Abstract
An in vitro single-compartment dilutional pharmacokinetic model was used to study the pharmacodynamics of ceftaroline against Staphylococcus aureus (both methicillin-susceptible S. aureus [MSSA] and methicillin-resistant S. aureus [MRSA]). Mean serum free concentrations of ceftaroline (the active metabolite of the prodrug ceftaroline fosamil) dosed in humans at 600 mg every 12 h (q12h) were simulated, and activities against 12 S. aureus strains (3 MSSA strains and 9 MRSA strains, 3 of which had a vancomycin-intermediate phenotype) were determined. Ceftaroline produced 2.5- to 4.0-log10-unit reductions in viable counts by 24 h with all strains and a 0.5- to 4.0-log-unit drop in counts at 96 h. The antibacterial effect could not be related to the strain MIC across the ceftaroline MIC range from 0.12 to 2.0 μg/ml. In dose-ranging studies, the cumulative percentage of a 24-h period that the free drug concentration exceeded the MIC under steady-state pharmacokinetic conditions (fTMIC) of 24.5% ± 8.9% was associated with a 24-h bacteriostatic effect, one of 27.8% ± 9.5% was associated with a −1-log-unit drop, and one of 32.1% ± 8.1% was associated with a −2-log-unit drop. The MSSA and MRSA strains had similar fTMIC values. fTMIC values increased with increasing duration of exposure up to 96 h. Changes in ceftaroline population analysis profiles were related to fTMIC. fTMICs of <50% were associated with growth on 4× MIC recovery plates at 96 h of drug exposure. These data support the use of ceftaroline fosamil at doses of 600 mg q12h to treat S. aureus strains with MICs of ≤2 μg/ml. An fTMIC of 25 to 30% would make a suitable pharmacodynamic index target, but fTMIC values of ≥50% are needed to suppress the emergence of resistance and require clinical evaluation.
INTRODUCTION
Ceftaroline fosamil (CPT) has been approved by the U.S. FDA for the treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia: market authorization in the European Union (EU) for complicated skin and soft tissue infections and community-acquired pneumonia is pending.
Ceftaroline fosamil (previously PP1-0903, T-91825, and TAK-599) is the prodrug of ceftaroline and is the first β-lactam with activity against methicillin-resistant Staphylococcus aureus (MRSA) marketed for clinical use in the United States. International surveillance studies conducted on MRSA strains in North America and the EU indicate that in the United States, the ceftaroline MIC50 and MIC90 are 1 and 1 μg/ml, respectively, with 5.2% of strains having MICs of >1 μg/ml. In contrast, in Europe the MIC50 and MIC90 are 1 and 2 μg/ml, respectively, with 17.3% of strains having MICs of >1 μg/ml. The FDA clinical breakpoint for susceptibility among S. aureus isolates is ≤1 μg/ml (1, 2). The proposed European (EUCAST) clinical breakpoint has not yet been published, but a breakpoint similar to that used in the United States will present significant problems in laboratory testing of ceftaroline, as it will cut through the MRSA MIC distribution.
Ceftaroline is approximately 2-fold more potent in vitro than ceftobiprole against S. aureus, the only other anti-MRSA β-lactam so far evaluated in phase III clinical trials (3). Ceftaroline has MICs of ≤1 μg/ml against community-associated MRSA, vancomycin-intermediate S. aureus (VISA), vancomycin-resistant S. aureus, heteroresistant VISA (hVISA), and daptomycin-nonsusceptible S. aureus strains isolated in the United States (4). The improved in vitro potency of ceftaroline against MRSA correlates with the drug's high affinity for MRSA PBP 2a (5).
Pharmacodynamic analysis of ceftaroline fosamil for the treatment of complicated skin and skin structure infections has indicated high target attainment rates for S. aureus strains when it is used at concentrations of up to 2 μg/ml (6). However, targets for the cumulative percentage of a 24-h period that the free drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (fTMIC) for S. aureus were based on only four S. aureus strains, and the antibacterial effects (ABEs) of prolonged ceftaroline dosing plus the risks of emergence of resistance were not assessed.
The aim of this study was to describe the antibacterial effect of ceftaroline against S. aureus strains with a range of ceftaroline MICs in 96-h experiments with doses simulating those in humans. In addition, the relationship between fTMIC and antibacterial effect and the risk of changes in population profiles were established for both methicillin-susceptible Staphylococcus aureus (MSSA) and MRSA strains.
MATERIALS AND METHODS
In vitro pharmacokinetic model.
A FerMac 310 fermentation system (ElectroLab, Tewkesbury, England) in vitro pharmacokinetic model was used to simulate serum free ceftaroline concentrations associated with intravenous (i.v.) dosing at 600 mg every 12 h (q12h) in humans. The apparatus, which has been described before (7), consists of a single central chamber connected to a reservoir containing broth. The central chamber (360 ml) is connected to a collecting vessel for overflow (7). The contents of the central chamber were diluted with broth using a peristaltic pump (Ismatec; Cole, Parmer, Hanwell, London, United Kingdom) at a flow rate of 96 ml/h. The temperature was maintained at 37°C, and the broth in the central chamber was agitated by a magnetic stirrer.
Media.
Cation-supplemented Mueller-Hinton broth (MHB; 100%) was used in all experiments. Nutrient agar plates (Oxoid, Basingstoke, England) were used to recover S. aureus strains from the model. Five microliters β-lactamase (kindly supplied by the University of Bristol) was used to neutralize ceftaroline. The β-lactamase-neutralized ceftaroline was added to nutrient agar plates up to a concentration of 75 μg/ml for recovery of total bacterial counts. No β-lactamase was added to the 1×, 2×, 4×, and 8× MIC plates used to assess changes in population structure.
Strains.
Twelve strains of S. aureus were used in the simulations of an i.v. dose of 600 mg q12h. Three strains were MSSA: clinical strain SMD 44099 (CPT MIC, 0.12 μg/ml/liter), clinical strain SMD 44100 (CPT MIC, 0.12 μg/ml), and JMI Laboratories (North Liberty, IA) strain SMD 43448 (CPT MIC, 1.0 μg/ml). Six strains were vancomycin-susceptible MRSA: clinical strain SMD 42690 (CPT MIC, 0.25 μg/ml), JMI Laboratories strain SMD 43450 (CPT MIC, 0.25 μg/ml), clinical strain SMD 42689 (CPT MIC, 0.5 μg/ml), JMI Laboratories strain SMD 43454 (CPT MIC, 1.0 μg/ml), clinical strain SMD 33815 (CPT MIC, 1.5 μg/ml), and JMI Laboratories strain SMD 43456 (CPT MIC, 2.0 μg/ml). Three strains of MRSA with a heteroresistant vancomycin-intermediate or vancomycin-intermediate phenotype were also tested: the VISA SMD 19898 Michigan strain (CPT MIC, 1.0 μg/ml), the VISA SMD 20201 Glasgow strain (CPT MIC, 1.0 μg/ml), and hVISA SMD 21286 LIM3 strain France (CPT MIC, 0.5 μg/ml).
Antibiotic.
Ceftaroline was supplied by AstraZeneca, Waltham, MA. Fresh solutions were prepared according to British Society for Antimicrobial Chemotherapy Guidelines (8).
MICs.
Ceftaroline MICs were determined by the standard broth dilution method according to CLSI guidelines (9). MICs were performed in 100% MHB and at nondoubling dilutions to more accurately determine MICs.
Pharmacokinetics.
The maximum concentration of drug in plasma (Cmax) was 19.0 mg/liter after a 1-h infusion, with a half-life (t1/2) of 2.5 h, and dosing was q12h for 96 h for human dosing simulations. In addition, between 7 and 16 doses were simulated per strain in dose-ranging experiments designed to achieve an fTMIC range of 0 to 100% for each strain to define the fTMIC--antibacterial effect relationship. In these experiments, the dose frequency was fixed at 12 h and various amounts of drug were added to achieve the targeted fTMIC. The concentrations of ceftaroline were determined by high-pressure liquid chromatography. Chromatography was performed on a Gemini-NXNM C18 110A column (Phenomenex, Macclesfield, England) using a mobile phase of 0.1 M sodium acetate–0.1 M glacial acetic acid–acetonitrile (86:2.8:11.2, vol/vol) at 254 nm. The flow rate was 1.8 ml/min. Detection was by UV absorbance. Intra- and interday accuracy and precision were assessed by use of quality control standards, with limits for accuracy of 10% and a coefficient of variation for precision of 5%.
ABEs.
Experiments to determine ABEs were performed at an initial inoculum of 106 CFU/ml prepared as previously described (10). Samples were taken throughout the simulation period for detection of viable counts. Bacteria were quantified by use of a spiral plater (Don Whitley Spiral Systems, Shipley, West Yorkshire, England). The minimum level of detection was 102 CFU/ml. Aliquots were stored at −70°C for ceftaroline measurement.
Emergence of resistance.
Ceftaroline resistance was assessed using population analysis profiles (7) at time zero (preexposure) and every 24 h postexposure. Samples were plated onto agar containing no antibiotic and antibiotic at 1×, 2×, 4×, and 8× MIC to quantify any resistant subpopulations. The limit of detection was 102 CFU/ml. The frequency of mutation of the strains in response to ceftaroline was not assessed.
All pharmacokinetic simulations of human doses to determine ABEs or changes in population profiles were performed at least in triplicate.
Pharmacodynamics and measurement of ABEs.
The ABE of ceftaroline was calculated by determining the log change in viable counts at 24 h (d24), 48 h (d48), 72 h (d72), and 96 h (d96). The area under the bacterial kill curve (AUBKC; log number of CFU/ml·h) was calculated using the log linear-trapezoidal rule four times from 0 to 24 h (AUBKC 24), 0 to 48 h (AUBKC 48) 0 to 72 h (AUBKC 72), and 0 to 96 h (AUBKC 96). The relationship between fTMIC and ABE was delineated using a Boltzmann sigmoid maximum effect model (Prism software; GraphPad Software, San Diego, CA).
RESULTS
Pharmacokinetic curves and pharmacodynamic index sizes.
The measured pharmacokinetic values for the serum free ceftaroline concentrations for simulation of an i.v. dose of 600 mg q12h are shown in Table 1. The fTMICs for ceftaroline given at 600 mg q12h by a 1-h infusion for the 12 strains tested are shown in Table 2.
Table 1.
Measured and target serum free ceftaroline concentrations for simulations with a dose of 600 mg q12h
| Parameter | Concn (mg/liter) at: |
t1/2 (h) | AUC0–12a (mg·h/liter) | |||
|---|---|---|---|---|---|---|
| 1 h | 4 h | 7 h | 12 h | |||
| Target | 23 | 10.4 | 4.6 | 1.1 | 2.8 | 63.9 |
| Measured | 21.1 ± 4.9 | 7.6 ± 2.3 | 3.4 ± 1.3 | 1.6 ± 0.9 | 2.0 ± 0.7 | 68.4 ± 8.2 |
AUC0–12, area under the concentration-time curve from time zero to 12 h.
Table 2.
ABE of ceftaroline at 600 mg q12h against 12 S. aureus strains with MICs in the range of 0.12 to 2 μg/ml in human simulations
| Strain | Phenotype | Ceftaroline MIC (μg/ml) | fTMIC (%) | Change in viable count (log no. of CFU/ml) at: |
AUBKC (log no. of CFU/ml) at: |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | ||||
| SMD 44099 | MSSA | 0.12 | 100 | −4.0 ± 0.5 | −33 ± 1.0 | −2.3 ± 1.9 | −1.9 ± 2.1 | 20 ± 2 | 29 ± 4 | 63 ± 32 | 116 ± 72 |
| SMD 44100 | MSSA | 0.12 | 100 | −4.2 ± 0.3 | −3.3 ± 1.2 | −2.2 ± 2.2 | −0.7 ± 1.1 | 23 ± 4 | 33 ± 6 | 69 ± 39 | 125 ± 83 |
| SMD 42690 | MRSA | 0.25 | 100 | −3.2 ± 0.8 | −3.0 ± 0.9 | −4.1 ± 0.4 | −4.1 ± 0.3 | 20 ± 2 | 35 ± 10 | 39 ± 10 | 44 ± 18 |
| SMD 43450 | MRSA | 0.25 | 100 | −4.0 ± 0.3 | −3.8 ± 0.5 | −3.6 ± 0.6 | −4.3 ± 0.1 | 18 ± 1 | 25 ± 3 | 33 ± 1 | 40 ± 2 |
| SMD 42689 | MRSA | 0.5 | 100 | −2.2 ± 1.1 | −2.5 ± 0.7 | NDa | ND | 29 ± 6 | 43 ± 6 | ND | ND |
| SMD 21286 | hVISA | 0.5 | 100 | −3.2 ± 0.8 | −3.6 ± 0.4 | ND | ND | 27 ± 18 | 35 ± 25 | ND | ND |
| SMD 43448 | MSSA | 1.0 | 100 | −4.1 ± 0.3 | −4.0 ± 0.5 | −3.9 ± 0.4 | −3.9 ± 0.7 | 15 ± 3 | 22 ± 5 | 29 ± 6 | 34 ± 6 |
| SMD 19898 | VISA | 1.0 | 100 | −3.7 ± 0.4 | −3.7 ± 0.3 | ND | ND | 20 ± 5 | 26 ± 10 | ND | ND |
| SMD 20201 | VISA | 1.0 | 100 | −3.8 ± 0.6 | −3.9 ± 0.4 | ND | ND | 21 ± 1 | 25 ± 4 | ND | ND |
| SMD 4345 4 | MRSA | 1.0 | 100 | −4.3 ± 0.1 | −3.8 ± 0.5 | −4.0 ± 0.5 | −3.7 ± 0.6 | 13 ± 2 | 20 ± 1 | 28 ± 4 | 35 ± 6 |
| SMD 33815 | MRSA | 1.5 | 90 | −2.4 ± 0.9 | −3.7 ± 0.3 | −3.3 ± 1.1 | −2.6 ± 1.5 | 36 ± 11 | 45 ± 13 | 48 ± 19 | 76 ± 45 |
| SMD 43456 | MRSA | 2.0 | 82 | −4.3 ± 0.2 | −3.5 ± 1.0 | −2.6 ± 0.8 | −2.0 | 33 ± 4 | 40 ± 8 | 61 ± 12 | 89 ± 27 |
ND, not done.
Antibacterial effect of ceftaroline over 96 h.
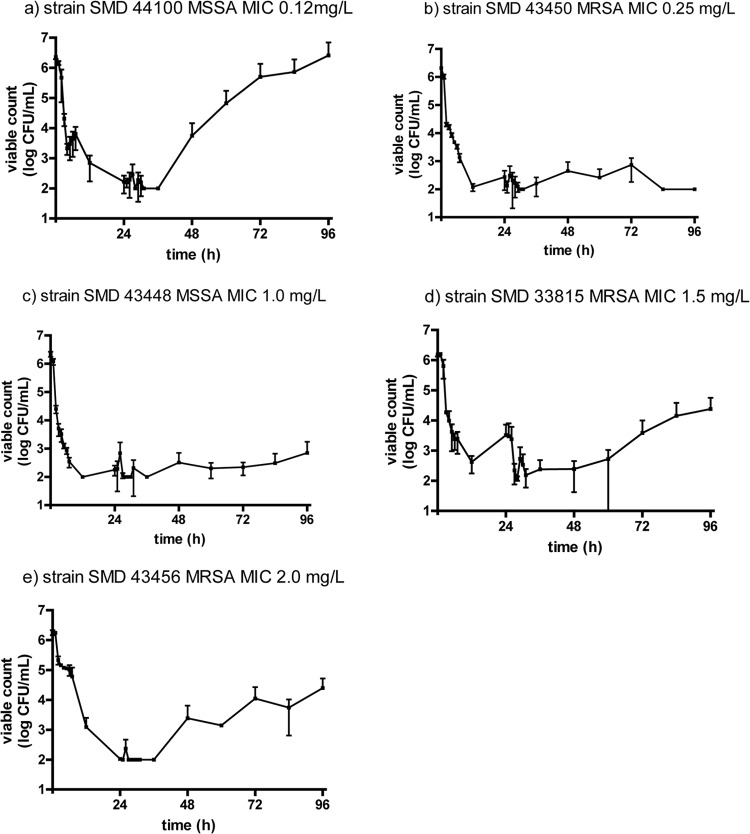
The activity of human serum free CPT concentrations associated with dosing at 600 mg q12h i.v. over 48 h (4 strains) or 96 h (8 strains) against 12 S. aureus strains with CPT MICs over the range of 0.12 to 2.0 μg/ml was tested (Table 2; Fig. 1). CPT produced a >2-log10-unit drop in the staphylococcal viable count at 24 h with all strains and a ≥4-log10-unit drop with six strains. The MIC was not related to the log drop in viable count. At 48 h, drops were >3 log10 units with 11 strains, and drops were >2 log10 units with six of eight strains at 96 h. The log change in the viable count AUBKC was not related to the CPT MIC. There was no emergence of resistance with any simulation, as indicated by the growth of S. aureus on media containing ceftaroline at 2× MIC or 4× MIC over 96 h; however, regrowth after 24 h was apparent with strain SMD 44100 (Fig. 1a), strain SMD 33815 (Fig. 1d), and strain SMD 43456 (Fig. 1e).
Fig 1.
Antibactericidal effect of serum free ceftaroline concentrations against S. aureus strains.
Dose-ranging studies with S. aureus.
A range of dosing simulations (n = 9 to 11) per strain was used to provide an fTMIC range of 0 to 100% for each of eight S. aureus strains (four MSSA and four MRSA strains). The antibacterial effect was measured from d24, d48, d72, and d96 (only data for d24, d48, and d96 are shown). Using d24 as the primary outcome measure, the fTMIC for a 24-h static effect for all S. aureus strains was 24.5% ± 8.9% (mean ± standard deviation); that for a −1-log-unit reduction was 27.8% ± 9.5%, increasing to 32.1% ± 8.1% for a −3-log-unit reduction in count (Table 3). The relationship of fTMIC to the antibacterial effect for ceftaroline at 48 h is shown in Table 4. The fTMICs were increased for static and bactericidal effects at 96 h: the fTMIC for a static effect at 96 h was 42.9% ± 13.5%, that for a −1-log-unit reduction in count was 46.1% ± 15.0%, and that for a −3-log-unit reduction in count was 53.7% ± 20.4% for all S. aureus strains (Table 5).
Table 3.
fTMIC relationship to ABE for ceftaroline at 24 h
| ABE at 24 h |
fTMIC (%)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSSA |
MRSA |
All S. aureus strains | |||||||||
| Strain SMD 44100 (0.12) | Strain SMD 44099 (0.12) | Strain SMD 43450 (0.25) | Strain SMD 43448 (1.0) | All MSSA strains | Strain SMD 42690 (0.25) | Strain SMD 43454 (1.0) | Strain SMD 33815 (1.5) | Strain SMD 43456 (2.0) | All MRSA strains | ||
| Static | 40.0 | 26.2 | 24.0 | 17.0 | 26.8 ± 9.6 | 29.0 | 31.0 | 12.8 | 17.0 | 22.4 ± 8.9 | 24.5 ± 8.9 |
| −1-log-unit drop | 47.1 | 28.5 | 29.0 | 19.0 | 30.9 ± 11.7 | 29.0 | 32.0 | 20.1 | 18.0 | 24.8 ± 6.8 | 27.8 ± 9.5 |
| −2-log-unit drop | 31.5 | 32.0 | 21.0 | 28.2 ± 6.2 (n = 3) | 30.0 | 33.0 | 28.2 | 18.5 | 27.4 ± 6.2 | 27.7 ± 5.7 (n = 7) | |
| −3-log-unit drop | 36.9 | 39.0 | 23.0 | 32.9 ± 8.9 (n = 3) | 30.0 | 34.0 | 41.6 | 20.0 | 31.4 ± 9.0 (n = 3) | 32.1 ± 8.1 (n = 7) | |
| −4-log-unit drop | 51.0 | 29.0 | 31.0 | 38.0 | 69.1 | 46.0 ± 20.3 (n = 3) | 37.6 ± 18.6 (n = 5) | ||||
Data in parentheses are MICs (in micrograms per milliliter).
Table 4.
fTMIC relationship to ABE for ceftaroline at 48 h
| ABE at 48 h |
fTMIC (%)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSSA |
MRSA |
All S. aureus strains | |||||||||
| Strain SMD 44100 (0.12) | Strain SMD 44099 (0.12) | Strain SMD 43450 (0.25) | Strain SMD 43448 (1.0) | All MSSA strains | Strain SMD 42690 (0.25) | Strain SMD 43454 (1.0) | Strain SMD 33815 (1.5) | Strain SMD 42456 (2.0) | All MRSA strains | ||
| Static | 44.3 | 36.2 | 34.0 | 28.0 | 35.6 ± 6.7 | 38.0 | 31.0 | 34.9 | 19.0 | 30.7 ± 8.3 | 33.2 ± 7.5 |
| −1-log-unit drop | 52.3 | 41.6 | 36.0 | 29.0 | 39.7 ± 9.8 | 39.0 | 31.0 | 44.3 | 19.5 | 33.4 ± 10.8 | 36.6 ± 10.1 |
| −2-log-unit drop | 67.1 | 48.3 | 38.0 | 30.0 | 45.9 ± 16.0 | 40.0 | 31.0 | 53.0 | 20.0 | 36.0 ± 14.0 | 40.9 ± 14.9 |
| −3-log-unit drop | 59.1 | 42.0 | 31.0 | 44.0 ± 14.2 (n = 3) | 42.0 | 31.0 | 63.1 | 20.5 | 39.1 ± 18.2 | 41.2 ± 14.9 (n = 7) | |
| −4-log-unit drop | 32.0 | 79.2 | |||||||||
Data in parentheses are MICs (in micrograms per milliliter).
Table 5.
fTMIC relationship to ABE for ceftaroline at 96 h
| ABE at 96 h |
fTMIC (%)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSSA |
MRSA |
All S. aureus strains | |||||||||
| Strain SMD 44100 (0.12) | Strain SMD 44099 (0.12) | Strain SMD 43450 (0.25) | Strain SMD 43448 (1.0) | All MSSA strains | Strain SMD 42690 (0.25) | Strain SMD 43454 (1.0) | Strain SMD 33815 (1.5) | Strain SMD 43456 (2.0) | All MRSA strains | ||
| Static | 71.1 | 38.9 | 43.0 | 38.0 | 47.8 ± 15.8 | 38.0 | 36.0 | 52.3 | 26.2 | 38.1 ± 10.8 | 42.9 ± 13.5 |
| −1-log-unit drop | 76.6 | 48.3 | 45.0 | 39.0 | 52.2 ± 16.7 | 39.0 | 37.0 | 56.4 | 27.5 | 40.0 ± 12.0 | 46.1 ± 15.0 |
| −2-log-unit drop | 81.2 | 58.4 | 48.0 | 41.0 | 57.2 ± 17.6 | 40.0 | 38.0 | 59.7 | 28.5 | 41.6 ± 13.1 | 49.3 ± 16.6 |
| −3-log-unit drop | 91.3 | 71.1 | 51.0 | 42.0 | 63.8 ± 22.0 | 41.0 | 39.0 | 64.4 | 30.2 | 43.7 ± 14.6 | 53.7 ± 20.4 |
| −4-log-unit drop | 62.0 | 46.0 | 86.6 | 34.9 | 57.3 ± 22.4 (n = 4) | ||||||
Data in parentheses are MICs (in micrograms per milliliter).
Comparison of the fTMIC targets for static or bactericidal effects at 24 h, 48 h, or 96 h indicated that there were no differences in the fTMIC targets between the MSSA strains and MRSA strains tested (Tables 3 to 5).
Changes in population profiles.
S. aureus population profiles changed as a result of the fTMIC exposures in the dose-ranging experiments (Table 6). At 48 h for fTMIC exposures of <50%, growth occurred on 2× MIC recovery plates but not 4× MIC plates. For experiments with fTMICs over the range of ≥15 to <30%, the majority of experiments had isolates recovered from 2× MIC plates. At 96 h, all fTMIC exposures of <90% had colonies recovered from 2× MIC plates. These occurred in most experiments with fTMICs of <50%, with >5-log-unit mean counts associated with fTMIC exposures of <40%. In contrast to the findings at 48 h, at 96 h colonies were also recovered from 4× MIC plates, with mean counts being >4 log10 units over the fTMIC range of ≥20 to <40%.
Table 6.
Changes in ceftaroline population profiles in S. aureus at 48 h and 96 h of drug exposure
| Time of drug exposure and TMIC (%) | No. of expts | No. of expts with growth on 2× MIC plates | Count on 2× MIC plates (log CFU/ml) | No. of expts with growth on 4× MIC plates | Count on 4× MIC plates (log CFU/ml) |
|---|---|---|---|---|---|
| At 48 h | |||||
| ≥80 | 15 | 0 | <2 | 0 | <2 |
| 70–79 | 6 | 0 | <2 | 0 | <2 |
| 50–69 | 8 | 0 | <2 | 0 | <2 |
| 40–49 | 5 | 1 | 2.7 | 1 | 2.1 |
| 30–39 | 8 | 2 | 3.9 | 0 | <2 |
| 25–29 | 8 | 4 | 4.4 ± 2.0 | 0 | <2 |
| 20–24 | 8 | 5 | 4.3 ± 1.8 | 0 | <2 |
| 15–19 | 7 | 4 | 5.2 ± 2.1 | 0 | <2 |
| 10–14 | 7 | 2 | 4.7 | 0 | <2 |
| At 96 h | |||||
| ≥80 | 15 | 0 | <2 | 0 | <2 |
| 70–79 | 6 | 1 | 2.5 | 0 | <2 |
| 50–69 | 8 | 1 | 2.5 | 0 | <2 |
| 40–49 | 5 | 3 | 3.4 ± 1.6 | 1 | 2.1 |
| 30–39 | 8 | 5 | 7.1 ± 1.0 | 4 | 4.8 ± 2.3 |
| 25–29 | 8 | 6 | 5.5 ± 1.4 | 4 | 4.6 ± 2.3 |
| 20–24 | 8 | 6 | 6.1 ± 1.7 | 4 | 4.3 ± 1.6 |
| 15–19 | 7 | 5 | 5.6 ± 2.6 | 3 | 3.9 ± 1.7 |
| 10–14 | 7 | 5 | 6.4 ± 2.3 | 4 | 3.7 ± 1.4 |
DISCUSSION
The pharmacodynamics of cephalosporins, including ceftaroline, are well understood. In time-kill studies, ceftaroline is bactericidal against S. aureus strains at 2×, 4×, and 8× MICs (11) and has an in vitro postantibiotic effect (PAE) of 0.7 to 2.2 h for staphylococci (12). In vivo fTMIC is the dominant pharmacodynamic index and the in vivo PAE is 0.8 to 7.2 h. fTMIC magnitudes of 20% have been associated with a 24-h bacteriostatic effect for cefazolin against MSSA; a 2-log-unit kill was associated with a TMIC of 40%, and a 3-log-unit drop was associated with a TMIC of 50 to 60% (13). Similar observations have been made with anti-MRSA cephalosporins; for example, the fTMIC for a 24-h static effect against eight strains of S. aureus (five MRSA and three MSSA strains) for ceftobiprole was 21.1% ± 3.9%, and the equivalent value for a 2-log-unit kill was 29.3% ± 4.6% (14). For ceftaroline, using a similar neutropenic thigh/lung infection model but only four S. aureus strains (two MRSA and two MSSA strains), the fTMIC for a 24-h static effect was 26% ± 8% and that for a 2-log-unit kill was 45% ± 13% (15). Our data are in keeping with these findings, indicating for eight S. aureus strains (four MRSA and four MSSA strains) that the 24-h bacteriostatic fTMIC is 24.5% ± 8.9%; our fTMIC associated with a −2-log-unit kill was 27.7% ± 5.7%, which is shorter than that reported in animal experiments with ceftaroline but very similar to values from animal models with ceftobiprole (14, 15). We continued studying the fTMIC required for static and bactericidal effects beyond 24 h. As may be expected, the fTMIC targets increase with increasing time of drug exposure. However, the fTMIC for a static effect at 96 h was only 42.9% ± 13.5% and that for a −2-log-unit kill was 49.3% ± 16.6%; similar data have also been reported with the anti-MRSA carbapenem razupenem (16).
The appropriate fTMIC target for mathematical modeling of clinical breakpoints is an ongoing topic for debate. For ceftaroline and S. aureus, our data would suggest an fTMIC target of 25 to 30% for a −2-log-unit kill at 24 h; this is somewhat less than the animal data for ceftaroline but in keeping with the animal data for ceftobiprole. Target attainment modeling using an fTMIC target of 26% or 33% indicated that for MIC values of ≤2 μg/ml, target attainment rates were >90% for ceftaroline administered at 600 mg q12h (6). Animal modeling of average human serum concentrations of ceftaroline administered at 600 mg q12h indicated a similar response with S. aureus strains with MICs over the range of 0.125 to 4 μg/ml, whatever the MIC (17). Our data obtained using an in vitro model and longer dose exposures are similar, indicating that for S. aureus strains with MICs of 0.12 to 2 μg/ml and 96-h dose exposures, there was no difference in antibacterial effect, as MICs increased up to 2 μg/ml, probably in relation to the high (>80%) fTMICs in all these simulations. Although regrowth occurred with some strains, this was not related to the ceftaroline MIC or detectable changes in population analysis profiles. Like others, the S. aureus strains with the hVISA and VISA phenotypes that we tested responded well to ceftaroline (18, 19). These in vitro data indicate, with other pharmacodynamic experiments, that ceftaroline fosamil at 600 mg q12h should be adequate therapy for S. aureus strains with MICs up to ≤2 μg/ml; indeed, comparison of the activity of ceftaroline administered at 600 mg q12h to that of ceftaroline administered at 600 mg every 8 h against S. aureus in an in vitro model indicated no advantage to the higher dose (18).
Unlike other investigators, we were able to perform long-term experiments to 96 h and study changes in ceftaroline population analysis profiles. With simulations with a dose of 600 mg q12h, there were no changes over 96 h. In contrast, in dose-ranging experiments, isolates able to grow on 4× MIC plates at fTMIC exposures of <50% emerged by 96 h.
Preclinical-clinical pharmacokinetics-pharmacodynamics correlates so far are poor for the use of cephalosporins to treat S. aureus infection; however, Kimko et al. (20) reported that a ceftobiprole fTMIC of ≥30% was associated with clinical cure in skin and skin structure infections. Such data are in reasonable agreement with the proposed fTMIC target of ≥25 to 30% based on our data. Ceftaroline fosamil has been shown to be noninferior to vancomycin plus aztreonam in two phase III randomized, double-blind studies (NCT00424190 and NCT00423657) in patients with complicated skin and skin structure infections (21, 22). The MIC90s for ceftaroline in these studies were 1 μg/ml and 0.5 μg/ml (for all strains with MICs of ≤0.5 μg/ml), respectively; hence, as yet, strains with MICs of 2 μg/ml are rare in clinical studies.
Therefore, the predicted good responses for S. aureus strains with a ceftaroline MIC of 2 μg/ml, based on preclinical data, have not yet been validated in clinical studies. This is an important issue, as 5.2% of the MRSA strains in the United States and 17.3% of the MRSA strains in Europe have MICs of >1 μg/ml.
In conclusion, these data from an in vitro pharmacokinetic model validate the use of ceftaroline fosamil at 600 mg q12h i.v. to treat S. aureus strains with MICs of ≤2 μg/ml. A suitable fTMIC target for clinical breakpoint setting is 25 to 30%, corresponding to a 1- to 2-log-unit kill in S. aureus after 24 h. In long-term experiments, fTMICs of ≤50% were associated with changes in ceftaroline population profiles in S. aureus.
ACKNOWLEDGMENTS
We thank Jane Ambler for her helpful advice, support, and discussions during the study. We thank JMI Laboratories, North Liberty, IA, for providing some of the S. aureus strains.
This study was funded by AstraZeneca, Waltham, MA.
Footnotes
Published ahead of print 4 March 2013
REFERENCES
- 1. Forest Laboratories Inc 2010. Full prescribing information. Teflaro 69-1020308-W-APR11. Forest Laboratories Inc, St. Louis, MO [Google Scholar]
- 2. Jones RN, Mendes R, Sader HS. 2010. Ceftaroline activity against pathogens associated with complicated skin and skin structure infections: results from an international surveillance study. J. Antimicrob. Chemother. 65(Suppl 4):iv17–iv31 [DOI] [PubMed] [Google Scholar]
- 3. Karlowsky JA, Adam HJ, Decorby MR, Lagacé-Wiens PR, Hoban DJ, Zhanel GG. 2011. In vitro activity of ceftaroline against Gram-positive and Gram-negative pathogens isolated from patients in Canadian hospitals in 2009. Antimicrob. Agents Chemother. 55:2837–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saravolatz L, Pawlak J, Johnson L. 2010. In vitro activity of ceftaroline against community-associated methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, and daptomycin-nonsusceptible Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 54:3027–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moisan HM, Pruneau M, Malouin F. 2010. Binding of ceftaroline to penicillin-binding problems of Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob. Chemother. 65:713–716 [DOI] [PubMed] [Google Scholar]
- 6. Drusano GL. 2010. Pharmacodynamics of ceftaroline fosamil for complicated skin and skin structure infection: rationale for improved anti-methicillin resistant Staphylococcus aureus activity. J. Antimicrob. Chemother. 65(Suppl 4):iv33–iv39 [DOI] [PubMed] [Google Scholar]
- 7. MacGowan AP, Rogers CA, Holt HA, Bowker KE. 2003. Activities of moxifloxacin against, and emergence of resistance in, Streptococcus pneumoniae and Pseudomonas aeruginosa in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 47:1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. British Society for Antimicrobial Chemotherapy Working Party 2001. Antimicrobial susceptibility testing. J. Antimicrob. Chemother. 46(Suppl 51):5–16 [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2005. Performance standards for antimicrobial susceptibility testing. CLSI document M100-516. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. MacGowan AP, Bowker KE, Noel AR. 2008. Pharmacodynamics of the antibacterial effect and emergence of resistance to tomopenem, formerly RO4908463/CS-023, in an in vitro pharmacokinetic model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 52:1401–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones RN, Fritsche TR, Ge Y, Kaniga K, Sader HS. 2005. Evaluation of PP1-0903M (T91825), a novel cephalosporin: bactericidal activity, effects of modifying in vitro testing parameters and optimisation of disc diffusion tests. J. Antimicrob. Chemother. 56:1047–1052 [DOI] [PubMed] [Google Scholar]
- 12. Pankuch GA, Appelbaum PC. 2009. Postantibiotic effect of ceftaroline against Gram-positive organisms. Antimicrob. Agents Chemother. 53:4537–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig WA. 1988. Correlation of antimicrobial pharmacokinetic parameters and therapeutic efficacy in an animal model. J. Infect. Dis. 158:831–847 [DOI] [PubMed] [Google Scholar]
- 14. Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK 599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacGowan AP, Noel AR, Tomaselli S, Elliott H, Bowker KE. 2011. Pharmacodynamics of razupenem (PZ601) studied in an in vitro pharmacokinetic model of infection. Antimicrob. Agents Chemother. 55:1436–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keel RA, Crandon JL, Nicolau DP. 2011. Efficacy of human simulated exposures of ceftaroline administered at 600 mg every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:4028–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vidaillac C, Leonard SN, Rybak MJ. 2009. In vitro activity of ceftaroline against methicillin-resistant Staphylococcus aureus and heterogeneous vancomycin-intermediate S. aureus in a hollow fiber model. Antimicrob. Agents Chemother. 53:4712–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhanel GG, Rossnagel E, Nichol K, Cox L, Karlowsky JA, Zelenitsky S, Noreddin AM, Hoban DJ. 2011. Ceftaroline pharmacodynamic activity versus community associated and healthcare-associated methicillin-resistant Staphylococcus aureus, hetero-resistant vancomycin-intermediate S. aureus, vancomycin-intermediate S. aureus and vancomycin resistant S. aureus using an in vitro model. J. Antimicrob. Chemother. 66:1301–1305 [DOI] [PubMed] [Google Scholar]
- 20. Kimko H, Xu X, Nandy P, Samtani MN, Strauss RS, Bagchi P, Noel GJ. 2009. Pharmacodynamic profiling of ceftobiprole for the treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 53:3371–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corey GR, Wilcox MH, Talbot GH, Thye D, Friedland D, Baculik T, CANVAS 1 Investigators 2010. CANVAS 1: the first phase III, randomised, double linked study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl 4):iv41–iv51 [DOI] [PubMed] [Google Scholar]
- 22. Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, Baculik T, CANVAS 2 Investigators 2010. CANVAS 2: the second phase III, randomised, double-blind, study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J. Antimicrob. Chemother. 65(Suppl 4):iv53–iv65 [DOI] [PubMed] [Google Scholar]