Abstract
The effect of delayed antifungal therapy in critically ill infants with invasive candidiasis has not been studied. Our objective was to evaluate the effect of time to initiation of antifungal therapy (TIA) on mortality, disseminated disease, and postinfection hospital stay. We conducted a cohort study of critically ill infants with cultures positive for Candida from 1990 to 2008. TIA was defined as the number of hours from the collection of the first positive culture until the start of antifungal therapy. Of 96 infants, 57% were male, the median gestational age was 27 weeks (range, 23 to 41 weeks), and the median birth weight was 956 g (range, 415 to 6,191 g). Most subjects received amphotericin B deoxycholate. TIA was ≤24 h for 35% of infants, between 25 and 48 h for 42%, and >48 h for 23%. Eleven subjects died during hospitalization, and 22% had disseminated candidiasis. The median duration of hospital stay postinfection was 53 days (range, 6 to 217 days). Both univariate and multivariate analyses demonstrated that TIA was not associated with mortality, disseminated disease, or hospital stay postinfection. However, ventilator use for >60 days significantly increased the risk of death (odds ratio [OR], 9.5; 95% confidence interval [CI], 2.2 to 66.7; P = 0.002). Prolonged candidemia increased the risk of disseminated disease by 10% per day of positive culture (OR, 1.1; 95% CI, 1.08 to 1.2; P = 0.007), and low gestational age was associated with increased neonatal intensive care unit (NICU) stay after the first positive Candida culture by 0.94 weeks (95% CI, 0.70 to 0.98; P < 0.001). The TIA was not associated with all-cause mortality, disseminated candidiasis, and postinfection length of hospital stay.
INTRODUCTION
Neonatal candidiasis is associated with significant morbidity and mortality. Despite the availability of antifungal agents, the mortality rate attributable to neonatal candidemia ranges from 10 to 25% (1, 2). Up to one-half of infected infants may develop adverse neurodevelopmental outcomes, a rate which is significantly higher than that for those without candidemia (1). In addition, neonates with candidemia have prolonged hospital stays and increased associated costs (3, 4).
Delayed initiation of antibiotics has been associated with increased durations of hospitalization, intensive care unit (ICU) stay, and mechanical ventilation in critically ill children (5) and adults (6–8). Three studies, all conducted with adult patients with invasive candidiasis, have explored the temporal effect of initiation of antifungal therapy on patient outcomes, including mortality, ICU stay, length of hospitalization after isolation of the organism, and treatment failure (9–11). Two studies demonstrated that patients with a delay in antifungal therapy (primarily fluconazole) by 12 to 72 h after collection of positive cultures were 1.5 to 2 times more likely to die than those without a delay (9, 11). One study also showed prolonged ICU stay in those starting antifungal therapy ≥12 h after collection of positive cultures (11). A decrease in the response rate and increases in both time to achieve clinical stability and hospital stay postinfection were also observed when caspofungin initiation was delayed for 72 h after collection of positive cultures (10).
To date, there have been no published studies evaluating the effect of time to initiation of antifungal therapy (TIA) in critically ill infants, a vulnerable population at high risk for significant morbidity and mortality secondary to invasive candidiasis. Our study aimed to determine the association of TIA in neonates with invasive candidiasis to treatment outcomes, including mortality, disseminated candidiasis, and length of neonatal ICU (NICU) stay postinfection.
MATERIALS AND METHODS
In this historical cohort study, we evaluated the medical records of critically ill infants diagnosed with candidiasis at Miller Children's Hospital, a community-based, tertiary care teaching hospital with 68 NICU beds. Patients were identified from hospital records based on ICD-9 (International Classification of Diseases, Ninth Revision) diagnostic codes and pharmacy records. All infants in the NICU who had at least one positive Candida culture from any sterile site (e.g., blood or cerebrospinal fluid [CSF]) obtained 72 h or more after hospitalization or birth between 1 January 1990 and 30 December 2008 were eligible for inclusion. Only the first positive culture was considered for subjects with multiple cultures positive for Candida. After receiving notification from the laboratory of a blood culture positive for yeast, another blood culture was obtained, and the patient was started on amphotericin B deoxycholate therapy at 1 mg/kg of body weight/day. Subjects were excluded if they received an antifungal medication within 2 weeks prior to study admission or had candiduria only. The study period encompassed the time from the collection of the first positive culture to hospital discharge or death. The institutional review board approved the study.
Data collected on standardized case report forms included demographic information; preexisting medical conditions; antifungal therapy, including exact timing of initiation; microbiological and laboratory values; and patient outcomes (mortality, disseminated candidiasis, length of NICU stay, and microbiological cure). Patient information was obtained from medical records and pharmacy-regulated monitoring forms. All blood cultures collected were entered into a computer system (TDS Eclipsys) with a time of collection. Specimens that were culture positive were updated in the computer system with the time when this occurred.
TIA was defined as the number of hours from the collection of the first positive culture until the start of antifungal therapy and was stratified into three groups: <24 h, 24 to 48 h, and >48 h. Data were analyzed by using one-way analysis of variance (ANOVA) (with Bonferroni adjustment if significant) or univariate logistic regression, depending on the type of variable. In addition, we stratified subjects into two categories: ≤24 h and >24 h as well as ≤48 h and >48 h. Patient characteristics of these two groups were compared by using the Student t test or Mann-Whitney U rank sum test for continuous variables and the chi-square or Fisher's exact test for categorical variables. Multivariate analysis to identify clinical parameters predictive of all-cause mortality and disseminated disease was performed by using logistic regression and, for length of NICU stay postinfection, by using linear regression. A significance level of 0.05 for a two-sided test was assumed when calculating results. All statistical analyses were performed by using R version 2.15.1.
RESULTS
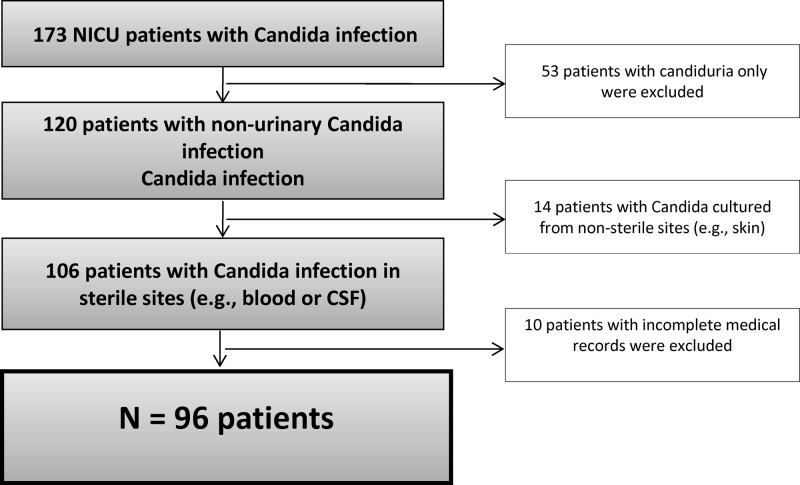
The medical records of 173 NICU infants with a Candida infection were reviewed. Of these, 77 were excluded from analysis for the following reasons: 53 had candiduria alone, 14 had positive Candida cultures only at nonsterile sites, and 10 had incomplete medical records. The remaining 96 patients were included in our study (Fig. 1).
Fig 1.
Exclusion algorithm.
Overall, 57% of subjects were male (Table 1). The median gestational age was 27 weeks (range, 23 to 41 weeks), and the birth weight was 956 g (range, 415 to 6,191 g). The median age at onset of infection was 28 days (range, 4 to 177 days). All but 3 subjects were initiated on amphotericin B therapy that consisted primarily of the deoxycholate formulation; 35% were subsequently changed to another agent, mainly fluconazole (Table 2). The mean time to positive cultures was 27 ± 24 h. The most common species isolated were Candida albicans (41%), C. parapsilosis (41%), and C. tropicalis (16%).
Table 1.
Clinical characteristics
| Characteristic | Value for group |
P value | ||
|---|---|---|---|---|
| All patients (n = 96) | Antifungal initiation ≤24 h (n = 34) | Antifungal initiation >24 h (n = 62) | ||
| Median gestational age (wk) (range) | 27 (23–41) | 26 (24–38) | 27 (23–41) | 0.7 |
| No. (%) of patients <28 wk of gestational age | 56 (58) | 24 (71) | 32 (52) | 0.1 |
| Median birth wt (g) (range) | 956 (415–6,191) | 953 (513–3,700) | 970 (415–6,191) | 0.3 |
| No. (%) of patients with birth wt of <1,000 g | 54 (56) | 22 (65) | 32 (52) | 0.2 |
| No. (%) of male patients | 55 (57) | 20 (59) | 35 (56) | 1.0 |
| No. (%) of patients with maternal race of: | 0.7 | |||
| Hispanic | 40 (42) | 13 (38) | 27 (44) | |
| Caucasian | 27 (28) | 10 (29) | 17 (27) | |
| African-American | 13 (14) | 4 (12) | 9 (15) | |
| Asian/Pacific Islander | 5 (5) | 1 (3) | 4 (6) | |
| Other | 10 (10) | 5 (15) | 5 (8) | |
| No. (%) of patients with underlying medical condition | ||||
| Congenital heart disease | 28 (29) | 15 (44) | 13 (21) | 0.03 |
| Necrotizing enterocolitis | 25 (26) | 9 (26) | 16 (26) | 1.0 |
| Renal disease | 10 (10) | 5 (15) | 5 (8) | 0.3 |
| Genetic defect | 13 (14) | 4 (12) | 9 (15) | 1.0 |
| Median age at onset of infection (days) (range) | 28 (4–177) | 25 (9–177) | 30 (4–146) | 0.3 |
| No. (%) of patients on ventilatora | 84 (88) | 30 (88) | 54 (87) | 1.0 |
| Median no. of days on ventilator (range) | 38 (2–206) | 43 (2–202) | 36 (2–206) | 0.7 |
| No. (%) of patients with cathetera | 82 (85) | 28 (82) | 54 (87) | 0.6 |
| Median no. of days with catheter (range) | 56 (0–220) | 47 (6–220) | 64 (5–206) | 0.7 |
| Median no. of days with catheter before infection (range) | 19 (0–169) | 17 (3–169) | 19 (1–123) | 0.6 |
| No. (%) of patients on H2 blockera | 34 (35) | 10 (29) | 24 (39) | 0.4 |
| No. (%) of patients on steroida | 29 (30) | 9 (26) | 20 (32) | 0.7 |
| No. (%) of patients with abdominal surgerya | 25 (26) | 7 (21) | 18 (29) | 0.5 |
| No. (%) of patients with concurrent bacterial infection | 35 (36) | 10 (30) | 25 (40) | 0.3 |
Assessed prior to the first positive culture.
Table 2.
Microbiological data
| Variable | Value for group |
P value | ||
|---|---|---|---|---|
| All patients (n = 96) | Antifungal initiation ≤24 h (n = 34) | Antifungal initiation >24 h (n = 62) | ||
| No. (%) of patients with site of infectiona | 0.4 | |||
| Blood | 93 (97) | 33 (97) | 60 (97) | |
| Cerebrospinal fluid | 1 (1) | 0 | 1 (2) | |
| Peritoneal | 1 (1) | 0 | 1 (2) | |
| Wound | 1 (1) | 1 (3) | 0 | |
| No. (%) of patients with Candida infectiona | 0.7 | |||
| C. albicans | 39 (41) | 14 (41) | 25 (40) | |
| C. parapsilosis | 39 (41) | 13 (38) | 26 (42) | |
| C. tropicalis | 15 (16) | 6 (18) | 9 (15) | |
| Other | 3 (3) | 1 (3) | 2 (3) | |
| Median duration of positive cultures (days) (range)a | 3 (1–45) | 3 (1–45) | 3 (1–28) | 0.9 |
| No. (%) of patients with persistent candidemia forb: | ||||
| >3 days | 57 (59) | 22 (65) | 35 (56) | 0.6 |
| >5 days | 41 (43) | 15 (44) | 26 (42) | 1.0 |
| No. (%) of patients with initial antifungal | 0.3 | |||
| Amphotericin B deoxycholate | 91 (95) | 32 (94) | 59 (95) | |
| Lipid-based amphotericin B | 2 (2) | 0 | 2 (3) | |
| Fluconazole | 1 (1) | 1 (3) | 0 | |
| Itraconazole | 1 (1) | 0 | 1 (2) | |
| No. (%) of patients with change to fluconazole | 32 (33) | 12 (35) | 20 (32) | 0.8 |
| Mean duration of antifungal therapy (days) ± SD | 21 ± 11 | 20 ± 13 | 21 ± 10 | 0.6 |
Denominators for each site of infection and type of infecting species vary.
Days from first to last positive blood cultures.
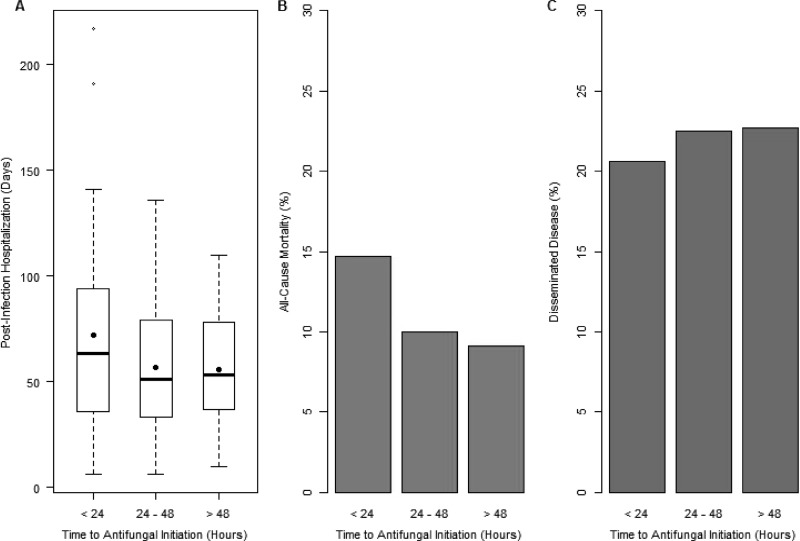
The proportion of subjects with TIA of ≤24 h was 35% (14% with TIA of <12 h), the proportion of subjects with TIA of between 25 and 48 h was 42%, and the proportion of subjects with TIA after >48 h was 23% (with 3% after 72 h). Based on these three categories, TIA did not contribute to all-cause mortality (P = 0.8), disseminated disease (P = 0.5), or postinfection hospital stay in the NICU (P = 0.08) (Fig. 2). By using comparative analyses of subjects with TIA of ≤24 h and those with TIA of >24 h, demographics, clinical characteristics, and microbiological data were similar (Tables 1 and 2). Clinical outcomes, including mortality, disseminated disease, and postinfection hospital stay, were also similar between these two groups (Table 3). Comparative analyses of all study subjects with TIAs of ≤48 h and >48 h also demonstrated a lack of association between TIA and clinical outcomes. Among the 39 subjects with C. albicans infection, these clinical outcomes were also similar between subjects with TIA of ≤24 h and those with TIA of >24 h.
Fig 2.
Clinical outcomes based on time to initiation of antifungal therapy. Dots in panel A represent means. P values were 0.081 for postinfection hospital stay (A), 0.5 for mortality (B), and 0.8 for disseminated disease (C).
Table 3.
Clinical outcomes
| Variable | Value for group |
P value | ||
|---|---|---|---|---|
| All patients (n = 96) | Antifungal initiation ≤24 h (n = 34) | Antifungal initiation >24 h (n = 62) | ||
| Length of NICU stay (days) | ||||
| Mean ± SD | 99 ± 46 | 105 ± 49 | 95 ± 44 | 0.3 |
| Median (range) | 97 (11–232) | 97 (20–230) | 95 (11–206) | |
| Length of postinfection NICU stay (days) | ||||
| Mean ± SD | 62 ± 37 | 72 ± 47 | 56 ± 29 | 0.08 |
| Median (range) | 53 (6–217) | 64 (6–217) | 53 (6–136) | |
| No. (%) of patients with disseminated disease | 21 (22) | 7 (21) | 14 (23) | 1.0 |
| No. (%) of patients with microbiological cure at 72 ha | 30 (31) | 8 (24) | 22 (35) | 0.6 |
| No. (%) of all-cause case fatalities | 11 (11) | 5 (15) | 6 (10) | 0.5 |
| No. (%) of attributed deaths | 5 (5) | 2 (6) | 3 (5) | 1.00 |
Only 74 subjects had repeated cultures.
Eleven (11%) infants died during hospitalization, and five of these deaths were attributed to invasive candidiasis. Compared to infants who survived, those who died were significantly younger (median gestational ages, 27 weeks [range, 23 to 41 weeks] and 24 weeks [range, 23 to 32 weeks], respectively; P = 0.039) and had longer ventilator use (medians, 36 days [range, 17 to 196 days] and 161 days [range, 58 to 206 days], respectively; P < 0.01). Using multivariable logistic regression analysis, TIA of >24 h, gestational age, and disease dissemination were not independent predictors of all-cause mortality (P > 0.2 for all variables). However, ventilator use for >60 days significantly increased the risk of death (odds ratio [OR], 9.5; 95% confidence interval [CI], 2.2 to 66.7; P = 0.002 by the likelihood ratio test).
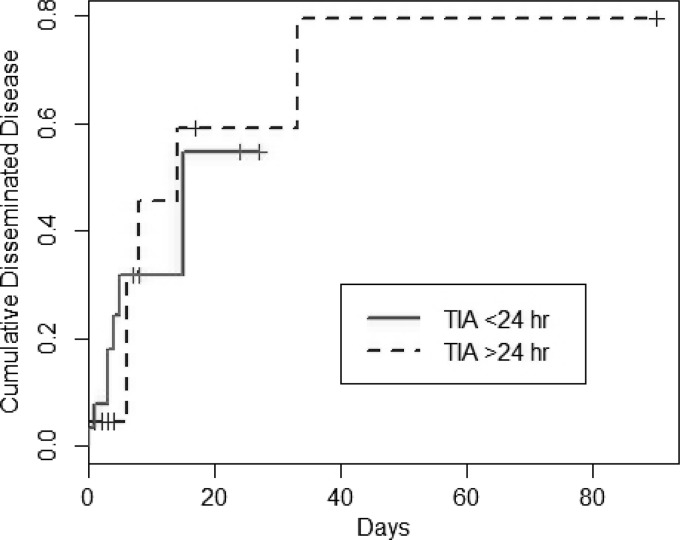
The development of disseminated candidiasis was not associated with TIA (Fig. 3). However, gestational age was found to be significantly different among those with and those without disseminated disease (medians, 26 weeks [range, 3 to 37 weeks] and 27 weeks [range, 23 to 41 weeks], respectively; P = 0.03). While not statistically significant, the duration of positive Candida cultures was longer for subjects with disseminated disease than for those without (medians, 3 days [range, 1 to 45 days] and 2 days [range, 1 to 28 days], respectively; P = 0.06). Upon multivariate analysis, TIA and gestational age were not associated with disseminated disease. However, for each additional day of positive culture, the risk of disseminated disease was increased by 10% (OR, 1.1; 95% CI, 1.08 to 1.2; P = 0.007 by the likelihood ratio test).
Fig 3.
Time to disseminated disease.
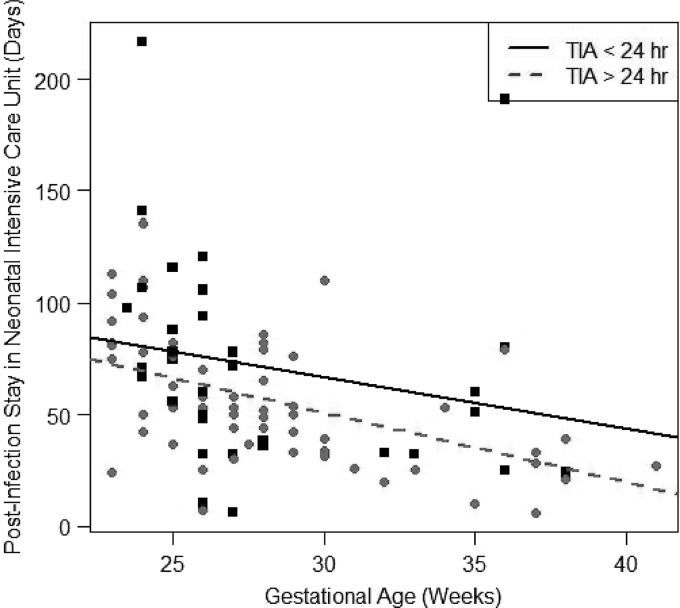
Based on univariate linear regression, subjects with TIA of ≤24 h had a significantly longer NICU stay postinfection than subjects with TIA of >24 h, by ∼1 day (95% CI, 0.2 to 1.0; P = 0.047). However, after adjusting for gestational age, TIA was not a significant predictor for NICU stay postinfection (P = 0.052) (Fig. 4). Subjects with low gestational age had a longer NICU stay after the first positive Candida culture, by 0.94 days per every week of age (95% CI, 0.70 to 0.98; P < 0.001).
Fig 4.
Postinfection stay in the neonatal intensive care unit by TIA and gestational age.
DISCUSSION
Our study demonstrated that there was no association between TIA, primarily amphotericin B deoxycholate, and three treatment outcomes: mortality, disseminated disease, and postinfection NICU stay. Three studies have explored the temporal effect of antifungal treatment on clinical outcomes in adult patients with candidemia. In one study comparing 157 adults with candidemia who received antifungal treatment (predominantly fluconazole) within 12 h and those who received treatment after 12 h, there were no significant differences in mortality (11% versus 33%; P = 0.169), microbiological clearance (100% versus 98%; P = 0.55), and length of hospitalization (mean, 40 ± 18 versus 31 ± 30 days; P = 0.056) (11). However, length of ICU stay was found to be significantly increased for those who received treatment after 12 h (mean, 0.4 ± 1.3 versus 9.4 ± 19.4 days; P = 0.019). In addition, administration of antifungal treatment 12 h after collection of the first positive blood culture was an independent predictor of hospital mortality (OR, 2.09; 95% CI, 1.5 to 2.8; P = 0.018) based on multivariate logistic regression analysis.
In another study that evaluated 230 adults who were hospitalized for candidemia and received fluconazole stratified by the number of days to initiation of therapy relative to the time when the culture was obtained, a delay in therapy significantly impacted mortality (9). In fact, results indicated a strong association between mortality and days to initiation of fluconazole (15% on day 0, 24% on day 1, 35% on day 2, and 41% on day ≥3; P = 0.0009). Length of ICU stay was significantly longer for those who received treatment after day 0 (mean, 6 ± 10 versus 10 ± 17 days; P = 0.03). Moreover, multivariate logistic regression revealed a delay in fluconazole initiation to be a predictor of mortality (OR, 1.5; 95% CI, 1.1 to 2.1; P = 0.014), along with APACHE II scores.
A more recent study evaluated the impact of the timing of caspofungin administration on clinical outcomes in 169 adults with invasive candidiasis, including those with candidemia and intra-abdominal infections (10). Compared to those who received caspofungin ≤72 h after collection of positive cultures, those with delayed initiation of >72 h had a significantly lower response rate and longer length of hospital stay after isolation of the organism (mean, 28 ± 23 versus 21 ± 17 days; P = 0.007). Timing of caspofungin initiation did not influence mortality.
Studies on the effect of TIA on clinical outcomes in neonates are limited. One recent retrospective national database study evaluated the impact of empirical antifungal therapy on extremely low birth weight infants with Candida infections. Empirical therapy was defined as receipt of a systemic antifungal on the day of or the day before collection of the positive culture. In that study, there was no difference in deaths or evidence of disseminated disease between infants who received empirical therapy and those who did not. This is similar to the results observed in our study. However, there was a lower combined incidence of death or neurodevelopmental impairment by 18 to 22 months of age in the group treated empirically, indicating that empirical treatment may have benefit (12).
Some of the reasons why TIA may have had an impact in the adult studies include differences in the Candida species causing infection and differences in the antifungals used. Internationally, the most common Candida species causing bloodstream infections among infants with low birth weight is C. albicans (4, 13–17). However, one surveillance study reported a significant decline in C. albicans and increase in non-albicans Candida infections from 1995 to 2004 in NICUs across the United States (18). Our species distribution was similar to those of other studies (19), with almost one-half of our isolates being C. parapsilosis. C. parapsilosis is a less virulent organism than C. albicans, the most common cause of fungal infections in adult studies (20, 21). The timing of treatment of a less virulent organism may not be as critical, so it may be difficult to show an effect on outcome.
Antifungal use differed in the adult studies that attempted to correlate TIA to clinical outcomes. Studies of adults evaluated fluconazole and echinocandins, whereas our study as well as a study by Greenberg et al. assessed amphotericin B (12). Amphotericin is the broadest-spectrum antifungal agent with fungicidal activity and hence may have contributed to the lack of correlation between TIA and outcomes. In addition, one adult study evaluated a later time to initiation of caspofungin therapy (i.e., 72 h), as opposed to TIA of 24 to 48 h in our study. It is possible that a delay in antifungal therapy by 72 h may have produced adverse clinical outcomes in neonates, but our study sample size was too small to detect this. Nonetheless, a delay of initiation of caspofungin therapy by 72 h did not influence mortality in adults, a finding similar to those of our study assessing the delay of amphotericin B treatment by 24 to 48 h (10).
Our study has several limitations. We evaluated infants over a prolonged period of time, thereby possibly introducing historical bias. However, during the study period, neonates with invasive Candida infections were treated very similarly. While fluconazole came into use later in the study, it served as step-down therapy once the organism and susceptibilities were identified. Initiation of therapy was done almost exclusively with amphotericin B deoxycholate. In addition, this study was retrospective, so it is challenging to ascertain if the infants who had TIA of <24 h may have been more ill and thereby more likely to experience adverse outcomes despite the timing of antifungal therapy.
In this historical cohort study, TIA was not associated with mortality, dissemination of invasive candidiasis, and length of NICU stay postinfection in critically ill infants. Prolonged duration of ventilator use and duration of positive Candida cultures significantly contributed to mortality and disseminated disease, respectively. Low gestational age, an indicator of severity of underlying illness, contributed to longer NICU stay postinfection. Further studies, with a larger sample size, are needed to corroborate our findings and to assess the effects of TIA of other antifungal agents, including fluconazole and echinocandins, on outcomes in infants with invasive candidiasis (17).
ACKNOWLEDGMENTS
We acknowledge Pamela A. Ny, Willis Shu, Daniel Liou, Gene Nguyen, and Helen Xu for assisting in data collection and Kelsey Schmidt for table and figure preparation.
This study was supported by an investigator-initiated grant from Astellas Pharma Global Development, Inc. (Deerfield, IL), and the Memorial Medical Center Foundation (Long Beach, CA). J.L. has received support from the National Institutes of Health (K23) and investigator-initiated research grants from Cubist, Pfizer, and Astellas.
Footnotes
Published ahead of print 18 March 2013
REFERENCES
- 1. Benjamin DK, Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84–92 [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leleu G, Aegerter P, Guidet B. 2002. Systemic candidiasis in intensive care units: a multicenter, matched-cohort study. J. Crit. Care 17:168–175 [DOI] [PubMed] [Google Scholar]
- 4. Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 41:1232–1239 [DOI] [PubMed] [Google Scholar]
- 5. Muszynski JA, Knatz NL, Sargel CL, Fernandez SA, Marquardt DJ, Hall MW. 2011. Timing of correct parenteral antibiotic initiation and outcomes from severe bacterial community-acquired pneumonia in children. Pediatr. Infect. Dis. J. 30:295–301 [DOI] [PubMed] [Google Scholar]
- 6. Aronin SI, Peduzzi P, Quagliarello VJ. 1998. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann. Intern. Med. 129:862–869 [DOI] [PubMed] [Google Scholar]
- 7. Battleman DS, Callahan M, Thaler HT. 2002. Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community-acquired pneumonia: link between quality of care and resource utilization. Arch. Intern. Med. 162:682–688 [DOI] [PubMed] [Google Scholar]
- 8. Lepur D, Barsic B. 2007. Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection 35:225–231 [DOI] [PubMed] [Google Scholar]
- 9. Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25–31 [DOI] [PubMed] [Google Scholar]
- 10. Hsu DI, Nguyen M, Nguyen L, Law A, Wong-Beringer A. 2010. A multicentre study to evaluate the impact of timing of caspofungin administration on outcomes of invasive candidiasis in non-immunocompromised adult patients. J. Antimicrob. Chemother. 65:1765–1770 [DOI] [PubMed] [Google Scholar]
- 11. Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greenberg RG, Benjamin DK, Jr, Gantz MG, Cotten CM, Stoll BJ, Walsh MC, Sanchez PJ, Shankaran S, Das A, Higgins RD, Miller NA, Auten KJ, Walsh TJ, Laptook AR, Carlo WA, Kennedy KA, Finer NN, Duara S, Schibler K, Ehrenkranz RA, Van Meurs KP, Frantz ID, III, Phelps DL, Poindexter BB, Bell EF, O'Shea TM, Watterberg KL, Goldberg RN, Smith PB, Eunice Kennedy Shriver National Institute of Child Health, Human Development Neonatal Research Network 2012. Empiric antifungal therapy and outcomes in extremely low birth weight infants with invasive candidiasis. J. Pediatr. 161:264–269.e2 doi:10.1016/j.jpeds.2012.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feja KN, Wu F, Roberts K, Loughrey M, Nesin M, Larson E, Della-Latta P, Haas J, Cimiotti J, Saiman L. 2005. Risk factors for candidemia in critically ill infants: a matched case-control study. J. Pediatr. 147:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, Blumberg HM, Patterson JE, Rinaldi M, Edwards JE, Wenzel RP, Jarvis W. 2000. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr. Infect. Dis. J. 19:319–324 [DOI] [PubMed] [Google Scholar]
- 15. Shetty SS, Harrison LH, Hajjeh RA, Taylor T, Mirza SA, Schmidt AB, Sanza LT, Shutt KA, Fridkin SK. 2005. Determining risk factors for candidemia among newborn infants from population-based surveillance: Baltimore, Maryland, 1998-2000. Pediatr. Infect. Dis. J. 24:601–604 [DOI] [PubMed] [Google Scholar]
- 16. Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. 2004. Risk factors for disseminated candidiasis in children with candidemia. Pediatr. Infect. Dis. J. 23:635–641 [DOI] [PubMed] [Google Scholar]
- 17. Steinbach WJ, Roilides E, Berman D, Hoffman JA, Groll AH, Bin-Hussain I, Palazzi DL, Castagnola E, Halasa N, Velegraki A, Dvorak CC, Charkabarti A, Sung L, Danziger-Isakov L, Lachenauer C, Arrieta A, Knapp K, Abzug MJ, Ziebold C, Lehrnbecher T, Klingspor L, Warris A, Leckerman K, Martling T, Walsh TJ, Benjamin DK, Jr, Zaoutis TE, International Pediatric Fungal Network 2012. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr. Infect. Dis. J. 31:1252–1257 [DOI] [PubMed] [Google Scholar]
- 18. Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. 2006. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995-2004. Pediatrics 117:1680–1687 [DOI] [PubMed] [Google Scholar]
- 19. Farmaki E, Evdoridou J, Pouliou T, Bibashi E, Panagopoulou P, Filioti J, Benos A, Sofianou D, Kremenopoulos G, Roilides E. 2007. Fungal colonization in the neonatal intensive care unit: risk factors, drug susceptibility, and association with invasive fungal infections. Am. J. Perinatol. 24:127–135 [DOI] [PubMed] [Google Scholar]
- 20. Chow BD, Linden JR, Bliss JM. 2012. Candida parapsilosis and the neonate: epidemiology, virulence and host defense in a unique patient setting. Expert Rev. Anti Infect. Ther. 10:935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trofa D, Gacser A, Nosanchuk JD. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 21:606–625 [DOI] [PMC free article] [PubMed] [Google Scholar]