyprogesterone acetate was confirmed by the presence of a mucified vaginal epithelium, desquamated epithelial cells, and mucus (Fig. 8c, d, and h).
Fig 8.
Representative photomicrography of vaginal tissue sections. (a, b) Group 1 (infected, untreated) showing denuded epithelium (arrow) and multinucleated cell (arrowhead); (c, d) group 7 (infected, MI-S pretreatment), vaginal orifice (boxed); (e, f) group 8 (infected, vehicle pretreatment); (g, h) group 3 (uninfected, MI-S treatment) showing mucified columnar epithelium (arrow) and mucus with epithelial cells in the lumen (arrowhead). Slides were stained with H&E; magnification for first and second rows, ×100 and ×400, respectively.
Figure 8a, which is from an infected untreated animal (group 1), shows a partially denuded mucosal epithelium with dying cells and inflammatory infiltrates consisting of neutrophils and mononuclear cells in the lumen. Multinucleated cells with intranuclear inclusion bodies, characteristic of HSV-2 infection, were also seen (Fig. 8b). Figure 8c and d show representative sections from animals of group 7 (MI-S pretreatment) displaying normal epithelia and a lack of inflammation. Sloughed epithelial cells were also seen in the lumen of the MI-S pretreated mice (Fig. 8d). Figure 8g and h show sections from uninfected animals treated with MI-S. The normal modified columnar epithelium with mucus and epithelial cells indicates that MI-S alone was not toxic and did not induce abnormalities in the tissue. In the mice infected and treated only with vehicle (Fig. 8e and f), we found severe epithelial exfoliation and inflammatory infiltrate, indicating that the vehicle did not inhibit the infection.
DISCUSSION
Sulfated polysaccharides have long been known to have antiviral effects (27). These long-chain anionic polymers predominantly inhibit viral entry but also may interfere with later stages of the viral life cycle, and they are potential candidates to treat and/or prevent herpes infections (28–35) and may have potential utility as microbicides to block transmission of sexually transmitted viral infections (10, 11). Recently, we described the in vitro anti-HSV-1 and HSV-2 activity of a sulfated derivative of Agaricus brasiliensis mycelial polysaccharide MI-S (17). The current paper presents a comprehensive evaluation of the in vivo activity of MI-S using ocular, cutaneous, and genital HSV infections as well as of the in vitro spectrum of activity of MI-S against several strains of HSV.
The results presented in Table 1 clearly show that MI-S was active against several strains of HSV (a panel of ocular HSV-1 isolates, as well as HSV-1 KOS and HSV-2 333), but the EC50s were higher than previously obtained results (17). This can be explained by the fact that, in the previous study, we used an MOI of 0.002 in a plaque reduction assay compared to the current study, in which an MOI of 1.0 was used in a yield reduction assay. The improved antiviral activity found in the simultaneous treatment compared to the postinfection treatment is most likely related to the fact that MI-S primarily acts in the early stages of the virus cycle, inhibiting the attachment and entry of HSV (17).
For the ocular model, topical application (0.015 mg/day) of MI-S did not significantly reduce the ocular disease scores or the viral titers in comparison to the vehicle-treated groups. Previously, a sulfated polysaccharide from the red microalga Porphyridium sp. was shown to be effective against HSV-1 ocular infection (30). Differences in the chemical features of the polysaccharides, the dosing and treatment schedule, and the animal model may contribute to the discrepancy. The authors used rabbits and higher doses of the sulfated polysaccharide (0.12 mg/day), and they began treatments immediately after infection. In another study, oral administration of fucoidan (9 kDa) in mice, at a dose of 5 mg/day for 1 week before virus inoculation to 1 week postinfection, or for 1 week postinfection, effectively suppressed the HSV ocular infection (34), whereas in our study oral administration of MI-S (2 mg/day) had no effect on ocular herpes.
Similar to the topical treatment for ocular HSV, we found that topical application in the cutaneous model had no effect on the disease scores. Skin swabs of the infected area did not yield infectious virus, so we were unable to determine if there was an effect on viral titers. Although MI-S was not effective in the topical cutaneous treatment model, mice treated orally with 2 mg of MI-S per day had significantly lower disease scores after day 9, suggesting that oral administration accelerated healing of the lesions. Showing results similar to ours, Cooper et al. (31) reported that the ingestion of a preparation of the brown alga Undaria pinnatifida, enriched in sulfated polyanions such as galactofucan, increased the healing rates of active herpes infections in humans and reduced reactivation in patients with latent infections. Previous studies have shown that dextran sulfates (10 to 500 kDa) can cross the intestinal epithelial layer in a cell culture system (36) and dermatan sulfate (475 kDa) is bioavailable following oral administration to humans (37). The human oral bioavailability of other sulfated polysaccharides such as heparin (7 kDa) and chondroitin sulfate (16 kDa) has also been demonstrated (38). The oral bioavailability of MI-S has not been determined, but since it is a polymer with an intermediate molecular mass of 86 kDa (17), it might display some degree of oral bioavailability. However, this needs to be evaluated.
One potential explanation for the effect of orally administered MI-S on cutaneous HSV infection is that the polysaccharide stimulates one or more components of the innate immune system and/or enhances adaptive immune responses. A number of different fungal extract components, including polysaccharides, have been shown to stimulate cytokine production, enhance activation of NK cells and T cells, and increase nitric oxide production by macrophages (34, 39, 40). Further studies are needed to determine if MI-S has immunostimulatory properties when administered orally and if this is responsible for the antiviral activity following oral administration.
Genital herpes infections cause significant morbidity and are a cofactor for transmission of HIV; thus, agents that could prevent sexual transmission of HSV are needed. When we tested the effect of postinfection topical treatment with MI-S in the genital model, we found that disease scores and viral titers were not significantly different from the vehicle-only control group. This finding is consistent with the lack of effect of topical treatments in the ocular and cutaneous models. When MI-S was tested in a microbicidal model in which it was prepared in a gel and administered intravaginally 20 min prior to infection, we found a significant reduction in the vaginal mean disease scores (all days), viral titers (day 1), and mortality compared to the control groups (untreated and vehicle treated). Since MI-S was shown to be a potent in vitro HSV entry inhibitor (17), we propose that this was the mechanism by which MI-S prevented the in vivo genital infection. A single dose of 500 μg of MI-S reduced the mortality by approximately 50%. This in vivo protective effect occurred at a dose that was at least 2 × 103 times higher than the in vitro EC50 (Table 1; HSV-2 333 EC50 = 4.73 μg/ml; 0.055 μM), which corresponded to a 0.24-μg dose. According to Zeitlin et al. (41), the in vivo 50% effective concentration of the sulfated polysaccharides carrageenan, dextran sulfate, fucoidan, heparin, and heparin sulfate against HSV-2 genital infection in mice ranged from 101 to 105 times higher than in cell culture. Other sulfated polysaccharides have previously been shown to have anti-HSV-2 activity in the murine genital model with increased effectiveness occurring when mice received intravaginal treatments both pre- and postinfection (28, 32), but this combined-treatment regimen was not tested in this work.
There is a significant need for antiviral preparations that women can use without the necessary involvement of their partner, and a great deal of effort and funding has been invested in developing microbicide products. Several clinical trials have recently been conducted using a variety of products, with mixed results. Some of these included sulfated polysaccharides (e.g., carrageenan [Carraguard]) or other polyanions. Carraguard was not effective, and this trial was terminated early (15). Other trials were halted early because of a lack of sufficient power in the design (16). The fact that these previous trials have yielded mixed results could lead one to conclude that sulfated polysaccharides or other polyanions should not be developed further. However, it is clear that all sulfated polysaccharides are not equivalent in their antiviral activity. For example, MI-S was protective in the genital mouse model, whereas dextran sulfate was not (29). The biological activity of sulfated polysaccharides depends on different factors such as the sugar composition, the molecular weight, the carbohydrate linkages and conformation, and the degree of substitution and position of the sulfate groups. Therefore, before concluding that sulfated polysaccharides should not be pursued for development, several different preparations, including MI-S, need to be tested.
In summary, we have demonstrated the in vivo potential of MI-S for the oral treatment of cutaneous herpes. We have also shown that MI-S was active against genital HSV-2 infection when administered before vaginal challenge and therefore has a potential to be developed as a microbicide. Further in vivo studies to determine the mechanism of action, including potential immunomodulatory activities, the pharmacokinetics, toxicity, and activity against other sexually transmitted pathogens, including HIV-1, are warranted in order to fully explore the potential use of MI-S as an antiviral microbicide.
ACKNOWLEDGMENTS
This study was supported by research fellowships from the Brazilian funding agency CNPq (Process numbers 143226/2008-8 and 201264/2011-0), as well as by NIH grant EY018597 (C.R.B.), Core Grant for Vision Research P30 EY16665 (C.R.B.), and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences at the University of Wisconsin—Madison.
C.R.B. is the Retina Research Foundation Walter Helmerich Professor.
Footnotes
Published ahead of print 18 March 2013
REFERENCES
- 1. Roizman B, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses, p 2502–2601 In Knipe DM, Howley PM, Griffin D, Lamb R, Martin M, Roizman B, Straus SE. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2. Liesegang TJ. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13 [DOI] [PubMed] [Google Scholar]
- 3. Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973 [DOI] [PubMed] [Google Scholar]
- 4. Clemens SAC, Farhat CK. 2010. Seroprevalence of herpes simplex 1-2 antibodies in Brazil. Rev. Saude Publica 44:726–734 [DOI] [PubMed] [Google Scholar]
- 5. Cowan FM, French RS, Mayaud P, Gopal R, Robinson NJ, Artimos De Oliveira S, Faillace T, Uusküla A, Nygard-Kibur M, Ramalingam S, Sridharan G, El Aouad R, Alami K, Rbai M, Sunil-Chandra NP, Brown DW. 2003. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex. Transm. Infect. 79:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fatahzadeh M, Schwartz RA. 2007. Human Herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 57:737–763 [DOI] [PubMed] [Google Scholar]
- 7. Gupta R, Warren T, Wald A. 2007. Genital herpes. Lancet 370:2127–2137 [DOI] [PubMed] [Google Scholar]
- 8. Van de Perre P, Segondy M, Foulongne V, Ouedraogo A, Konate I, Huraux J-M, Mayaud P, Nagot N. 2008. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect. Dis. 8:490–497 [DOI] [PubMed] [Google Scholar]
- 9. Piret J, Boivin G. 2011. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 55:459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buckheit RW, Jr, Watson KM, Morrow KM, Ham AS. 2010. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Res. 85:142–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Obiero J, Mwethera PG, Wiysonge CS. 2012. Topical microbicides for prevention of sexually transmitted infections. Cochrane Database Syst. Rev. 6:CD007961 doi:10.1002/14651858.CD007961.pub2 [DOI] [PubMed] [Google Scholar]
- 12. Van Damme L, Szpir M. 2012. Current status of topical antiretroviral chemoprophylaxis. Curr. Opin. HIV AIDS 7:520–525 [DOI] [PubMed] [Google Scholar]
- 13. Mertenskoetter T, Kaptur PE. 2011. Update on microbicide research and development—seeking new HIV prevention tools for women. Eur. J. Med. Res. 16:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdool Karim SS, Baxter C. 2012. Overview of microbicides for the prevention of human immunodeficiency virus. Best Pract. Res. Clin. Obst. Gynaecol. 26:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977–1987 [DOI] [PubMed] [Google Scholar]
- 16. Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, Deese J, Crucitti T, Taylor D. 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 359:463–472 [DOI] [PubMed] [Google Scholar]
- 17. Cardozo FTGS, Camelini CM, Mascarello A, Rossi MJ, Nunes RJ, Barardi CR, de Mendonça MM, Simões CMO. 2011. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antiviral Res. 92:108–114 [DOI] [PubMed] [Google Scholar]
- 18. Cardozo FTGS, Camelini CM, Cordeiro MNS, Mascarello A, Malagoli BG, Larsen I, Rossi MJ, Nunes RJ, Braga FC, Brandt CR, Simões CMO. 17 March 2013. Characterization and cytotoxic activity of sulfated derivatives of polysaccharides from Agaricus brasiliensis. Int. J. Biol. Macromol. [Epub ahead of print.] pii: S0141-8130(13)00112-8. doi:10.1016/j.ijbiomac.2013.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grau DR, Visalli RJ, Brandt CR. 1989. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Invest. Ophthalmol. Vis. Sci. 30:2474–2480 [PubMed] [Google Scholar]
- 20. Berdugo M, Larsen IV, Abadie C, Deloche C, Kowalczuk L, Touchard E, Dubielzig R, Brandt CR, Behar-Cohen F, Combette JM. 2012. Ocular distribution, spectrum of activity, and in vivo viral neutralization of a fully humanized anti-herpes simplex virus igg fab fragment following topical application. Antimicrob. Agents Chemother. 56:1390–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandt CR, Akkarawongsa R, Altmann S, Jose G, Kolb AW, Waring AJ, Lehrer RI. 2007. Evaluation of a theta-defensin in a murine model of herpes simplex virus type 1 keratitis. Invest. Ophthalmol. Vis. Sci. 48:5118–5124 [DOI] [PubMed] [Google Scholar]
- 22. Chuanasa T, Phromjai J, Lipipun V, Likhitwitayawuid K, Suzuki M, Pramyothin P, Hattori M, Shiraki K. 2008. Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antiviral Res. 80:62–70 [DOI] [PubMed] [Google Scholar]
- 23. Brandt CR, Spencer B, Imesch P, Garneau M, Deziel R. 1996. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob. Agents Chemother. 40:1078–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park HJ, Kurokawa M, Shiraki K, Nakamura N, Choi JS, Hattori M. 2005. Antiviral activity of the marine alga Symphyocladia latiuscula against herpes simplex virus (HSV-1) in vitro and its therapeutic efficacy against HSV-1 infection in mice. Biol. Pharm. Bull. 28:2258–2262 [DOI] [PubMed] [Google Scholar]
- 25. Bernstein DI, Goyette N, Cardin R, Kern ER, Boivin G, Ireland J, Juteau JM, Vaillant A. 2008. Amphipathic DNA polymers exhibit antiherpetic activity in vitro and in vivo. Antimicrob. Agents Chemother. 52:2727–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gill N, Rosenthal KL, Ashkar AA. 2005. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J. Virol. 79:4470–4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green RH, Woolley DW. 1947. Inhibition by certain polysaccharides of hemagglutination and of multiplication of influenza virus. J. Exp. Med. 86:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohta Y, Lee JB, Hayashi K, Hayashi T. 2009. Isolation of sulfated galactan from Codium fragile and its antiviral effect. Biol. Pharm. Bull. 32:892–898 [DOI] [PubMed] [Google Scholar]
- 29. Piret J, Lamontagne J, Bestman-Smith J, Roy S, Gourde P, Désormeaux A, Omar RF, Juhász J, Bergeron MG. 2000. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J. Clin. Microbiol. 38:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huheihel M, Ishanu V, Tal J, Arad S. 2002. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 50:189–200 [DOI] [PubMed] [Google Scholar]
- 31. Cooper R, Dragar C, Elliot K, Fitton JH, Godwin J, Thompson K. 2002. GFS, a preparation of Tasmanian Undaria pinnatifida is associated with healing and inhibition of reactivation of Herpes. BMC Complement. Altern. Med. 2:11 doi:10.1186/1472-6882-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carlucci MJ, Scolaro LA, Noseda MD, Cerezo AS, Damonte EB. 2004. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res. 64:137–141 [DOI] [PubMed] [Google Scholar]
- 33. Baba M, Snoeck R, Pauwels R, De Clercq E. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayashi K, Nakano T, Hashimoto M, Kanekiyo K, Hayashi T. 2008. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 8:109–116 [DOI] [PubMed] [Google Scholar]
- 35. Herold BC, Siston A, Bremer J, Kirkpatrick R, Wilbanks G, Fugedi P, Peto C, Cooper M. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang E, Kabcenell AK, Coleman JR, Robson J, Ruffles R, Yazdanian M. 2001. Permeability measurement of macromolecules and assessment of mucosal antigen sampling using in vitro converted M cells. J. Pharmacol. Toxicol. Methods 46:93–101 [DOI] [PubMed] [Google Scholar]
- 37. Dawes J, Hodson BA, Pepper DS. 1989. The absorption, clearance and metabolic fate of dermatan sulphate administered to man—studies using a radioiodinated derivative. Thromb. Haemostasis 62:945–949 [PubMed] [Google Scholar]
- 38. Volpi N, Cusmano M, Venturelli T. 1995. Qualitative and quantitative studies of heparin and chondroitin sulfates in normal human plasma. Biochim. Biophys. Acta 1243:49–58 [DOI] [PubMed] [Google Scholar]
- 39. Yang T, Jia M, Zhou S, Pan F, Mei Q. 2012. Antivirus and immune enhancement activities of sulfated polysaccharide from Angelica sinensis. Int. J. Biol. Macromol. 50:768–772 [DOI] [PubMed] [Google Scholar]
- 40. Brandt CR, Piraino F. 2000. Mushroom antivirals. Recent Res. Dev. Antimicrob. Agents Chemother. 4:11–26 [Google Scholar]
- 41. Zeitlin L, Whaley KJ, Hegarty TA, Moench TR, Cone RA. 1997. Tests of vaginal microbicides in the mouse genital herpes model. Contraception 56:329–335 [DOI] [PubMed] [Google Scholar]