Abstract
pHN1122-1 carrying blaCTX-M-55, from an Escherichia coli isolate from a dog, was completely sequenced. pHN1122-1 has an IncI2 replicon and typical IncI2-associated genetic modules, including mok/hok-finO-yafA/B, nikABC, and two transfer regions, tra and pil, as well as a shufflon. blaCTX-M-55 is found within a 3.084-kb ISEcp1 transposition unit that includes a fragment of IncA/C plasmid backbone. pHN1122-1 and closely related plasmids were identified in other E. coli isolates from animals in China.
TEXT
The dissemination of CTX-M enzymes conferring resistance to extended-spectrum β-lactam antibiotics around the world has been referred to as the “CTX-M pandemic” (1). Within this group, the most dominant variants are blaCTX-M-15 and blaCTX-M-14, found in isolates from humans, animals, and the environment all over the world (2, 3). In China, CTX-M-14 is the dominant extended-spectrum β-lactamase (ESBL) among human and animal isolates (4–6), but CTX-M-55, rather than CTX-M-15, has become the second most frequently identified CTX-M gene, especially in Escherichia coli from animals (5–8). Like blaCTX-M-15, blaCTX-M-55 encodes an ESBL with enhanced activity against ceftazidime and is generally found in an ISEcp1-mediated transposition unit carried by plasmids (9). However, no such plasmid has been fully sequenced. Here, a representative plasmid carrying blaCTX-M-55, isolated from a dog (5) and designated pHN1122-1, was fully sequenced and other plasmids carrying blaCTX-M-55 were compared with pHN1122-1 by PCR and restriction digestion.
Previously, 24 of 240 E. coli isolates from pets were found to produce CTX-M-55 and 15 gave transconjugants carrying blaCTX-M-55 that were all resistant to cefotaxime and ceftazidime (5). None of these 15 isolates produced amplicons by PCR-based replicon typing (PBRT) using the 18 primer pairs described previously (10). Plasmid DNA from a transconjugant of E. coli 122 recovered from a dog in Guangzhou in 2007 (Table 1) (5) was extracted using a Qiagen Midi kit (Qiagen, Hilden, Germany) and sequenced on an ABI 3730xl sequencer (Applied Biosystems). Assembly with the Phred/Phrap/Consed software suite (University of Washington, Seattle, WA) gave four contigs. Combinatorial PCR to assemble contigs and fill in gaps gave a complete 62.196-kb plasmid.
Table 1.
Characteristics of isolates and plasmids carrying blaCTX-M-55a
| Isolate | Origin | Date of isolation | Locationb | Spacer size (bp)c | Plasmid features |
Reference | |
|---|---|---|---|---|---|---|---|
| Replicon type | ApaLI | ||||||
| 122 | Dog feces | 2007.11 | GDPH1 | 45 | IncI2 | A1 | 5 |
| 203 | Dog feces | 2007.12 | GDPH1 | 45 | IncI2 | A1 | 5 |
| 107 | Dog feces | 2008.1.18 | GDPH3 | 45 | IncI2 | A1 | 5 |
| 129 | Dog feces | 2008.1.19 | GDPH2 | 45 | IncI2 | A3 | 5 |
| 124 | Dog feces | 2008.1.19 | GDPH2 | 45 | IncI2 | A3 | 5 |
| 143 | Dog feces | 2008.1.22 | GDPH2 | 45 | IncI2 | A3 | 5 |
| 160 | Dog feces | 2008.1.24 | GDPH3 | 45 | IncI2 | A1 | 5 |
| 212 | Dog feces | 2008.2.22 | GDPH2 | 45 | IncI2 | A2 | 5 |
| 02R | Dog feces | 2008.2.21 | GDPH2 | 45 | IncI2 | A5 | 5 |
| 304 | Dog feces | 2008.3.28 | GDPH1 | 45 | IncI2 | A1 | 5 |
| 312 | Dog feces | 2008.3.31 | GDPH1 | 45 | IncI2 | A1 | 5 |
| 408 | Dog skin | 2008.4.12 | GDPH1 | 45 | IncI2 | A2 | 5 |
| 066 | Cat feces | 2008.5.6 | GDPH1 | 45 | IncI2 | A3 | 5 |
| 069 | Dog feces | 2008.5.6 | GDPH1 | 45 | IncI2 | A5 | 5 |
| 096 | Cat feces | 2008.5.9 | GDPH1 | 45 | IncI2 | A3 | 5 |
| D71 | Dog feces | 2007.4.5 | GDPH1 | 45 | IncI2 | A5 | 20 |
| HD96 | Duck feces | 2006.12 | GD | 45 | IncI2 | A3 | 7 |
| HD101 | Duck feces | 2006.12 | GD | 45 | IncI2 | A4 | 7 |
| FW122 | Duck feces | 2006.12 | GD | 45 | IncI2 | A3 | 7 |
| HD111 | Duck feces | 2006.12 | GD | 45 | IncI2 | A3 | 7 |
| RD175 | Duck feces | 2006.12 | GD | 45 | IncI2 | A4 | 7 |
| RD197 | Duck feces | 2006.12 | GD | 45 | IncI2 | A3 | 7 |
| RD212 | Duck feces | 2006.12 | GD | 45 | IncI2 | A3 | 7 |
| GS11 | Chicken feces | 2009.5 | GS | 45 | IncI2 | A1 | 6 |
| JX138 | Pig feces | 2009.8 | JX | 45 | IncI2 | A5 | 6 |
| SD06 | Chicken liver | 2009.5 | SD | 45 | IncI2 | A1 | 6 |
| D40 | Dog | 2006.12.4 | GDPH1 | 48 | IncI1 | B1 | 20 |
| RD174 | Duck feces | 2006.12 | GD | 48 | IncI1 | B1 | 7 |
| HLJ9 | Calf feces | 2009.6 | HLJ | 48 | IncI1 | B1 | 6 |
| SD15 | Chicken liver | 2009.5 | SD | 48 | IncI1 | B2 | 6 |
| HN10 | Chicken feces | 2009.7 | HN | 48 | IncI1 | C | 6 |
| ZQ06 | Pigeon | 2009.7 | GD | 48 | F2:A−:B− | D | 6 |
| HN21 | Pigeon feces | 2009.7 | HN | 48 | F2:A−:B− | D | 6 |
| SD13 | Chicken liver | 2009.5 | SD | 127 | F33:A−:B− | E | 6 |
| ZCG09 | Pigeon | 2009.7 | GD | 127 | IncN | F | 6 |
All of the isolates listed gave transconjugants carrying blaCTX-M-55. For isolates transferring multiple plasmids (underlined), single plasmids for digestion were obtained by transformation. All transconjugants/transformants carrying blaCTX-M-55 and a single plasmid from all isolates were resistant to cefotaxime and ceftazidime and were susceptible to gentamicin, amikacin, tetracycline, chloramphenicol, ciprofloxacin, and trimethoprim-sulfamethoxazole by agar dilution according to EUCAST (http://www.eucast.org/clinical_breakpoints).
Different provinces are indicated as follows: GD, Guangdong; GS, Gansu; JX, Jiangxi; SD, Shangdong; HN, Hunan; HLJ, Heilongjiang. GDPH1 to GDPH3 indicate different pet hospitals.
Length of the spacer region between the left end of ISEcp1 and the blaCTX-M-55 start codon.
BLASTn searches revealed that the gross structure of pHN1122-1 is closely related to that of the IncI2 plasmids R721 (GenBank accession no. AP002527) from E. coli, pSH146_65 from Salmonella Heidelberg (JN983044) (11), pChi7122-3 from E. coli APEC strain 7122 (O78:K80:H9) (FR851304) (12), and a plasmid from E. coli O157:H7 strain FRIK2000 (NZ_ACXO01000104, referred to as pFRIK2000 here) (13) (Fig. 1).
Fig 1.
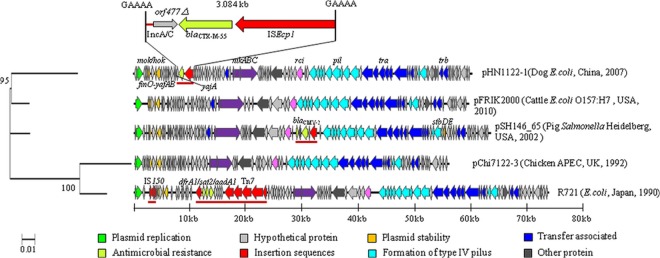
Linear comparisons of pHN1122-1 with other sequenced IncI2 plasmids. Mobile elements and/or resistance regions are underlined in red. The phylogeny was constructed in MEGA5 by the neighbor-joining method from the entire sequences of the five IncI2 plasmids after the removal of any mobile elements. Bootstrap consensus trees were inferred by using 500 replicates (values are shown next to nodes). Sequences with the following GenBank accession numbers were obtained: R721, AP002527; pSH146_65, JN983044; pChi7122-3, FR851304; pFRIK2000, NZ_ACXO01000104; pHN1122-1, JN797501. The maps were drawn by using Vector NTI-11.5.
The full sequence of R721, which is not yet published, was used as a reference for the annotation of pHN1122-1 (see Table S1 in the supplemental material). The RepA replicon protein of pHN1122-1 shows >98% amino acid identity with RepA of R721, pChi7122-3, and pFRIK2000 and has only two nucleic acid differences from repA of pSH146_65. Like R721, pHN1122-1 carries genes encoding plasmid stability functions, including mok/hok and yafA/yafB (parA). The mok/hok genes have only three nucleotide differences from those of pSH146_65 and R721.
Like IncI1 plasmids (14), IncI2-type plasmids produce two types of pili. The thick pilus encoded by the tra operon is the primary pilus for conjugation transfer. The thin pilus encoded by the pil locus is similar to other type IV secretion systems and contributes to increased conjugation rates in liquid medium (15, 16). Both IncI1- and IncI2-type plasmids also contain a shufflon region that undergoes complex rearrangements mediated by Rci, a plasmid-encoded site-specific recombinase (17, 18). Like the four plasmids listed above, pHN1122-1 has the tra and pil loci and a shufflon (Fig. 1). Phylogenetic analysis using entire sequences of the five IncI2 plasmids after the removal of any mobile elements or concatenated sequences of genes shared by all five IncI2 plasmids (see Table S1 in the supplemental material) indicates that they fall into three lineages (Fig. 1; see Fig. S1 in the supplemental material). One contains pHN1122-1 and pFRIK2000, another contains pSH146_65 only, and a third contains pChi7122-3 and R721. Comparison with proteins from the typical IncI1 plasmid R64 showed that only a few have some similarity to those of IncI2 plasmids (see Table S1).
blaCTX-M-55, the only known antibiotic resistance gene carried by pHN1122-1, is located in a 3.084-kb ISEcp1 transposition unit. This consists of a fragment related to the typical ISEcp1-blaCTX-M-15 transposition unit, except with a 45-bp spacer instead of the usual 48 bp and a 112-bp fragment matching IncA/C plasmid backbones beyond orf477Δ. This whole structure is inserted near yajA and is flanked by 5-bp direct repeats (GAAAA) characteristic of ISEcp1 transposition (Fig. 1). This configuration could be explained by the insertion of an ISEcp1-blaCTX-M-55 transposition unit into an IncA/C backbone and capture of the adjacent fragment in a subsequent transposition event (19).
R721 carries three resistance genes, dfrA1, sat2, and aadA1, in a class 2 integron associated with Tn7, while pSH146_65 carries blaCMY-2 also associated with ISEcp1. The sites of these insertions in these three plasmids are different (Fig. 1), and pChi7122-3 and pFRIK2000 do not carry any known antibiotic resistance genes.
The remaining 14 transconjugants carrying blaCTX-M-55 and 20 additional E. coli isolates carrying blaCTX-M-55 from pets (n = 2) and food animals (n = 18; Table 1) from our other previous studies (6, 7, 20) were also examined for pHN1122-1-like plasmids. All 20 of the additional isolates gave transconjugants carrying blaCTX-M-55 in streptomycin-resistant E. coli C600 with selection on cefotaxime (2 μg/ml) and streptomycin (2,000 μg/ml). Examination of alkaline lysis extracts of plasmid DNA from the 34 transconjugants on agarose gels revealed that some had multiple plasmids. For these isolates (Table 1), transformation into E. coli DH5α with selection on 2 μg/ml cefotaxime was used to obtain a single plasmid carrying blaCTX-M-55, as verified by PCR and gel analysis.
Plasmids from the 24 transconjugants or transformants carrying blaCTX-M-55 were analyzed for replicon type (10, 21). Primers designed to amplify conserved regions of the IncI2 backbone (repA, rci, pilO, nikB, and finO; Table 2) revealed that all 14 transconjugants/transformants from the original set of isolates and 11 from the additional 20 had IncI2 plasmids. Sequencing revealed that the repA genes of all 25 were 100% identical to repA of pHN1122-1. Further PCR and sequencing identified the same ISEcp1–blaCTX-M-55 transposition unit with a 45-bp spacer in the same location as in pHN1122-1 in all 25. Digestion of plasmid DNA extracted from transconjugants or transformants with ApaLI (TaKaRa Biotechnology, China) revealed five slightly different plasmid types among the IncI2 plasmids (Table 1). These minor variations in ApaLI patterns may be due to mutations or recombination. ISEcp1-blaCTX-M-55 structures with a 48-bp or 127-bp spacer in the other nine transconjugants/transformants were associated with IncF, IncI1, or IncN plasmids (Table 1), suggesting that blaCTX-M-55 genes with different spacer regions have been disseminated by different plasmid scaffolds.
Table 2.
Primers used to detect pHN1122-1-like regions
| Primer | Nucleotide sequence (5′→3′) | Target DNA sequence | Position in pHN1122-1 |
|---|---|---|---|
| RepA-F | CTGTCGGCATGTCTGTCTC | repA gene | 923–941 |
| RepA-R | CTGGCTACCAGTTGCTCTAA | repA gene | 1475–1456 |
| CHP1-F | GCTAAATGCTTCGCAGGAG | Upstream of orf477Δ | 7093–7111 |
| Orf477-R | ATTCAGCACCACGAAACGA | orf477Δ gene | 8034–8052 |
| ISEcp-F | TATTGTAGCATCGGTTTCC | tnpA of ISEcp1 | 10274–10292 |
| HP2-R | TGTTGTCCCGTATCCTTAT | Downstream of ISEcp1 | 11595–11577 |
| finO-F | CCCGTGATTGTGGTCAAA | finO gene | 3280–3297 |
| finO-R | GGGTTATCCGCCAGGTAT | finO gene | 3597–3614 |
| nikB-F | ATCCAACCTACAGACGCCTTAC | nikB gene | 20234–20255 |
| nikB-R | CTGCGACCTGTGCTTGCT | nikB gene | 21151–21169 |
| rci-F | TGCCCGTTTCTGTTCTCG | rci gene | 30214–30231 |
| rci-R | TGCCCTGTTGTCATCATTATTC | rci gene | 30867–30888 |
| pilQ-F | CGTTGGCGTGTAAGGTCG | pilQ gene | 36773–36790 |
| pilQ-R | CCTGGCGAAAGCAAACAA | pilQ gene | 37513–37530 |
The detection of pHN1122-1-like plasmids in isolates from companion and food animals from different geographic areas of China suggests that these plasmids are effective vectors for the dissemination of blaCTX-M-55 among bacteria in different animal host species. As pHN1122-1 contains no obvious virulence or other antibiotic resistance genes, its persistence and spread may be attributed to either constant β-lactam exposure or its stability in the absence of any antimicrobial selection pressure. The addiction systems (mok/hok) and partitioning genes (yafA/yafB) that promote plasmid maintenance during vertical transmission, as well as the type IV pilus and shufflon, which have been shown to play a role in plasmid conjugation, epithelial cell adherence, and adherence to abiotic surfaces (17, 22), might contribute to the successful dissemination of these pHN1122-like plasmids.
Unlike IncI1 plasmids, IncI2 plasmids have not yet been well studied (11, 12), but this work suggests that they play an important role in the spread of blaCTX-M-55. IncI2 plasmids carrying blaCMY-2 (pSH146_65) and other resistance genes (R721) have also been identified, suggesting that IncI2 plasmids should be included in the group of plasmids involved in the dissemination of important drug resistance genes (23). Considering that IncI2 plasmids are widespread among different animal species but are probably currently missed in PBRT surveys, we suggest the inclusion of PCR screening for IncI2 plasmids to identify any associations with other important resistance genes. Further studies should also examine the prevalence and spread of these plasmids in the environment and human clinical settings.
Nucleotide sequence accession number.
The nucleotide sequence of pHN1122-1 reported in this study has been deposited in the GenBank nucleotide sequence database under accession no. JN797501.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grant U1031004 from the National Natural Science Foundation of China and grant 2013CB127203 from the National Key Basic Research Program of China.
We acknowledge Qiang Li for his assistance in the comparative analysis of genes/proteins and phylogenetic analysis of plasmids.
Footnotes
Published ahead of print 11 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02155-12.
REFERENCES
- 1. Cantón R, Coque TM. 2006. The CTX-M β-lactamase pandemic. Curr. Opin. Microbiol. 9:466–475 [DOI] [PubMed] [Google Scholar]
- 2. Bush K, Fisher JF. 2011. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from Gram-negative bacteria. Annu. Rev. Microbiol. 65:455–478 [DOI] [PubMed] [Google Scholar]
- 3. Cantón R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front. Microbiol. 3:110 doi:10.3389/fmicb.2012.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hawkey PM, Jones AM. 2009. The changing epidemiology of resistance. J. Antimicrob. Chemother. 64(Suppl. 1):i3–i10 [DOI] [PubMed] [Google Scholar]
- 5. Sun Y, Zeng Z, Chen S, Ma J, He L, Liu Y, Deng Y, Lei T, Zhao J, Liu JH. 2010. High prevalence of blaCTX-M extended-spectrum β-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin. Microbiol. Infect. 16:1475–1481 [DOI] [PubMed] [Google Scholar]
- 6. Zheng H, Zeng Z, Chen S, Liu Y, Yao Q, Deng Y, Chen X, Lv L, Zhuo C, Chen Z, Liu JH. 2012. Prevalence and characterisation of CTX-M β-lactamases amongst Escherichia coli isolates from healthy food animals in China. Int. J. Antimicrob. Agents 39:305–310 [DOI] [PubMed] [Google Scholar]
- 7. Ma J, Liu JH, Lv L, Zong Z, Sun Y, Zheng H, Chen Z, Zeng ZL. 2012. Characterization of extended-spectrum β-lactamase genes found among Escherichia coli isolates from duck and environmental samples obtained on a duck farm. Appl. Environ. Microbiol. 78:3668–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu F, Chen Q, Yu X, Li Q, Ding B, Yang L, Chen C, Qin Z, Parsons C, Zhang X, Huang J, Luo Y, Wang L, Pan J. 2011. High prevalence of extended-spectrum β lactamases among Salmonella enterica Typhimurium isolates from pediatric patients with diarrhea in China. PLoS One 6:e16801 doi:10.1371/journal.pone.0016801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao WH, Hu ZQ. 2013. Epidemiology and genetics of CTX-M extended-spectrum β-lactamases in Gram-negative bacteria. Crit. Rev. Microbiol. 39:79–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins K, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 11. Han J, Lynne AM, David DE, Tang H, Xu J, Nayak R, Kaldhone P, Logue CM, Foley SL. 2012. DNA sequence analysis of plasmids from multidrug resistant Salmonella enterica serotype Heidelberg isolates. PLoS One 7:e51160 doi:10.1371/journal.pone.0051160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mellata M, Maddux JT, Nam T, Thomson N, Hauser H, Stevens MP, Mukhopadhyay S, Sarker S, Crabbe A, Nickerson CA, Santander J, Curtiss R., III 2012. New insights into the bacterial fitness-associated mechanisms revealed by the characterization of large plasmids of an avian pathogenic E. coli. PLoS One 7:e29481 doi:10.1371/journal.pone.0029481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dowd SE, Crippen TL, Sun Y, Gontcharova V, Youn E, Muthaiyan A, Wolcott RD, Callaway TR, Ricke SC. 2010. Microarray analysis and draft genomes of two Escherichia coli O157:H7 lineage II cattle isolates FRIK966 and FRIK2000 investigating lack of Shiga toxin expression. Foodborne Pathog. Dis. 7:763–773 [DOI] [PubMed] [Google Scholar]
- 14. Johnson TJ, Shepard SM, Rivet B, Danzeisen JL, Carattoli A. 2011. Comparative genomics and phylogeny of the IncI1 plasmids: a common plasmid type among porcine enterotoxigenic Escherichia coli. Plasmid 66:144–151 [DOI] [PubMed] [Google Scholar]
- 15. Kim SR, Komano T. 1997. The plasmid R64 thin pilus identified as a type IV pilus. J. Bacteriol. 179:3594–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradley DE. 1984. Characteristics and function of thick and thin conjugative pili determined by transfer-derepressed plasmids of incompatibility groups I1, I2, I5, B, K and Z. J. Gen. Microbiol. 130:1489–1502 [DOI] [PubMed] [Google Scholar]
- 17. Garcillán-Barcia MP, Francia MV, de la Cruz F. 2009. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 33:657–687 [DOI] [PubMed] [Google Scholar]
- 18. Kim SR, Komano T. 1992. Nucleotide sequence of the R721 shufflon. J. Bacteriol. 174:7053–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Partridge SR. 2011. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol. Rev. 35:820–855 [DOI] [PubMed] [Google Scholar]
- 20. Deng Y, He L, Chen S, Zheng H, Zeng Z, Liu Y, Sun Y, Ma J, Chen Z, Liu JH. 2011. F33:A−:B− and F2:A−:B− plasmids mediate dissemination of rmtB-blaCTX-M-9 group genes and rmtB-qepA in Enterobacteriaceae isolates from pets in China. Antimicrob. Agents Chemother. 55:4926–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Villa L, García-Fernández A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 65:2518–2529 [DOI] [PubMed] [Google Scholar]
- 22. Dudley EG, Abe C, Ghigo JM, Latour-Lambert P, Hormazabal JC, Nataro JP. 2006. An IncI1 plasmid contributes to the adherence of the atypical enteroaggregative Escherichia coli strain C1096 to cultured cells and abiotic surfaces. Infect. Immun. 74:2102–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53:2227–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


