Abstract
Fosfomycin targets the first step of peptidoglycan biosynthesis in Streptococcus pneumoniae catalyzed by UDP-N-acetylglucosamine enolpyruvyltransferase (MurA1). We investigated whether heteroresistance to fosfomycin occurs in S. pneumoniae. We found that of 11 strains tested, all but 1 (Hungary19A) displayed heteroresistance and that deletion of murA1 abolished heteroresistance. Hungary19A differs from the other strains by a single amino acid substitution in MurA1 (Ala364Thr). To test whether this substitution is responsible for the lack of heteroresistance, it was introduced into strain D39. The heteroresistance phenotype of strain D39 was not changed. Furthermore, no relevant structural differences between the MurA1 crystal structures of heteroresistant strain D39 and nonheteroresistant strain Hungary19A were found. Our results reveal that heteroresistance to fosfomycin is the predominant phenotype of S. pneumoniae and that MurA1 is required for heteroresistance to fosfomycin but is not the only factor involved. The findings provide a caveat for any future use of fosfomycin in the treatment of pneumococcal infections.
INTRODUCTION
Pneumococci have responded to antimicrobial treatment by acquiring resistance to antibiotics, particularly penicillin (1). However, although knowledge of classical resistance mechanisms is increasing (2, 3), understanding of the penicillin heteroresistance phenomenon described in pneumococci remains elusive (4).
Heteroresistance is the ability of a clonal population to grow one or several subpopulations at a frequency of 10−7 to 10−3 in the presence of a higher antibiotic concentration than that predicted to be effective by measurement of the MIC (4). This phenomenon may allow some bacteria to grow at higher antibiotic concentrations without the fitness cost required by a genetically fixed resistance mechanism. Furthermore, heteroresistance allows some bacteria to survive antimicrobial treatment while at the same time making conventional resistance testing unreliable (4, 5). Heteroresistance to different antibiotics has been described in pathogens of various species (6–9), but even for the most extensively studied (heteroresistance to methicillin, oxacillin, and vancomycin in staphylococci [10–16]), the mechanism is unknown. Understanding heterogeneity between single cells is complicated by the fact that conventional assays of microbial populations consider an averaged value of thousands or millions of cells in a sample (17).
Fosfomycin (18) is not currently used in the treatment of pneumococcal infections, but in this era of multidrug resistance, studies of this antibiotic may be valuable to inform decisions on future antibiotic use (19). Fosfomycin is a drug of particular interest for its synergy with benzylpenicillin, by decreasing the production of penicillin-binding proteins. This is beneficial in the treatment of infections with β-lactam-resistant pneumococci (20). Combinations of other antibiotics with fosfomycin are used successfully in the treatment of infections with other organisms, such as Staphylococcus aureus (21). The target of fosfomycin is MurA (UDP-N-acetylglucosamine enolpyruvyltransferase, EC 2.5.1.7), an enzyme catalyzing the first step of the peptidoglycan biosynthetic pathway of Gram-positive and Gram-negative microorganisms. MurA transfers the enolpyruvyl group of phosphoenolpyruvate (PEP) to UDP-N-acetylglucosamine (UDP-GlcNAc), producing enolpyruvyl UDP-N-acetylglucosamine (EP-UDP-GlcNAc) (22, 23). Gram-positive bacteria have two genes, murA1 and murA2, both encoding active MurA enzymes with similar catalytic parameters and conserved major structural features that are both inhibited by fosfomycin (24).
Mutagenesis experiments have revealed that fosfomycin covalently binds to Cys115 of MurA, suggesting that this amino acid plays an important role in the catalytic mechanism (25, 26). The Cys115 residue has been suggested to be the general acid catalyst, protonating C-3 of PEP prior to nucleophilic attack by UDP-GlcNAc to form the tetrahedral intermediate (27, 28), although other experiments indicate that a different amino acid (Asp) plays this role (29). Recent studies have shown that MurA forms dormant complexes with UDP-N-acetylmuramic acid (UDP-MurNAc), which is the product of MurB, the second enzyme participating in peptidoglycan synthesis. This supports the idea that Cys115 provides tight product regulation and protection, which are absent from species containing aspartic acid in this position (30).
At the structural level, MurA can exist in open and closed conformations (31, 32). Native enzymes are in the open conformation and then close when UDP-GlcNAc enters the catalytic pocket. This closure consists of the movement of the loop containing Cys115 over UDP-GlcNAc, creating the optimal environment for the addition-elimination reaction.
After describing heteroresistance to penicillin in Streptococcus pneumoniae (4), we investigated whether the heteroresistance phenomenon also exists in an early step of peptidoglycan biosynthesis. Therefore, we tested a representative selection of pneumococcal strains for heteroresistance to fosfomycin, which acts earlier in the peptidoglycan synthesis pathway than penicillin. Here we report for the first time that heteroresistance to fosfomycin occurs in S. pneumoniae and investigate the role of MurA1 in this phenotype.
MATERIALS AND METHODS
Bacterial strains.
Eleven strains including five clinical isolates of S. pneumoniae from two nationwide surveillance programs collecting nasopharyngeal and invasive isolates, five international reference strains, and laboratory strain D39, all listed in Table 1, were used for this study.
Table 1.
Frequency of subpopulations with higher fosfomycin and penicillin resistance levels in selected S. pneumoniae strains
| Strain | MLST type | Serotype | Fosfomycin MIC (μg/ml)a | Frequency of cells with higher fosfomycin resistanceb | Penicillin MIC (μg/ml)c | Frequency of cells with higher penicillin resistancec | Reference or source |
|---|---|---|---|---|---|---|---|
| Wild types | |||||||
| 207.41 | 179 | 19F | 32 | 10−4–10−5 | 0.064 | 0 | 48 |
| 208.39 | 276 | 19F | 3.0 | 10−4–10−5 | 0.75 | 10−3–10−5 | 4 |
| 304.80 | 179 | 19F | 3.0 | 10−2–10−5 | 0.75 | 10−3–10−5 | 48 |
| 106.44 | 344 | nt | 8.0 | 10−5–10−6 | 1.0 | 10−4–10−5 | 49 |
| 110.58 | 344 | nt | 8.0 | 10−3–10−5 | 0.19 | 10−6 | 49 |
| Spain9V-3 | 156 | 9V | 24 | 10−4 | 2.0 | 10−3–10−6 | ATCC 700671 |
| Hungary19A-6 | 268 | 19A | 6.0 | 0 | 1.0 | 10−3–10−5 | ATCC 700673 |
| Finland6B-12 | 270 | 6B | 3.0 | 10−3–10−6 | 0.38 | 10−3 | ATCC 700903 |
| Taiwan23F-15 | 242 | 23F | 12 | 10−6 | 1.5 | 10−3–10−6 | ATCC 700906 |
| South Africa19A-7 | 75 | 19A | 12 | 10−4–10−6 | 0.38 | 0 | ATCC 700674 |
| D39 | 128 | 2 | 16 | 10−5–10−6 | 0.023 | 0 | 50 |
| ΔmurA1 mutants | |||||||
| 106.44ΔmurA1 | 344 | nt | 1.5 | 0 | 0.5 | 10−5–10−6 | This study |
| Taiwan23FΔmurA1 | 242 | 23F | 1.5 | 0 | 0.5 | 10−2–10−5 | This study |
| D39ΔmurA1 | 128 | 2 | 2.0 | 0 | 0.016 | 0 | This study |
| Hungary19AΔmurA1 | 268 | 19A | 2.0 | 0 | 0.5 | 10−3–10−5 | This study |
| Switch mutants | |||||||
| D39::murA1Hungary | 128 | 2 | 16.0 | 10−5–10−6 | 0.032 | 0 | This study |
| Hungary::murA1D39 | 268 | 19A | 8.0 | 0 | 0.5 | 10−4–10−6 | This study |
| D39::murA1+murBHungary | 128 | 2 | 16.0 | 10−5–10−6 | 0.016 | NDd | This study |
| Hungary::murA1+murBD39 | ND | 19A | 8.0 | 0 | 0.75 | NDd | This study |
| Back transformants | |||||||
| D39::murA1D39 | 128 | 2 | 16.0 | 10−5–10−6 | 0.016 | 0 | This study |
| Hungary::murA1Hungary | 268 | 19A | 8.0 | 0 | 0.75 | 10−3–10−5 | This study |
Construction of mutants.
For the construction of murA1 knockout mutants, murA1 was disrupted with the bicistronic cassette Janus (33). Transformation of strains 106.44, Taiwan23F, Hungary19A, and D39 was performed as described elsewhere (34). Insertion of the cassette was confirmed by PCR. Mutant Hungary::murA1D39 and D39::murA1D39 back transformants were obtained by the insertion of a construct with murA1D39 fused to a spectinomycin resistance cassette to allow selection as described previously (35). Mutant D39::murA1Hungary19A and Hungary::murA1Hungary19A back transformants were obtained after the insertion of the murA1Hungary19A-spectinomycin resistance cassette construct, which was created by the introduction of mutation G1090A into the murA1D39-spectinomycin resistance cassette construct. Switch mutants of murB, i.e., Hungary::murA1+murBD39 and D39::murA1+murBHungary19A, were obtained by the introduction of a construct with murBD39 or murBHungary19A, respectively, fused to a kanamycin resistance cassette into murA1 switch mutants. The murA1 and murB sequences of all switch mutants and back transformants were confirmed as described before (36). For more details about the procedures used, see Materials and Methods in the supplemental material.
Susceptibility testing and PAP.
Fosfomycin MICs were determined by the Etest method (bioMérieux, Geneva, Switzerland) according to the manufacturer's protocol. Population analysis profiles (PAPs) were prepared as described earlier (4). Briefly, bacteria were cultured to mid-log phase (optical density at 600 nm [OD600] of 0.7) and dilutions of 10−2 to 10−4 and 10−6 in phosphate-buffered saline (pH 7.4) were prepared. A 100-μl volume was plated on Müller-Hinton broth plates with 5% sheep blood containing fosfomycin (fosfomycin disodium; Chemical Abstracts Service no. 26016-99-9) in concentrations ranging from 0 to 500 μg/ml. Colonies were counted by eye after 48 h of incubation at 37°C in 5% CO2.
Gene expression studies.
Bacteria were grown overnight in 5% brain heart infusion broth (BHI)–fetal calf serum (FCS) for 9 h at 37°C, and then 200 μl of the overnight culture was rediluted in 20 ml of BHI-FCS and grown to an OD600 0.6. A 20-ml volume of RNAprotect (Qiagen) was then added, RNA was extracted, and the expression of murA1 and murA2 was quantified by real-time reverse transcription (RT)-PCR as described elsewhere (37). For the primers and probes used, see Table S1 in the supplemental material. Quantification of murA1 and murA2 expression in wild-type strains was done four times, and quantification of murA2 expression in ΔmurA1 mutant strains was measured in triplicate.
Recombinant protein expression.
The murA1 sequences of strains D39 and Hungary19A were cloned into pGEX-6p-1 and expressed in Escherichia coli BL21 cells. Purification of recombinant protein was performed by glutathione Sepharose 4 Fast Flow chromatography (GE Healthcare, Stockholm, Sweden) as suggested by the manufacturer. Protein samples were analyzed by SDS-PAGE and were >95% of the expected protein. Identities of recombinant proteins were confirmed by mass spectrometric peptide fingerprinting. For more details, see Materials and Methods in the supplemental material.
MurA1D39 and MurA1Hungary19A crystallization and data collection and processing.
Crystallization conditions were screened (PACT Suite and JCSG Suite from Qiagen, Hilden, Germany; JBScreen Classic 1-4 from Jena Bioscience, Jena, Germany; Crystal Screen, Crystal Screen 2, and Index HT from Hampton Research) by high-throughput techniques with a NanoDrop robot and Innovadyne SD-2 microplates (Innovadyne Technologies Inc.). Conditions were optimized by a hanging-drop vapor diffusion method, mixing of 1 μl of protein solution and 1 μl of precipitant solution and equilibration against 500 μl of reservoir solution.
MurA1D39 protein crystals were obtained in 50 mM CaCl2–100 mM HEPES (pH 7.5)–24% (wt/vol) polyethylene glycol (PEG) 4000 at a concentration of 14 mg/ml. MurA1Hungary19A crystals were obtained in 0.2 M ammonium acetate–0.1 M sodium citrate tribasic dihydrate (pH 5.6)–30% (wt/vol) PEG 4000 at a concentration of 7.5 mg/ml. Crystals of both proteins were cryoprotected in 20% (vol/vol) glycerol before collection of diffraction data by the use of synchrotron radiation at beamline PX3 in the Swiss Light Source (SLS), Zurich, Switzerland. MurA1D39 crystals diffracted up to 2.9 Å resolution and belonged to the P212121 orthorhombic space group (a = 46.62 Å, b = 81.11 Å, c = 104.88 Å, α = β = γ = 90°). One single monomer was found in the asymmetric unit, yielding a Matthews coefficient (38) of 2.18 Å3 Da−1 and a solvent content of 43.5%. MurA1Hungary19A crystals diffracted up to 1.8 Å resolution and belonged to the P21212 orthorhombic space group (a = 106.58 Å, b = 70.36 Å, c = 45.24 Å, α = β = γ = 90°). One single monomer was found in the asymmetric unit, yielding a Matthews coefficient of 1.86 Å3 Da−1 and a solvent content of 33.9%. Diffraction data sets were processed with XDS (39) and SCALA (40, 41).
MurA1D39 and MurA1Hungary19A structural determination and refinement.
MurA1D39 and MurA1Hungary19A crystal structures were obtained by molecular replacement, which was performed with Phaser (42) by using MurA from Enterobacter cloacae (31) (Protein Data Bank [PDB] code 1NAW) as the initial model for the MurA1Hungary19A data set and then MurA1Hungary19A as the model for MurA1D39. Refinement and model building of both structures were performed with Phenix (43) and Coot (44), respectively. The refinement converged to the final values of Rwork = 0.16 and Rfree = 0.20 for MurA1D39 and Rwork = 0.18 and Rfree = 0.24 for MurA1Hungary19A (Table 2). Analysis of these models with MolProbity showed good stereochemistry (45). The quality of the electron density map allowed the modeling of MurA1D39 from Met1 to Asp419, although some residues belonging to a flexible loop were not included in the final model (from Gly114 to Pro120). The modeling of MurA1Hungary19A involved residues Met1 to Glu418, although two regions were not included in the final models (from Pro113 to Cys116 and from Tyr85 to Lys87). All graphic representations were prepared with PyMOL.
Table 2.
X-ray data collection and refinement statistics for MurA1D39 and MurA1Hungary19A
| Parameter | Measurementa |
|
|---|---|---|
| MurA1D39 | MurA1Hungary19A | |
| Space group | P212121 | P21212 |
| a (Å) | 46.62 | 106.58 |
| b (Å) | 81.11 | 70.36 |
| c (Å) | 104.88 | 45.24 |
| α = β = γ (°) | 90 | 90 |
| Resolution range (Å) | 44.0–2.9 | 45.2–1.8 |
| Beamline | PX3 (SLS) | PX3 (SLS) |
| Wavelength (Å) | 1 | 1 |
| No. of reflections | ||
| Total | 185,839 | 420,364 |
| Unique | 14,373 | 32,258 |
| Rpimb | 0.114 (0.427) | 0.039 (0.351) |
| Mean I/σ (I) | 7.0 (1.9) | 16.3 (2.3) |
| Completeness (%) | 100 | 99.7 |
| Redundancy | 6.5 (6.2) | 12.7 (13.0) |
| Refinement | ||
| No. of atoms (non-H) | 3,132 | 3,506 |
| Rwork/Rfreec | 0.18/0.24 | 0.16/0.20 |
| RMSd bond deviations | ||
| Length (Å) | 0.002 | 0.007 |
| Angle (°) | 0.504 | 1.07 |
| PDB code | 3HZ3 | 3HZ4 |
Values in parentheses are for the highest-resolution shell.
Rpim = ∑hkl [1/(N − 1)] 1/2 ∑i | Ii(hkl) − [I(hkl)]|/∑hkl ∑i Ii(hkl), where Ii(hkl) is the ith measurement of reflection hkl, [I(hkl)] is the weighted mean of all measurements, and N is the redundancy of the hkl reflection.
Rwork/Rfree = ∑hkl | Fo − Fc |/∑hkl | Fo |, where Fc is the calculated and Fo is the observed structure factor amplitude of reflection hkl for the working/free (5%) set, respectively.
RMS, root mean square.
Nucleotide sequence accession numbers.
The atomic coordinates and structure factors for MurA1D39 and MurA1Hungary19A have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University (http://www.rcsb.org/), under codes 3ZH3 and 3ZH4, respectively. The GenBank accession numbers for MurA1 of D39 and Hungary19A are YP_816443.1 and YP_001694508.1, respectively.
RESULTS AND DISCUSSION
Heteroresistance to fosfomycin was exhibited by the majority of the strains studied.
Heteroresistance to penicillin has been described in S. pneumoniae and is hypothesized to be due to a combination of auxiliary genes acting in concert with one or more pbp genes, the products of which are inhibited by penicillin (4). Here we investigated whether we could observe heteroresistance to fosfomycin, an antibiotic acting on an earlier step of peptidoglycan biosynthesis than penicillin. Fosfomycin inhibits MurA1 and MurA2; enzymes that catalyze the first step of peptidoglycan biosynthesis. MurA1 and MurA2 have conserved major structural features and similar catalytic parameters and are both inhibited by fosfomycin with comparable efficiency (24).
Within the 11 strains we tested, representing Swiss clinical strains, international reference strains and laboratory strains, we found that heteroresistance to fosfomycin was the predominant phenotype as all of the strains but one, Hungary19A, were heteroresistant to fosfomycin (Table 1). Heteroresistance to penicillin, although the predominant phenotype, was exhibited by only 8 of the 11 strains. This suggests that different mechanisms cause the heterogeneous expression of resistance to penicillin and fosfomycin.
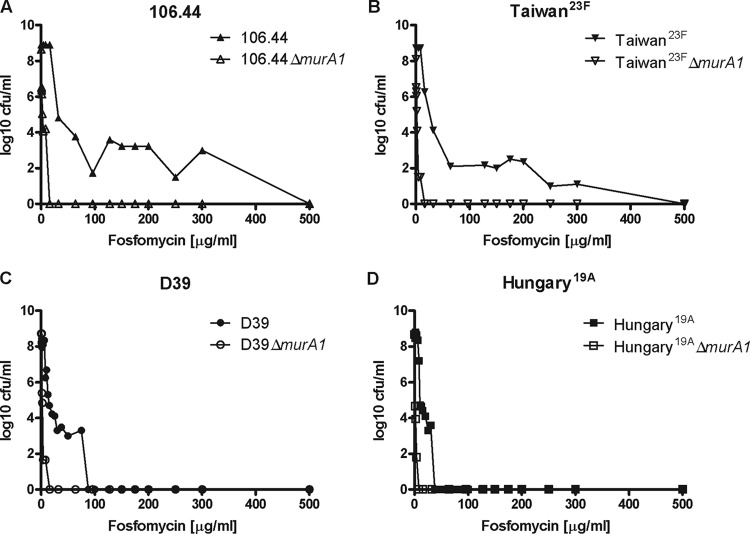
Fosfomycin is not currently used as a treatment for pneumococcal infections, so the predominance of the heteroresistance phenotype in the absence of selective pressure was unexpected. Still, the fosfomycin MICs determined all ranged from 3 to 32 μg/ml (Table 1), which reflects the usual distribution of fosfomycin MICs for pneumococci (http://mic.eucast.org/Eucast2/). However, growth of heteroresistant strains at up to 300 μg/ml fosfomycin was observed (Fig. 1), demonstrating the threat that heteroresistance could pose, although its clinical significance remains controversial (5).
Fig 1.
PAPs of S. pneumoniae wild-type and murA1 knockout strains. An experiment representative of three independent experiments is shown. The concentration of fosfomycin used to select subpopulations with higher fosfomycin resistance levels is shown against the frequency of bacteria able to grow at that concentration. Wild-type and murA1 knockout mutant forms of Swiss clinical strain 106.44 (A), international reference strain Taiwan23F (B), laboratory strain D39 (C), and international reference strain Hungary19A (D) are shown. A horizontal plateau in the curve, such as that shown for strain 106.44 in panel A, indicates heteroresistance, in contrast to a steep drop in the curve indicating no heteroresistance, as shown for strains Hungary19A in panel D.
As a differentiating element unique to Hungary19A, we identified a mutation in murA1. Therefore, we investigated further the role of MurA1 in heteroresistance.
Deletion of murA1 abolished heteroresistance.
The influence of the fosfomycin target MurA1 on heteroresistance was analyzed by comparing the PAPs of wild-type and ΔmurA1 mutant strains. Knockout of murA1 leads to abolishment of the heteroresistance phenotype (Fig. 1) and a drastic reduction of the fosfomycin MIC (Table 1) for all of the strains tested, indicating that it does indeed mediate heteroresistance to this antibiotic. However, heteroresistance to penicillin was conserved in the mutants, supporting the hypothesis that distinct mechanisms determine heteroresistance to penicillin and fosfomycin.
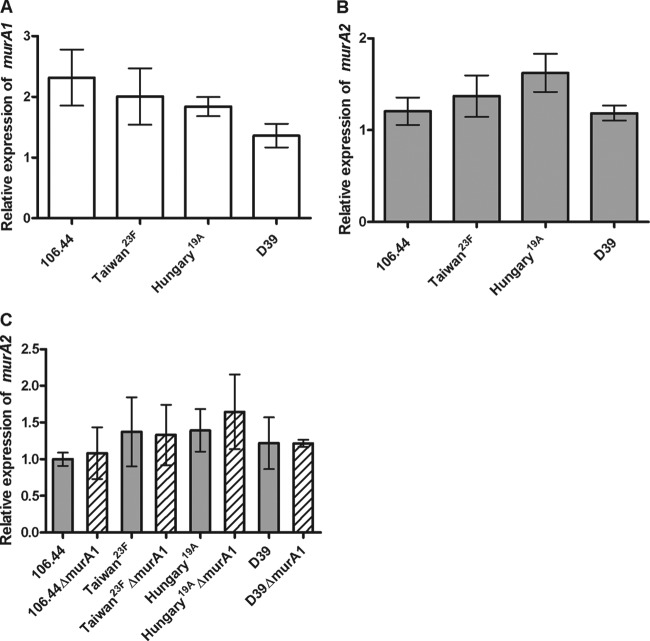
Expression of murA1 and murA2 did not differ between strains.
To determine whether heteroresistance to fosfomycin in the wild-type strains is due to differences in the expression of the fosfomycin targets MurA1 and MurA2, expression was quantified by real-time RT-PCR (Fig. 2A and B).
Fig 2.
Relative levels of murA1 (A) and murA2 (B) expression in S. pneumoniae wild-type strains. (C) Comparison of murA2 expression levels in wild-type (gray bars) and murA1 knockout (hatched bars) strains. Expression is displayed as a value relative to that of the isolate with the lowest expression level after normalization to 16S RNA gene expression. Values are means of three independent experiments. The standard error of the mean is shown. No significant differences were detected by analysis of variance.
There was no significant difference between strains in the expression of either murA1 or murA2, indicating that MurA1/A2 expression levels do not determine heteroresistance. In addition, knocking out murA1 did not result in a compensatory upregulation of murA2 for any of the strains tested (Fig. 2C). A limitation of these experiments is that expression levels were measured in liquid culture in the absence of antibiotic and therefore may not represent the expression of MurA within a highly resistant subpopulation in a heteroresistant strain. Experiments conducted with colonies grown in different antibiotic concentrations (not shown) showed extremely variable MurA1 expression patterns, making the results difficult to interpret, which is why Fig. 2 represents the results of liquid culture in the absence of antibiotic.
An Ala364Thr substitution in MurA1 did not influence heteroresistance.
Sequencing of MurA1 revealed a mutation leading to an Ala364Thr substitution in strain Hungary19A. To determine whether this mutation confers a significant conformational change, we solved the three-dimensional structures of S. pneumoniae MurA1D39 and MurA1Hungary19A at 2.9 and 1.8 Å resolutions, respectively.
The S. pneumoniae MurA1 enzyme consists of a single chain of 419 residues forming two globular domains (I and II) linked by a double-stranded hinge (residues 19 to 20 and 231 to 233) (Fig. 3). The overall structure of this protein follows the folding previously described for the E. cloacae MurA enzyme (31). The closest structural homologue of MurA1D39 is the open conformation of E. cloacae MurA (PDB code 1EJD), whereas for MurA1Hungary19A it is the closed conformation of Bacillus anthracis MurA (PDB code 3SG1) (see Fig. S1 in the supplemental material). The loop containing catalytic Cys116 (residues 110 to 122) presents some differences compared with that of close homologues (see Fig. S2). Most of this loop showed strong flexibility, in agreement with previous works in which this flexibility has been reported (31, 46).
Fig 3.
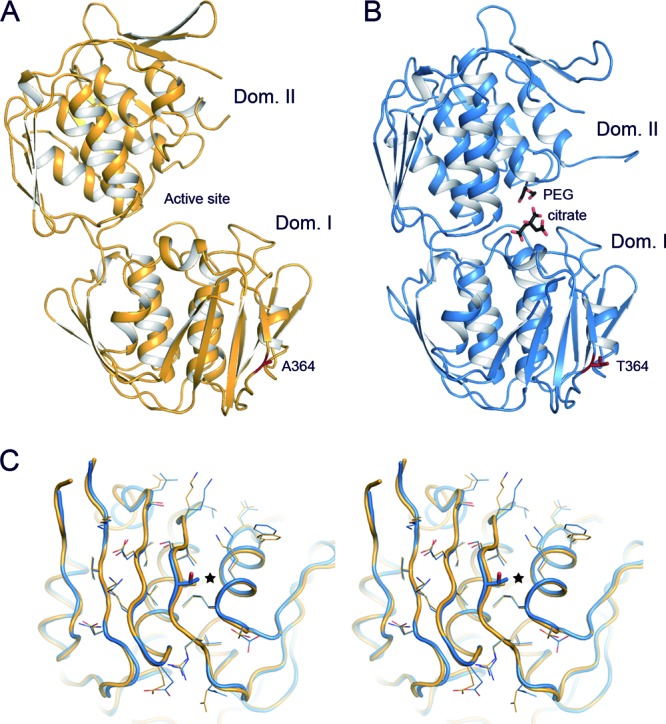
Crystal structure of pneumococcal MurA1. (A) Overall structure of S. pneumoniae MurA1D39 in an open conformation. Ala364 is represented as capped sticks and colored red. (B) Overall structure of S. pneumoniae MurA1Hungary19A in a closed conformation, showing a citrate and a PEG molecule in the catalytic pocket (black-capped sticks). Thr364 is represented as capped sticks and colored red. (C) Stereo view of the superposition of domain I of MurA1D39 (orange) and MurA1Hungary19A (blue). The Ala364Thr mutation is represented in sticks and highlighted (star). Side chains of residues around position 364 are drawn as lines to show that there are no relevant modifications upon mutation. Dom., domain.
The crystal structure of MurA1D39 presents an open conformation (Fig. 3A), while MurA1Hungary19A showed a closed conformation; this is probably due to the unexpected action of the citrate buffer in the catalytic pocket. Citrate buffer was found attached to the active site occupying the position of PEP and fosfomycin (Fig. 3B). The difference between the open and closed conformations is an angle of about 20° between their domains.
The only difference between MurA1D39 and MurA1Hungary19A concerns the Ala364Thr substitution. This mutation is located 25 Å away from the active site in domain I (Fig. 3). Structural inspection of this region reveals no significant changes in the main chain (root mean square deviation of 0.546 Å for the Cα atoms of domain I) or in the amino acid interaction networks that could propagate to the active site affecting catalytic activity (Fig. 3C). This structural similarity between MurA1D39 and MurA1Hungary19A suggests that the Ala364Thr substitution is not involved in the heteroresistance phenomenon. Notwithstanding the absence of significant conformation alteration by the mutation, the influence of the amino acid substitution on a differential dynamic contribution between the two proteins cannot be discounted, as has been described previously for a variant of the TEM-1 β-lactamase of E. coli (47).
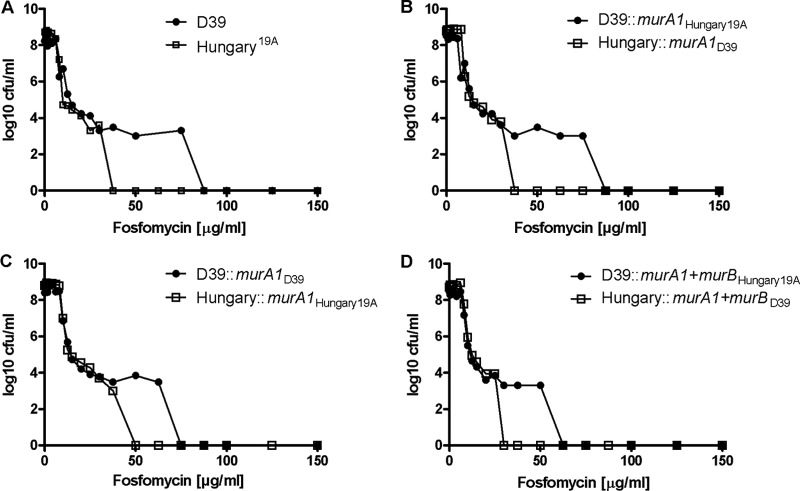
The findings of the structural analysis are confirmed by the assessment of murA1 switch mutants in PAPs. Compared to the wild-type strains (Fig. 4A), transfer of murA1Hungary19A did not lead to abolishment of the heteroresistance phenotype in D39, clearly distinguishable by the plateau in the PAP curve representing the growth of colonies over a fosfomycin concentration range of 12.5 to 75 μg/ml, nor did heteroresistance appear upon the insertion of murA1D39 into the Hungary19A background (Fig. 4B). The spectinomycin resistance cassette used for the selection of the mutants created did not influence the heteroresistance phenotype, as demonstrated by the comparison of back transformants with the wild-type strains (Fig. 4C).
Fig 4.
PAPs of D39 and Hungary19A wild-type strains, murA1 switch mutants, murA1 and murB switch mutants, and back transformants. Representative values of triplicate experiments are shown. (A) D39 compared to Hungary19A wild-type strain. (B) D39 switch mutant containing murA1 of Hungary19A and vice versa. (C) Back transformants containing the spectinomycin resistance cassette of the murA1 switch mutants. (D) Mutants containing switched murA1 and murB genes.
A previous study reported that the product of the MurB enzyme UDP-MurNAc of E. cloacae binds tightly to MurA, creating a dormant complex. Therefore, UDP-MurNAc could act as a negative feedback regulator of MurA when it is present in excess (30). Because the murB sequences of Hungary19A and D39 differ, we tested whether murB switch mutants of strains D39 (GenBank accession no. ABJ54032.1) and Hungary19A (ACA35854.1) exhibited any altered behavior in fosfomycin PAPs. This was not the case, as mutants carrying murA1 and murB from the strain with the opposite fosfomycin heteroresistance phenotype behaved as is characteristic of their respective backgrounds (Fig. 4D).
Our results indicate that MurA1 is required for heteroresistance to fosfomycin, but there is another factor within the genome that determines the heteroresistance phenotype. This conclusion is consistent with the findings of studies investigating the mechanism of heteroresistance to methicillin in staphylococci which identified a gene crucial to the heteroresistance phenotype but also proposed a role for an unknown factor X (13, 14) or chr* (15).
Here we have shown that heteroresistance to fosfomycin is the predominant phenotype of S. pneumoniae. We found that heteroresistance to fosfomycin can occur in the absence of heteroresistance to penicillin and vice versa, suggesting that different mechanisms cause heteroresistance to different antimicrobial agents.
Appreciation of the existence of heteroresistance of S. pneumoniae to antibiotics and understanding of the mechanisms by which it arises are important, as the phenomenon might represent a mechanism of bacterial survival of therapeutic concentrations of a given antibiotic resulting in clinical treatment failure. The predominance of heteroresistance to fosfomycin described here may influence decisions on the use of this antibiotic against pneumococcal infections in the future.
Supplementary Material
ACKNOWLEDGMENTS
We thank Suzanne Aebi and Marianne Küffer for excellent technical assistance and SLS staff for help in X-ray data collection. We also thank J. K. Trczinski (Harvard School of Public Health, Boston, MA) for the Janus cassette, Andrew Camilli (Tufts University, Boston, MA) for the pR412 plasmid, J. Frey (Institute of Veterinary Bacteriology, University of Bern, Bern, Switzerland) for the E. coli SURE and BL21 strains, Dalia Denapaite (Technical University of Kaiserslautern, Kaiserslautern, Germany) for advice on genetic constructions, and Andrea Endimiani for critical reading of the manuscript.
This study was supported by grants from the Spanish Ministry of Economy and Competitiveness (BFU2011-25326), the Madrid Regional Government (S2010/BMD-2457), and the Swiss Centre for Antibiotic Resistance (www.anresis.ch).
Footnotes
Published ahead of print 9 April 2013
We dedicate this work to the memory of Kathrin Mühlemann, who designed and guided research for this study, encouraging us to overcome difficulties of any kind. Her expertise and friendship are sorely missed.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00223-13.
REFERENCES
- 1. Hakenbeck R, Grebe T, Zahner D, Stock JB. 1999. β-Lactam resistance in Streptococcus pneumoniae: penicillin-binding proteins and non-penicillin-binding proteins. Mol. Microbiol. 33:673–678 [DOI] [PubMed] [Google Scholar]
- 2. Contreras-Martel C, Dahout-Gonzalez C, Martins Ados S, Kotnik M, Dessen A. 2009. PBP active site flexibility as the key mechanism for β-lactam resistance in pneumococci. J. Mol. Biol. 387:899–909 [DOI] [PubMed] [Google Scholar]
- 3. Zerfass I, Hakenbeck R, Denapaite D. 2009. An important site in PBP2x of penicillin-resistant clinical isolates of Streptococcus pneumoniae: mutational analysis of Thr338. Antimicrob. Agents Chemother. 53:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morand B, Mühlemann K. 2007. Heteroresistance to penicillin in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 104:14098–14103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falagas ME, Makris GC, Dimopoulos G, Matthaiou DK. 2008. Heteroresistance: a concern of increasing clinical significance? Clin. Microbiol. Infect. 14:101–104 [DOI] [PubMed] [Google Scholar]
- 6. Alam MR, Donabedian S, Brown W, Gordon J, Chow JW, Zervos MJ, Hershberger E. 2001. Heteroresistance to vancomycin in Enterococcus faecium. J. Clin. Microbiol. 39:3379–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung KH, Wang MC, Huang AH, Yan JJ, Wu JJ. 2012. Heteroresistance to cephalosporins and penicillins in Acinetobacter baumannii. J. Clin. Microbiol. 50:721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:2946–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rinder H, Mieskes KT, Loscher T. 2001. Heteroresistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 5:339–345 [PubMed] [Google Scholar]
- 10. de Lencastre H, Tomasz A. 1994. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 38:2590–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ender M, Berger-Bächi B, McCallum N. 2009. A novel DNA-binding protein modulating methicillin resistance in Staphylococcus aureus. BMC Microbiol. 9:15 doi:10.1186/1471-2180-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finan JE, Rosato AE, Dickinson TM, Ko D, Archer GL. 2002. Conversion of oxacillin-resistant staphylococci from heterotypic to homotypic resistance expression. Antimicrob. Agents Chemother. 46:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartman BJ, Tomasz A. 1986. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 29:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murakami K, Tomasz A. 1989. Involvement of multiple genetic determinants in high-level methicillin resistance in Staphylococcus aureus. J. Bacteriol. 171:874–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ryffel C, Strassle A, Kayser FH, Berger-Bächi B. 1994. Mechanisms of heteroresistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 38:724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomasz A, Nachman S, Leaf H. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 35:124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avery SV. 2006. Microbial cell individuality and the underlying sources of heterogeneity. Nat. Rev. Microbiol. 4:577–587 [DOI] [PubMed] [Google Scholar]
- 18. Perales A, Martínez-Ripoll M, Fayos J, von Carstenn-Lichterfelde C, Fernandez M. 1982. The absolute configuration of active and inactive fosfomycin. Acta Crystallogr. B 38:2763–2764 [Google Scholar]
- 19. Michalopoulos AS, Livaditis IG, Gougoutas V. 2011. The revival of fosfomycin. Int. J. Infect. Dis. 15:e732–e739 [DOI] [PubMed] [Google Scholar]
- 20. Kikuchi K, Totsuka K, Shimizu K, Ishii T, Yoshida T, Orikasa Y. 1995. Effects of combination of benzylpenicillin and fosfomycin on penicillin-resistant Streptococcus pneumoniae. Microb. Drug Resist. 1:185–189 [DOI] [PubMed] [Google Scholar]
- 21. Miró JM, Entenza JM, Del Rio A, Velasco M, Castaneda X, Garcia de la Maria C, Giddey M, Armero Y, Pericas JM, Cervera C, Mestres CA, Almela M, Falces C, Marco F, Moreillon P, Moreno A. 2012. High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 56:4511–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gunetileke KG, Anwar RA. 1968. Biosynthesis of uridine diphospho-N-acetylmuramic acid. II. Purification and properties of pyruvate-uridine diphospho-N-acetylglucosamine transferase and characterization of uridine diphospho-N-acetylenopyruvylglucosamine. J. Biol. Chem. 243:5770–5778 [PubMed] [Google Scholar]
- 23. Walsh CT, Benson TE, Kim DH, Lees WJ. 1996. The versatility of phosphoenolpyruvate and its vinyl ether products in biosynthesis. Chem. Biol. 3:83–91 [DOI] [PubMed] [Google Scholar]
- 24. Du W, Brown JR, Sylvester DR, Huang J, Chalker AF, So CY, Holmes DJ, Payne DJ, Wallis NG. 2000. Two active forms of UDP-N-acetylglucosamine enolpyruvyl transferase in gram-positive bacteria. J. Bacteriol. 182:4146–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wanke C, Amrhein N. 1993. Evidence that the reaction of the UDP-N-acetylglucosamine 1-carboxyvinyltransferase proceeds through the O-phosphothioketal of pyruvic acid bound to Cys115 of the enzyme. Eur. J. Biochem. 218:861–870 [DOI] [PubMed] [Google Scholar]
- 26. Marquardt JL, Brown ED, Lane WS, Haley TM, Ichikawa Y, Wong CH, Walsh CT. 1994. Kinetics, stoichiometry, and identification of the reactive thiolate in the inactivation of UDP-GlcNAc enolpyruvoyl transferase by the antibiotic fosfomycin. Biochemistry 33:10646–10651 [DOI] [PubMed] [Google Scholar]
- 27. Kim DH, Lees WJ, Walsh CT. 1995. Stereochemical analysis of the tetrahedral adduct formed at the active site of UDP-GlcNAc enolpyruvyl transferase from the pseudosubstrates, (E)- and (Z)-3-fluorophosphoenolpyruvate, in D2O. J. Am. Chem. Soc. 117:6380–6381 [Google Scholar]
- 28. Han H, Yang Y, Olesen SH, Becker A, Betzi S, Schonbrunn E. 2010. The fungal product terreic acid is a covalent inhibitor of the bacterial cell wall biosynthetic enzyme UDP-N-acetylglucosamine 1-carboxyvinyltransferase (MurA). Biochemistry 49:4276–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eschenburg S, Kabsch W, Healy ML, Schonbrunn E. 2003. A new view of the mechanisms of UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) and 5-enolpyruvylshikimate-3-phosphate synthase (AroA) derived from X-ray structures of their tetrahedral reaction intermediate states. J. Biol. Chem. 278:49215–49222 [DOI] [PubMed] [Google Scholar]
- 30. Zhu JY, Yang Y, Han H, Betzi S, Olesen SH, Marsilio F, Schonbrunn E. 2012. Functional consequence of covalent reaction of phosphoenolpyruvate with UDP-N-acetylglucosamine 1-carboxyvinyltransferase (MurA). J. Biol. Chem. 287:12657–12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schönbrunn E, Sack S, Eschenburg S, Perrakis A, Krekel F, Amrhein N, Mandelkow E. 1996. Crystal structure of UDP-N-acetylglucosamine enolpyruvyltransferase, the target of the antibiotic fosfomycin. Structure 4:1065–1075 [DOI] [PubMed] [Google Scholar]
- 32. Skarzynski T, Mistry A, Wonacott A, Hutchinson SE, Kelly VA, Duncan K. 1996. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure 4:1465–1474 [DOI] [PubMed] [Google Scholar]
- 33. Sung CK, Li H, Claverys JP, Morrison DA. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meier PS, Utz S, Aebi S, Mühlemann K. 2003. Low-level resistance to rifampin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:863–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shevchuk NA, Bryksin AV, Nusinovich YA, Cabello FC, Sutherland M, Ladisch S. 2004. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 32:e19 doi:10.1093/nar/gnh014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brugger SD, Hathaway LJ, Mühlemann K. 2009. Detection of Streptococcus pneumoniae strain cocolonization in the nasopharynx. J. Clin. Microbiol. 47:1750–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hathaway LJ, Battig P, Mühlemann K. 2007. In vitro expression of the first capsule gene of Streptococcus pneumoniae, cpsA, is associated with serotype-specific colonization prevalence and invasiveness. Microbiology 153:2465–2471 [DOI] [PubMed] [Google Scholar]
- 38. Matthews BW. 1968. Solvent content of protein crystals. J. Mol. Biol. 33:491–497 [DOI] [PubMed] [Google Scholar]
- 39. Kabsch W. 2010. XDS. Acta Crystallogr. D Biol. Crystallogr. 66:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans P. 2006. Scaling and assessment of data quality. Acta Crystallogr. D. Biol. Crystallogr. 62:72–82 [DOI] [PubMed] [Google Scholar]
- 41. Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS. 2011. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J. Appl. Crystallogr. 40:658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davies IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66:486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen VB, Arendall WB, III, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. 2010. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schönbrunn E, Eschenburg S, Krekel F, Luger K, Amrhein N. 2000. Role of the loop containing residue 115 in the induced-fit mechanism of the bacterial cell wall biosynthetic enzyme MurA. Biochemistry 39:2164–2173 [DOI] [PubMed] [Google Scholar]
- 47. Meroueh SO, Roblin P, Golemi D, Maveyraud L, Vakulenko SB, Zhang Y, Samama JP, Mobashery S. 2002. Molecular dynamics at the root of expansion of function in the M69L inhibitor-resistant TEM β-lactamase from Escherichia coli. J. Am. Chem. Soc. 124:9422–9430 [DOI] [PubMed] [Google Scholar]
- 48. Hauser C, Aebi S, Mühlemann K. 2004. An internationally spread clone of Streptococcus pneumoniae evolves from low-level to higher-level penicillin resistance by uptake of penicillin-binding protein gene fragments from nonencapsulated pneumococci. Antimicrob. Agents Chemother. 48:3563–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hathaway LJ, Stutzmann Meier P, Battig P, Aebi S, Mühlemann K. 2004. A homologue of aliB is found in the capsule region of nonencapsulated Streptococcus pneumoniae. J. Bacteriol. 186:3721–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bättig P, Mühlemann K. 2007. Capsule genes of Streptococcus pneumoniae influence growth in vitro. FEMS Immunol. Med. Microbiol. 50:324–329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.