Abstract
Nitazoxanide and three halogeno-thiazolides, RM-4850, RM-4865, and RM-5038, were tested against Cryptosporidium parvum in experimentally infected immunosuppressed Mongolian gerbils. Daily 400-mg/kg doses of the four test drugs for 5 to 8 consecutive days produced similar reductions of oocyst shedding. Using early-infected gerbils, a shorter 4-day treatment with RM-5038 reduced oocyst shedding by 95%, compared to 47% for nitazoxanide (P = 0.02), suggesting that RM-5038 is more effective than nitazoxanide under the experimental conditions used.
TEXT
We have previously reported the in vitro activities of several thiazolides against Cryptosporidium parvum and Sarcocystis neurona, two apicomplexan protozoa (1, 2). Recent tests of RM-5038 showed the same level of activity as its active circulating metabolite RM-4848, which, with RM-4850 and RM-4865, had exhibited the highest level of in vitro activity against C. parvum development. These compounds deserved to be tested in vivo to confirm their anticryptosporidial activity.
The in vivo activities of nitazoxanide and three halogeno-thiazolides were studied in experimentally Cryptosporidium parvum-infected gerbils (Meriones unguiculatus). Animals weighing 30 to 40 g at the beginning of the study were individually housed in plastic cages equipped with a grill ceiling providing rodent food granules and water ad libitum. The cage was protected by another top wrapped with sterile paper in order to comply with level II contamination requirements. Animals were handled according to the technical and ethical regulations of the French Ministry of Agriculture. Gerbils were immunosuppressed by injection of dexamethasone (Qualimed, Puteaux, France) at 0.8 mg every 2 days and fed a low-protein diet (white bread) for at least 10 consecutive days before infection, and dexamethasone was administered until the end of the experiment. Prior to the start of treatment, each gerbil was inoculated by oral gavage with C. parvum oocysts. The test compounds were suspended in pure dimethyl sulfoxide (DMSO) and administered at a dose of 200 mg/kg of body weight twice daily by oral gavage in a constant volume. One to 3 days after the cessation of treatment, all of the animals were killed by deep sodium thiopental anesthesia. To assess C. parvum infection, the volume of the cecal and colonic contents collected from each sacrificed gerbil was suspended in a 10% (wt/vol) formalin solution and homogenized. The suspension was vortexed for 30 s and allowed to settle for an additional 30 s. Ten percent of the suspension was diluted in phosphate-buffered saline (PBS) and centrifuged at 1,800 × g for 30 min. After washing, the pellet was resuspended in PBS and half of the oocyst-containing pellet was incubated with an oocyst-specific monoclonal antibody conjugated with fluorescein isothiocyanate (Crypt-a-Glo; Waterborne, New Orleans, LA) in the dark at 37°C for 30 min. A CytoSpin was prepared for each specimen, and oocysts were counted by epifluorescence microscopy at a magnification of ×400. The number of oocysts in 1 ml of colonic content was used for comparisons between gerbils and to assess C. parvum oocyst shedding inhibition in treated gerbils. A total of four sets of experiments were performed in which the oocyst challenge dose and the onset and duration of treatment differed. The duration of treatment was driven in part by the availability of the test agents. In the first set of experiments, on day 0, each gerbil received 6 × 105 C. parvum oocysts by oral gavage and chronic cryptosporidiosis was allowed to develop for 21 days before treatment with the test drugs. On day 21 after oocyst ingestion, the animals were divided into three treatment groups. In the first experiment, one group was treated with nitazoxanide, one group was treated with RM-4865, and one group received DMSO as a positive control. Gerbils received treatment for 8 consecutive days and were killed 4 days after the cessation of treatment (i.e., day 32 postinfection). In the second set of experiments, gerbils were challenged with oocysts 6 days before the onset of a 5-day treatment course with RM-4865, RM-4850 (at a 200-mg/kg or a 400-mg/kg dose), or nitazoxanide and were killed 2 days after the cessation of treatment. In the third set of experiments, where RM-5038 was tested in early-infected gerbils, treatment was administered for 4 days and gerbils were killed 1 day after the cessation of treatment. In the fourth set of experiments, gerbils received treatment 24 h after a challenge with a larger dose of oocysts and were treated for 5 days. Data from treated and control gerbils were compared by using the Mann-Whitney U test. P values of <0.05 were considered statistically significant.
In a first experiment, nitazoxanide and RM-4865 at a daily dose of 400 mg/kg body weight for 8 days produced a statistically significant reduction of oocyst shedding in treated versus control infected gerbils (P < 0.05). RM-4865 was as effective as nitazoxanide, producing 89 and 96% reductions of oocyst excretion, respectively (P > 0.05). In a second experiment, RM-4850 and RM-4865 at a daily dose of 400 mg/kg produced statistically significant reductions of oocyst shedding versus that of infected controls (P = 0.019 and P = 0.03, respectively). Treatment with RM-4850 resulted in an oocyst shedding reduction that was comparable to that obtained with nitazoxanide at the same dose. In the third experiment, a daily 400-mg/kg RM-5038 dose completely suppressed C. parvum oocyst excretion in two out of five gerbils versus zero out of five nitazoxanide-treated animals (P < 0.05) (Table 1; see Fig. S1 in the supplemental material). With RM-5038, oocyst shedding at day 5 postinfection, i.e., 1 day after the cessation of treatment, reached a 95% mean reduction versus a 47% reduction with nitazoxanide. In the fourth set of experiments, treatment was initiated 12 h after infection with a large dose of oocysts (1 × 106 oocysts, three times as many as in the third experiment) and gerbils treated with RM-5038 for 5 days exhibited a 61% mean reduction of oocyst shedding versus that of controls 1 day after the cessation of treatment. These results indicate that RM-5038, a new derivative in early clinical trials in the United States, could potentially be more effective than nitazoxanide in the treatment of cryptosporidial diarrhea. The compound is a halogeno-thiazolide where the nitro group was replaced with a halogen (Table 2), which resulted in the elimination of the effect of the drug against anaerobic bacteria (3). The antibacterial activity of nitazoxanide against anaerobic bacteria (4), which make up most of the intestinal flora, is not appropriate since it could itself cause diarrhea. The fact that RM-5038 is not effective against anaerobic bacteria should theoretically improve the clinical tolerance of the drug when it is given for a prolonged period of time. However, it should be mentioned that some halogeno-thiazolides have been shown to induce apoptosis in some proliferative human cells, which could affect the overall safety of the new compound (5, 6). RM-5038 is in early clinical trials in the United States, and the present study supports clinical trials of the compound against cryptosporidial diarrhea. Finally, these experiments have validated the gerbil model of cryptosporidiosis even if the infection does not cause diarrhea and they are restricted to the drug's effect on oocyst shedding. It remains a reliable animal model that correlates with our efficient in vitro screening system and allowed us to test two families of effective compounds, the isoflavones (7, 8) and the thiazolides (1).
Table 1.
In vivo inhibitory activities of nitazoxanide and halogeno-thiazolides RM-4865, RM-4850, and RM-5038 against C. parvum in immunosuppressed infected Mongolian gerbils
| Expt and agent | No. of gerbils | No. of oocysts given/gerbil | Daily dose (mg/kg), no. of days (time of treatment onset postinfection) | No. of animals with no oocyst shedding/total (day postinfection) | Reduction (%) of mean oocyst sheddinga (day postinfection), P value vs control |
|---|---|---|---|---|---|
| 1 | |||||
| None | 5 | 6 × 105 | None | 0/5 | |
| RM-4865 | 5 | 6 × 105 | 400, 8 (21 days) | 1/5 (32) | 89 (32), <0.05 |
| Nitazoxanide | 5 | 6 × 105 | 400, 8 (21 days) | 0/5 (32) | 96 (32), <0.05 |
| 2 | |||||
| None | 7 | 5 × 105 | None | 0/5 | |
| Nitazoxanide | 6 | 5 × 105 | 200, 12 (6 days) | 0/6 (7) | 65 (7), 0.03 |
| RM-4850 | 5 | 5 × 105 | 200, 5 (6 days) | 0/4 (7) | 13 (7), NSb |
| RM-4850 | 8 | 5 × 105 | 400, 5 (6 days) | 0/8 (7) | 65 (7), 0.019 |
| RM-4865 | 4 | 5 × 105 | 400, 5 (6 days) | 0/4 (7) | 52 (7), 0.03 |
| 3 | |||||
| None | 5 | 3 × 105 | None | 0/5 | |
| Nitazoxanide | 5 | 3 × 105 | 400, 4 (32 h) | 0/5 (6) | 47 (6), NS |
| RM-5038 | 5 | 3 × 105 | 400, 4 (32 h) | 2/5 (6) | 95 (6), 0.01 |
| 4 | |||||
| None | 4 | 1 × 106 | None | 0/4 | |
| RM-5038 | 5 | 1 × 106 | 400, 5 (12 h) | 0/5 (7) | 61 (6.5), 0.02 |
Calculated as follows: [(mean oocyst count in treated animals)/(mean oocyst count in untreated animals)] × 100.
NS, no statistically significant difference.
Table 2.
Chemical structures of nitazoxanide and the halogeno-thiazolide agents tested in this study
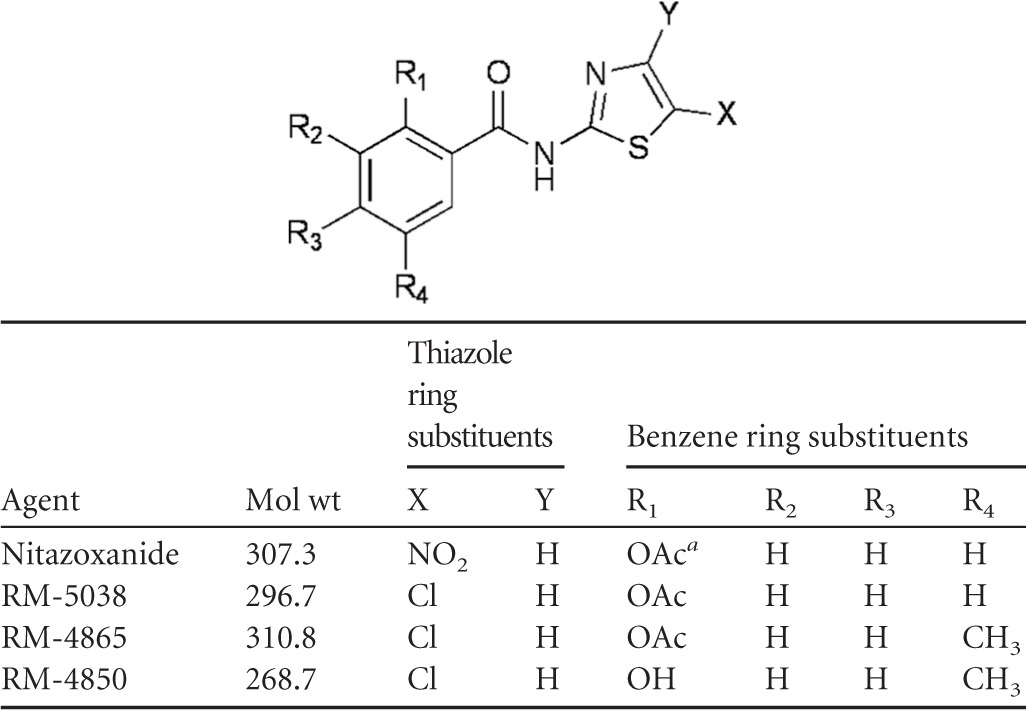
a OAc, acetate.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to R. Mancassola and M. Naciri, INRA, Nouzilly, France, for kindly providing C. parvum-infected calf feces. We are most grateful to A. V. Stachulski for supplying thiazolides.
Financial support was provided by Romark Laboratories, LC, Tampa, Florida, USA.
J. F. Rossignol owns equity interests in Romark Laboratories, LC, the pharmaceutical company that owns the patent for nitazoxanide.
Footnotes
Published ahead of print 11 March 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01538-12.
REFERENCES
- 1. Gargala G, Le Goff L, Ballet JJ, Favennec L, Stachulski AV, Rossignol JF. 2010. Evaluation of new thiazolide/thiadiazolide derivatives reveals nitro-group-independent efficacy against Cryptosporidium parvum. Antimicrob. Agents Chemother. 54:1315–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gargala G, Le Goff L, Ballet JJ, Favennec L, Stachulski AV, Rossignol JF. 2009. In vitro efficacy of nitro- and halogeno-thiazolide and thiadiazolide derivatives against Sarcocystis neurona. Vet. Parasitol. 162:230–235 [DOI] [PubMed] [Google Scholar]
- 3. Pankuch GA, Appelbaum PC. 2006. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob. Agents Chemother. 50:1112–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffman PS, Sisson G, Croxen MA, Welch K, Harman WD, Cremades N, Morash MG. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob. Agents Chemother. 51:868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sidler D, Brockmann A, Mueller J, Nachbur U, Corazza N, Renzulli P, Hemphill A, Brunner T. 2012. Thiazolide-induced apoptosis in colorectal cancer cells is mediated via the Jun kinase-Bim axis and reveal glutathione-S-transferase P1 as Achilles' heel. Oncogene 31:4095–4106 [DOI] [PubMed] [Google Scholar]
- 6. Müller J, Sidler D, Nachbur U, Wastling J, Brunner T, Hemphill A. 2008. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int. J. Cancer 123:1797–1806 [DOI] [PubMed] [Google Scholar]
- 7. Gargala G, Baishanbo A, Favennec L, François A, Ballet JJ, Rossignol JF. 2005. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob. Agents Chemother. 49:4628–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stachulski AV, Berry NG, Lilian Low AC, Moores SL, Row E, Warhurst DC, Adagu IS, Rossignol JF. 2006. Identification of isoflavone derivatives as effective anticryptosporidial agents in vitro and in vivo. J. Med. Chem. 49:1450–1454 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


