Abstract
Candida species other than Candida albicans are increasingly recognized as causes of biofilm-associated infections. This is a comprehensive study that compared the in vitro activities of all three echinocandins against biofilms formed by different common and infrequently identified Candida isolates. We determined the activities of anidulafungin (ANID), caspofungin (CAS), and micafungin (MFG) against planktonic cells and biofilms of bloodstream isolates of C. albicans (15 strains), Candida parapsilosis (6 strains), Candida lusitaniae (16 strains), Candida guilliermondii (5 strains), and Candida krusei (12 strains) by XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assay. Planktonic and biofilm MICs were defined as ≥50% fungal damage. Planktonic cells of all Candida species were susceptible to the three echinocandins, with MICs of ≤1 mg/liter. By comparison, differences in the MIC profiles of biofilms in response to echinocandins existed among the Candida species. Thus, C. lusitaniae and C. guilliermondii biofilms were highly recalcitrant to all echinocandins, with MICs of ≥32 mg/liter. In contrast, the MICs of all three echinocandins for C. albicans and C. krusei biofilms were relatively low (MICs ≤ 1 mg/liter). While echinocandins exhibited generally high MICs against C. parapsilosis biofilms, MFG exhibited the lowest MICs against these isolates (4 mg/liter). A paradoxical growth effect was observed with CAS concentrations ranging from 8 to 64 mg/liter against C. albicans and C. parapsilosis biofilms but not against C. krusei, C. lusitaniae, or C. guilliermondii. While non-albicans Candida planktonic cells were susceptible to all echinocandins, there were drug- and species-specific differences in susceptibility among biofilms of the various Candida species, with C. lusitaniae and C. guilliermondii exhibiting profiles of high MICs of the three echinocandins.
INTRODUCTION
Candida biofilms have been associated with many infections, including catheter-related fungemia, endocarditis, bone and joint infections, and others, with high morbidity and considerable mortality (1–3). In addition, oropharyngeal and vaginal in vivo models have demonstrated that mucosal Candida infections are due to the development of biofilms, a process controlled by morphogenetic and biofilm regulators (4, 5). Mature Candida biofilms consist of a complex 3-dimensional structure of fungal cells held together by pseudohyphae and a carbohydrate-rich extracellular matrix (6, 7).
While Candida albicans remains the predominant fungus responsible for bloodstream infections, in recent years there has been a trend of an increased incidence of candidemia caused by non-albicans Candida species. Overall, the most common non-albicans Candida sp. isolated from blood cultures is Candida parapsilosis; however, other Candida spp., including Candida krusei, Candida lusitaniae, and Candida guilliermondii, also cause candidemia (8–10). The biofilm-forming capacity of non-albicans Candida spp. has been implicated as a potential virulence factor in the development of candidemia for patients with vascular catheters, as the latter provide a suitable environment in which fungal organisms may grow and from which they may detach and colonize other body sites through the bloodstream (11, 12). In addition to vascular catheters, Candida spp. can cause difficult-to-treat infections in association with other inserted foreign bodies.
Echinocandins target (1→3)-β-d-glucan synthase, an enzyme absent in mammalian cells but essential for cell wall structural integrity and the function of many pathogenic fungi. Echinocandins are fungicidal for Candida planktonic cells (nonattached cells) and exhibit activity against mature biofilms of several Candida species (13–17). There have been several studies on the efficacy of echinocandins against Candida biofilms (18–21), the occurrence of the paradoxical effect observed with this class of antifungals (22, 23), and the role of echinocandin lock solutions in the management of candidiasis related to implanted devices (24); however, there are no comprehensive reports that have compared the in vitro activities of the three echinocandins against biofilms formed by different non-albicans Candida spp.
The aim of this study was to determine the antifungal activities of anidulafungin (ANID), caspofungin (CAS), and micafungin (MFG) against planktonic cells as well as biofilms formed by non-C. albicans Candida bloodstream isolates, such as C. parapsilosis, C. krusei, C. lusitaniae, and C. guilliermondii, in comparison to those of C. albicans.
(This study was presented in part at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 9 to 12 September 2012.)
MATERIALS AND METHODS
Clinical isolates and growth conditions.
A total of 54 bloodstream isolates of C. albicans and non-albicans Candida species recovered from pediatric and adult immunocompromised and critically ill patients were studied. Twenty-six isolates were obtained from the Laboratory of Infectious Diseases Collection, Aristotle University, Hippokration Hospital, and were isolated at the Hippokration Hospital of Thessaloniki between October 1996 and June 2007, and 28 were obtained from the UOA/HCPF929 Collection, University of Athens, and were isolated between January 2010 and January 2011. Of the non-albicans Candida species isolated, C. parapsilosis was one of the most frequently identified non-C. albicans Candida isolates, C. krusei was a less frequently identified but azole-resistant species, and C. lusitaniae and C. guilliermondii were uncommonly reported bloodstream isolates (25–27). Species identification of these isolates was performed using a Vitek II system or an API ID32C kit (both from bioMérieux, Marcy l'Etoile, France) according to the manufacturer's instructions. The clinical isolates from the Hippokration collection were also identified by the germ tube test in serum, whereas the isolates obtained from University of Athens were identified by sequencing of the internal transcribed spacer (ITS) 1 and 2 noncoding ribosomal regions and by sequencing of the 26S ribosomal DNA gene, variable region D1/D2 (28, 29). Stocks were maintained at −80°C in yeast-peptone-dextrose broth with 10% to 25% glycerol (Oxoid, Cambridge, United Kingdom) solution. The clinical isolates were subcultured after overnight incubation at 37°C on Sabouraud (Scharlau Chemie, S.A., Barcelona, Spain) agar plates containing 0.05 mg/ml chloramphenicol and 0.25 mg/ml gentamicin. Two to three colonies from each isolate were subsequently transferred to 20 ml of yeast nitrogen base (Difco Laboratories, Detroit, MI) medium supplemented with 2% glucose and incubated at 37°C overnight on a rocking table. The grown cultures were harvested by centrifugation at 2,000 rpm for 10 min, washed twice with 10 ml of phosphate-buffered saline (PBS) solution (0.02 M phosphate, 0.15 M NaCl; pH 7.2), and resuspended in RPMI 1640 medium (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) buffered to pH 7.2 with 3-(N-morpholino)propanesulfonic acid (Sigma-Aldrich Chemie GmbH). For all experiments, the organisms were used at a final concentration of 106/ml.
Biofilm formation.
Mature Candida biofilms were formed using 96-well microtiter polystyrene plates as previously described (30). Briefly, aliquots of 100 μl of yeast suspension were inoculated into each well and incubated for 48 h (C. albicans, C. lusitaniae, and C. guilliermondii) or 72 h (C. parapsilosis and C. krusei) at 37°C with shaking for the formation of comparable biofilms. Plates were then centrifuged at 4,000 rpm for 20 min and washed once with PBS to remove unattached cells, and 100 μl of fresh RPMI 1640 was subsequently added. Mature biofilm production was documented by staining the polysaccharide structure of the extracellular matrix of biofilms with 0.1% safranin for 5 min and measuring the optical absorbance at 492 nm with a microplate reader (ChroMate 4300; Awareness Technology, Inc., Palm City, FL) (31). The documented biofilm-producing strain C. albicans M61, obtained from an infected intravascular catheter and previously extensively studied (32), was used as a control of biofilm formation.
Antifungal agents.
The three echinocandins were obtained from their manufacturers: anidulafungin from Pfizer Inc. (New York, NY), caspofungin from Merck and Co. Inc. (Whitehouse Station, NJ), and micafungin from Astellas Pharma Inc. (Tokyo, Japan). Each antifungal was dissolved in sterile water to a final concentration of 3.3 mg/ml for ANID, 5 mg/ml for CAS, and 10 mg/ml for MFG and maintained as a stock solution at −35°C for up to 1 month. A portion from each antifungal stock was further diluted to 1,024 mg/liter in RPMI 1640 and used to prepare a series of 2-fold dilutions, as follows: ANID, 0.007 to 256 mg/liter; CAS, 0.03 to 256 mg/liter; MFG, 0.06 to 256 mg/liter.
Antifungal treatment and assessment of biofilm damage.
One hundred microliters of the above-indicated serially double-strength 2-fold-diluted concentrations of each drug was added to corresponding microtiter wells containing 100 μl mature biofilms or planktonic cells, and the plates were incubated at 37°C on a rocker table for an additional 24 h. For controls, biofilms or planktonic cells were incubated in the presence of 200 μl of RPMI 1640 without antifungals under otherwise identical conditions. The in vitro activity of each drug against biofilms or planktonic cells was assessed by using an XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] reduction assay as described previously with minor modifications (33). Briefly, 150 μl of XTT (0.25 g/liter; Sigma-Aldrich) containing coenzyme Q (40 mg/liter; Sigma-Aldrich) was added to microtiter plates after a washing step with PBS to remove antifungal agents or growth medium. The plates were then incubated for 30 to 50 min at 37°C, and the change in color, indicating the percent fungal damage effected by each antifungal, was measured in a microtiter plate reader at 450 nm with a reference wavelength of 690 nm. Antifungal activity was calculated according to the following formula: percent fungal damage = [1 − (X/C)] × 100, where X is the absorbance of experimental wells and C is the absorbance of control wells. The MICs for biofilms and planktonic cells were determined as the minimum antifungal concentration that caused ≥50% fungal damage compared to that for the untreated controls (17, 34).
Statistical analysis.
Each concentration of ANID, CAS, and MFG for every clinical isolate was tested in pentaplicate, and each untreated control was tested in 16 replicates per experiment. The average values of these replicates were used in the data analysis to determine the mean ± standard error (SE) for each condition. All isolates were retested at least twice in independent experiments. The documented biofilm-producing strain C. albicans M61, used as a control, was retested five times on separate days. Τhe differences in biofilm formation between M61 and each Candida species were examined by the Wilcoxon rank-sum test. The differences in fungal damage between planktonic cells and biofilms of the strains within each Candida species caused by ANID, CAS, and MFG and the differences in drug efficacy (MICs) for each echinocandin between planktonic cells and biofilms were assessed by the Wilcoxon matched-pairs test. Data were analyzed using Instat (version 3) biostatistics software (GraphPad Inc., San Diego CA). A two-tailed P value of <0.05 was considered significant.
RESULTS
Biofilm formation among Candida species.
All isolates used in this study were biofilm producers, but the degree of biofilm formation depended on the Candida species. In comparison to the biofilm production of the C. albicans M61 control strain, as measured by the mean optical density ± standard error of the mean (0.96 ± 0.02), C. albicans (0.95 ± 0.01), C. lusitaniae (0.94 ± 0.01), and C. guilliermondii (0.92 ± 0.02) isolates formed comparable biofilms. In contrast, biofilms of intermediate density compared to the density of the biofilms produced by the control strain were formed by C. parapsilosis (0.84 ± 0.02) and C. krusei (0.53 ± 0.01) (P < 0.01; data not shown).
Susceptibility of Candida planktonic cells and biofilms to echinocandins. (i) Planktonic cells.
The ANID and MFG MICs for C. albicans were ≤0.007 mg/liter and ≤0.06 mg/liter, respectively, whereas the MICs of these antifungals for both C. krusei and C. lusitaniae were 0.06 to 0.125 mg/liter and ≤0.06 mg/liter, respectively. The patterns of C. albicans, C. krusei, and C. lusitaniae planktonic cell susceptibility to ANID and MFG were similar, with MICs ranging from ≤0.007 to 0.125 mg/liter (Table 1). By comparison, the ANID and MFG MICs for C. parapsilosis were 0.03 to 0.125 mg/liter and 0.06 to 0.5 mg/liter, respectively, whereas for C. guilliermondii the MICs of these antifungals were 0.5 to 1 mg/liter and 0.06 to 0.125 mg/liter, respectively. Therefore, the corresponding mean MICs of ANID and MFG for C. parapsilosis and C. guilliermondii were somewhat higher and ranged from 0.06 to 1 mg/liter (Table 1). The MICs of CAS for planktonic cells of C. albicans and non-albicans Candida isolates were within similar ranges (Table 1; 0.25 to 0.5 mg/liter for C. albicans, 0.25 to 1 mg/liter for C. parapsilosis, 0.5 to 1 mg/liter for C. krusei and C. guilliermondii, and 0.25 to 1 mg/liter for C. lusitaniae).
Table 1.
MICs of echinocandins for planktonically grown cells and biofilms of different bloodstream Candida isolates determined by the XTT assay
| Species (n = 54) | Mean (range) MIC (mg/liter) |
|||||
|---|---|---|---|---|---|---|
| ANID |
CAS |
MFG |
||||
| Planktonic cells | Biofilms | Planktonic cells | Biofilms | Planktonic cells | Biofilms | |
| C. albicans (n = 15) | ≤0.007 | 0.06a (0.015–0.06) | 0.5 (0.25–0.5) | 0.5 (0.25–4) | ≤0.06 | 0.25a (0.06–2) |
| C. parapsilosis (n = 6) | 0.06 (0.03–0.125) | 32a,b (2–64) | 1 (0.25–1) | 64a,b (2–128) | 0.5 (0.06–0.5) | 4a,b (2–8) |
| C. krusei (n = 12) | 0.125 (0.06–0.125) | 0.125b (0.06–0.125) | 1 (0.5–1) | 1b (0.5–1) | ≤0.06 | 0.125a,b (0.06–0.25) |
| C. lusitaniae (n = 16) | 0.125 (0.03–0.125) | >256a | 1 (0.25–1) | 64a (16–64) | ≤0.06 | >256a |
| C. guilliermondii (n = 5) | 1 (0.5–1) | 32a (8–128) | 1 (0.5–1) | 64a (32–64) | 0.125 (0.06–0.125) | >256a |
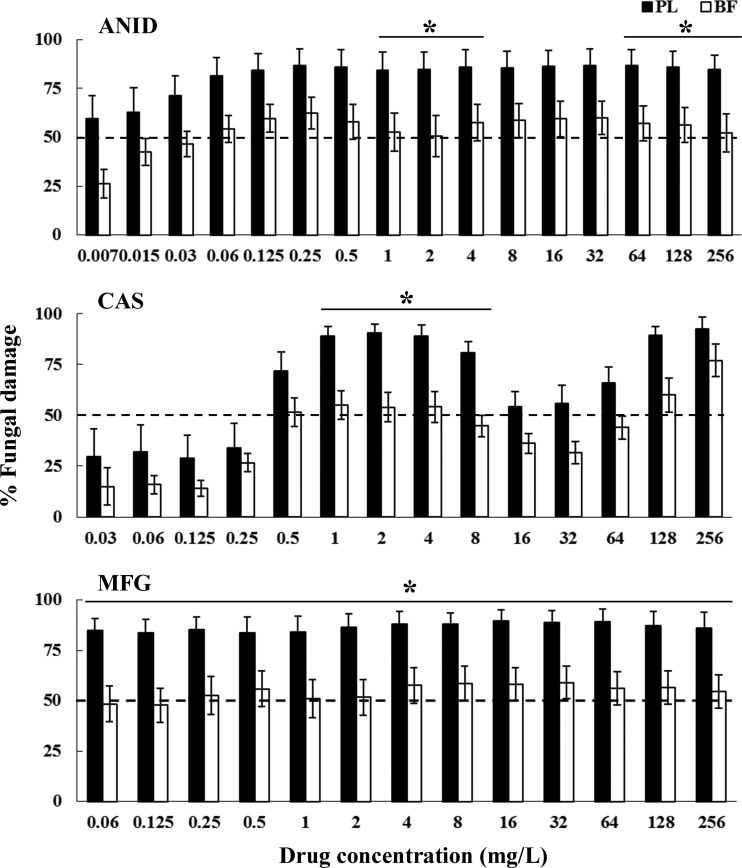
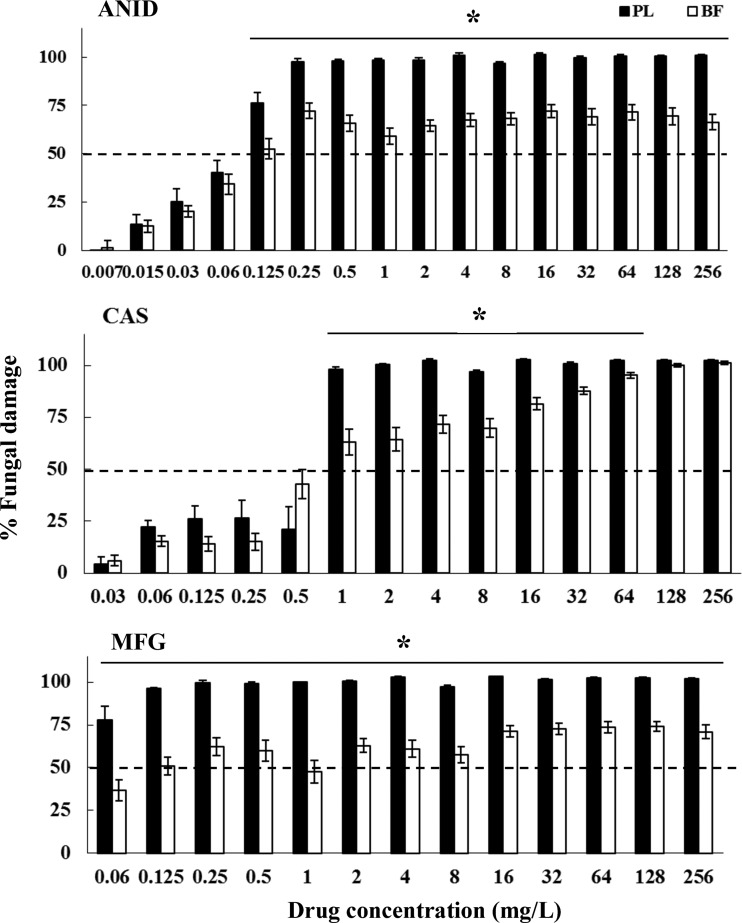
The three echinocandins demonstrated similar antifungal activities against planktonic cells of the 15 C. albicans isolates and those of the control M61 strain: ANID MIC, <0.007 versus 0.015 mg/liter; CAS MIC, 0.5 versus 0.25 mg/liter; and MFG MIC, ≤0.06 versus 0.06 mg/liter, respectively, while ANID and CAS exhibited >70% fungal damage against planktonic forms of all Candida isolates at ≥1 mg/liter (Fig. 1 to 5), MFG showed high antifungal efficacy at concentrations starting from ≥0.125 mg/liter against planktonic cells of C. albicans (Fig. 1; 84% ± 7%), C. krusei (Fig. 2; 96% ± 0.8%), C. lusitaniae (Fig. 4; 95% ± 1.8%), and C. guilliermondii (Fig. 5; 80% ± 5.3%). These results suggested that planktonically grown cells of Candida isolates share similar profiles of susceptibility to echinocandins.
Fig 1.
Fungal damage of planktonic cells (PL) and biofilms (BF) of C. albicans bloodstream isolates caused by different concentrations of ANID, CAS, and MFG. Fungal damage was assessed by XTT assay. Results are means ± SEs of the percent fungal damage of planktonic cells and biofilms of all isolates of a particular species and drug. Each of the 15 isolates was studied at least twice in independent experiments. Asterisks show significant differences between planktonic cells and the corresponding biofilms for the concentrations indicated by horizontal lines (P < 0.01). The discontinuous line denotes the MIC that caused ≥50% fungal damage compared to the untreated controls.
Fig 5.
Fungal damage of planktonic cells and biofilms of C. guilliermondii bloodstream isolates caused by different concentrations of ANID, CAS, and MFG. Fungal damage was assessed by the XTT assay. Results are means ± SEs of the percent fungal damage of planktonic cells and biofilms of all isolates of a particular species and drug. Each of 5 isolates was studied at least twice in independent experiments. Asterisks show significant differences between planktonic cells and the corresponding biofilms for the concentrations indicated by horizontal lines (P < 0.01). The discontinuous line denotes the MIC that caused ≥50% fungal damage compared to the untreated controls.
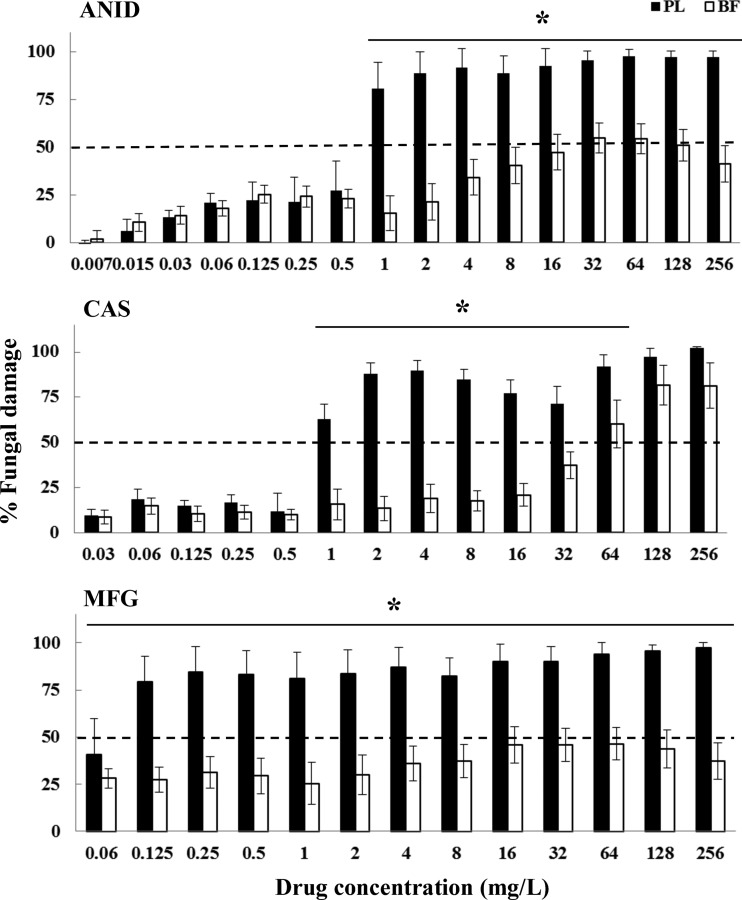
Fig 2.
Fungal damage of planktonic cells and biofilms of C. parapsilosis bloodstream isolates caused by different concentrations of ANID, CAS, and MFG. Fungal damage was assessed by the XTT assay. Results are means ± SEs of the percent fungal damage of planktonic cells and biofilms of all isolates of a particular species and drug. Each of the 6 isolates was studied at least twice in independent experiments. Asterisks show significant differences between planktonic cells and the corresponding biofilms for the concentrations indicated by horizontal lines (P < 0.01). The discontinuous line denotes the MIC that caused ≥50% fungal damage compared to the untreated controls.
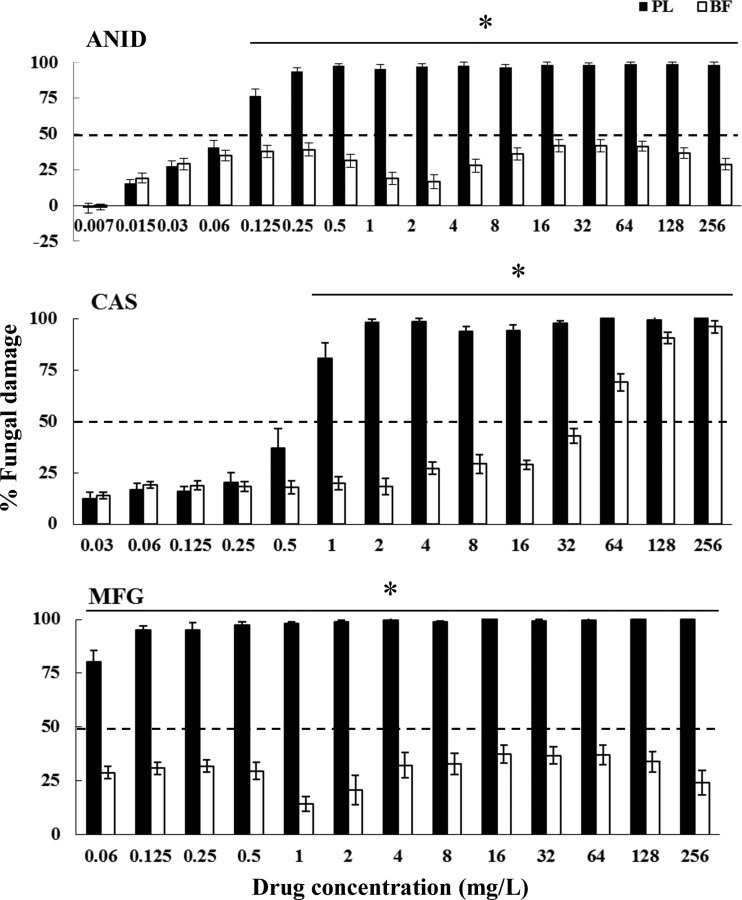
Fig 4.
Fungal damage of planktonic cells and biofilms of C. lusitaniae bloodstream isolates caused by different concentrations of ANID, CAS, and MFG. Fungal damage was assessed by the XTT assay. Results are means ± SEs of the percent fungal damage of planktonic cells and biofilms of all isolates of a particular species and drug. Each of 16 isolates was studied at least twice in independent experiments. Asterisks show significant differences between planktonic cells and the corresponding biofilms for the concentrations indicated by horizontal lines (P < 0.01). The discontinuous line denotes the MIC that caused ≥50% fungal damage compared to the untreated controls.
(ii) Biofilms.
Mature biofilms were more recalcitrant to the three echinocandins than their corresponding planktonic cells, especially those formed by C. parapsilosis, C. lusitaniae, and C. guilliermondii (Table 1; P < 0.01). All three echinocandins were active against C. albicans biofilms, with MICs of ≤0.5 mg/liter (Table 1). Significant echinocandin-specific differences in the antifungal activities of ANID, CAS, and MFG were observed against biofilms of isolates of the non-albicans Candida spp. While all three echinocandins were characterized by high MICs for C. parapsilosis (4 to 64 mg/liter), MFG had the lowest value among them (Table 1; 4 mg/liter; P < 0.01). The three echinocandins showed diminished activity against biofilms of C. krusei, with the lowest MIC characterizing ANID and MFG (Table 1; 0.125 mg/liter; P < 0.01). In contrast, increased activity of the three echinocandins was demonstrated against biofilms of C. lusitaniae and C. guilliermondii, with MICs of ≥32 to >256 mg/liter (Table 1).
In general, among Candida species, C. albicans and C. krusei biofilms exhibited the lowest MICs of all echinocandins. In contrast, biofilms of C. parapsilosis, C. lusitaniae, and C. guilliermondii were more refractory to the three antifungal agents (Table 1).
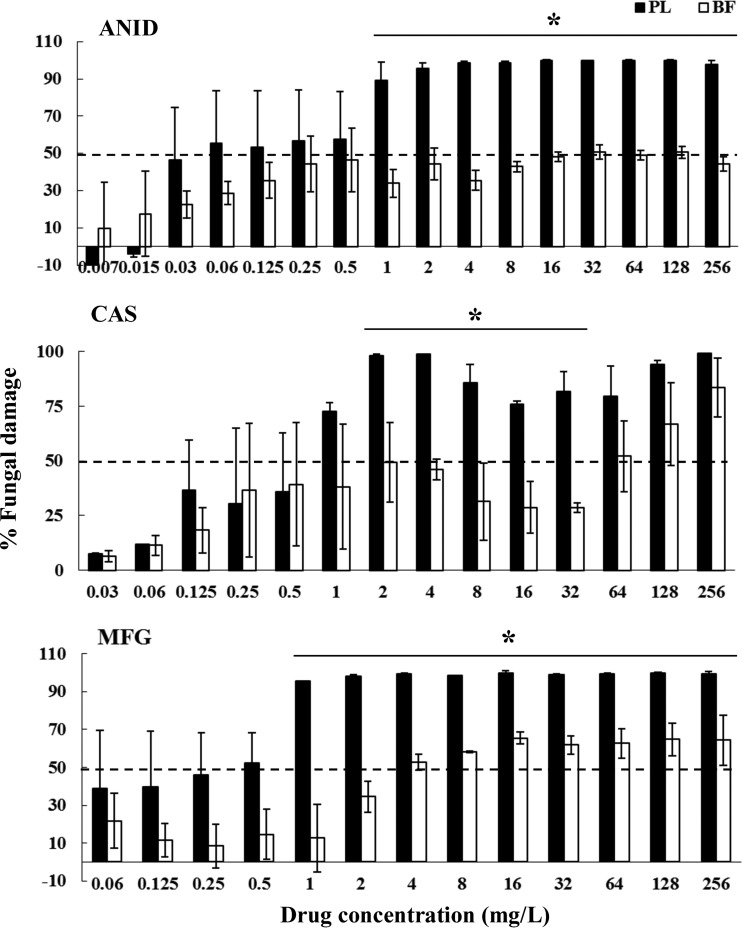
A paradoxical growth effect was observed with CAS but not with ANID and MFG, with concentrations ranging from 8 to 64 mg/liter against both planktonic cells and biofilms of C. albicans and C. parapsilosis but not against C. krusei, C. lusitaniae, or C. guilliermondii (Fig. 1 to 5). Of note, the fungal damage resulting from CAS against biofilms of the last three species was increased with increasing concentrations of CAS (for C. krusei, 43% to 100% over the range of 0.5 to 256 mg/liter [Fig. 3]; for C. lusitaniae, 29% to 96% over the range of 16 to 256 mg/liter [Fig. 4]; for C. guilliermondii, 21% to 81% over the range of 16 to 256 mg/liter [Fig. 5]). Nevertheless, except for the antibiofilm activity of CAS at ≥64 mg/liter for C. krusei and at ≥128 mg/liter for C. lusitaniae, greater than 90% fungal damage was not observed to be caused by CAS or the other echinocandins for any of the other organisms of this study.
Fig 3.
Fungal damage of planktonic cells and biofilms of C. krusei bloodstream isolates caused by different concentrations of ANID, CAS, and MFG. Fungal damage was assessed by the XTT assay. Results are means ± SEs of the percent fungal damage of planktonic cells and biofilms of all isolates of a particular species and drug. Each of 12 isolates was studied at least twice in independent experiments. Asterisks show significant differences between planktonic cells and the corresponding biofilms for the concentrations indicated by horizontal lines (P < 0.01). The discontinuous line denotes the MIC that caused ≥50% fungal damage compared to the untreated controls.
DISCUSSION
Due to their recalcitrant nature toward many antifungal agents and host immune mechanisms (35–37), fungal biofilm-associated infections have been linked to increased morbidity and mortality rates. C. albicans as well as non-albicans Candida species have been shown to form biofilms and contribute to the pathogenesis of Candida infections (38–40). The activities of individual echinocandins against Candida biofilms have been studied. However, in this study, all three echinocandins were compared for their activities against Candida biofilms caused by C. albicans and non-albicans Candida species.
We determined the MIC profiles of ANID, CAS, and MFG for planktonic cells and mature biofilms of C. albicans, C. parapsilosis, C. krusei, C. lusitaniae, and C. guilliermondii. Comparative analyses demonstrated the presence of species-specific and drug-specific differences in the MICs of the three echinocandins for biofilms. We found that while planktonic cells of Candida species were susceptible to the three echinocandins with mean MICs of ≤1 mg/liter, there were differences in the MICs of echinocandins for biofilms among Candida species. For the first time, to our knowledge, C. lusitaniae and C. guilliermondii biofilms were reported to have high MICs (≥32 mg/liter) to all three echinocandins. By comparison, C. albicans and C. krusei biofilms had relatively low MICs (≤1 mg/liter) to the three echinocandins. The C. parapsilosis biofilm was generally recalcitrant to echinocandins, but MFG exhibited the lowest MIC among them (4 mg/liter). In contrast, the MICs of CAS were either equal to or up to 6 dilution steps higher than those exhibited by ANID and MFG for planktonic cells of C. albicans and non-albicans Candida species. With the exception of CAS for planktonic cells of C. krusei, the resistance breakpoint MICs for the organisms examined did not exceed the Clinical and Laboratory Standards Institute (CLSI)-approved resistance breakpoint MICs of ≥1 mg/liter for C. albicans and of ≥8 mg/liter for C. parapsilosis, C. lusitaniae, and C. guilliermondii (41). Finally, in this study, a paradoxical growth of both planktonic cells and biofilms of C. albicans and C. parapsilosis was demonstrated with CAS concentrations ranging from 8 to 64 mg/liter; this paradoxical effect of CAS was not observed with C. krusei, C. lusitaniae, or C. guilliermondii.
The Candida isolates under investigation are all biofilm producers, but biofilm formation was species dependent, being the highest in C. lusitaniae, intermediate in C. albicans and C. guilliermondii, and the lowest in C. parapsilosis and C. krusei. These findings are consistent with previous observations (11, 18, 42, 43).
As demonstrated in this study and reported by others, the MIC profiles of Candida planktonic cells were within the susceptibility range of echinocandins (16, 18, 21, 44–46). However, in our study, significant differences between planktonic cell MICs and biofilm MICs as well as among biofilm MICs of echinocandins were observed for the C. albicans and non-albicans species.
The MICs of ANID, CAS, and MFG for C. parapsilosis biofilms in our study were 32, 64, and 4 mg/liter, respectively. In the literature, C. parapsilosis biofilms have shown variable MIC profiles for the three echinocandins. In a study by Fiori et al. (21), C. parapsilosis biofilms exhibited low MICs to ANID, ranging from 0.5 to 2 mg/liter, differing by at least 16-fold from the corresponding MIC value exhibited in our study (32 mg/liter). However, in another study investigating long-term trials of continuous-flow cultivation, C. parapsilosis biofilms demonstrated relatively high MIC profiles (MIC > 32 mg/liter) after 5 days of ANID exposure (47). Likewise, in our study and others, C. parapsilosis biofilms exhibited variable CAS and MFG MIC profiles, with MICs ranging from 1 to >16 mg/liter (19, 45, 48, 49). Several explanations for such variability have been offered. Cocuaud et al. have demonstrated that species-specific differences between C. albicans and C. parapsilosis could depend on the Candida species, biofilm age, and concentration of CAS used (50). Seidler et al. (31) have shown that profiles of high MICs could depend on the ability of Candida species to form biofilms on different substrates. In particular, the study demonstrated that MFG displays diminished activity (>16 mg/liter) against C. parapsilosis mature biofilms grown on polystyrene substrate but increased activity (<0.5 mg/liter) against biofilms grown on central venous catheter sections (31). Finally, interstudy differences could be due to either differences in the biofilm models used or the distinct clumping and irregular appearance of C. parapsilosis blastospores embedded within a shallow biofilm matrix that results in an easily dissociated biofilm structure (51, 52).
The potential for biofilm formation of C. lusitaniae was recently demonstrated by Pannanusorn et al. (53). They observed that C. tropicalis and C. lusitaniae had the greatest propensity for forming biofilms among all Candida spp. studied (53). Recent studies of C. lusitaniae indicate that virulence, cell wall formation, and antifungal tolerance are calcineurin dependent (54). Indeed, among echinocandin-resistant isolates, FK506, a calcineurin inhibitor, demonstrated synergistic activity with caspofungin. These observations may carry important implications for treatment and prevention of biofilm formation by C. lusitaniae.
Our study demonstrated that Candida guilliermondii also has the ability to be highly recalcitrant to the activity of echinocandins in biofilms. Walker and colleagues recently demonstrated that C. guilliermondii, C. krusei, C. albicans, and C. parapsilosis upregulate the expression of chitin via calcineurin and protein kinase C in response to subinhibitory concentrations of caspofungin (55). Such subinhibitory concentrations may be present in biofilms, allowing the increased expression of chitin and increased resistance to the effect of echinocandins. Further contributing to understanding the possible mechanisms of refractoriness of C. guilliermondii in biofilms is the study of Barchiesi et al. (56), who found that caspofungin was fungistatic against this organism only for the first 4 to 6 h of the assay, despite being present at a concentration 8 times above the MIC. By the end of the 24-h assay, caspofungin-treated C. guilliermondii displayed growth comparable to that of untreated controls. This limited activity contrasted with the more durable inhibitory effect of caspofungin against C. albicans and C. parapsilosis throughout the 24-h assays (56). The refractoriness of C. guilliermondii to echinocandins in biofilms may also be related to this limited activity.
Concerning the paradoxical growth phenomenon observed with CAS in our study and by others (20, 57, 58), the cells have been found to exhibit large amounts of chitin molecules in their cell wall and biofilm matrix, compensating for (1→3)-β-d-glucan depletion and in this way resisting killing by the antifungal (59, 60). A recent study evaluated the paradoxical growth effect using time-kill experiments and demonstrated that such a phenomenon is eliminated when Candida species are briefly exposed to CAS; additional data presented by the same investigators indicate that the paradoxical growth effect is unlikely to have a major impact on the clinical course of patients treated with echinocandins for candidemia (61). However, as suggested by Moriyama et al., the role of the paradoxical effect may be more relevant in the setting of biofilm (60).
In conclusion, our results suggest that echinocandins are active against planktonic cells of all Candida species examined and of biofilms of C. albicans and C. krusei bloodstream isolates. Furthermore, the recalcitrant nature of C. lusitaniae and C. guilliermondii biofilms has been demonstrated for the first time. Although the two non-albicans Candida species were resistant to the antifungal activity of echinocandins, CAS at high concentrations (≥64 mg/liter) was able to damage 70% to 96% of their biofilm structure, therefore being a good candidate for antifungal lock therapy for the containment of fungal growth due to C. lusitaniae and C. guilliermondii biofilms. The three echinocandins exhibited relatively high MICs for the C. parapsilosis biofilm; however, MFG exhibited somewhat greater antifungal activity. Laboratory animal studies are needed to extend these in vitro findings to therapeutic interventions for treatment of non-albicans Candida species with echinocandins for systemic and antifungal lock therapy. Comparative analysis of the 3-dimensional structure and biofilm-related gene expression between biofilms of C. albicans or C. parapsilosis and those of C. lusitaniae and C. guilliermondii may lead to the discovery of new targets for potent antifungal therapy against Candida biofilms.
ACKNOWLEDGMENTS
This work was supported by institutional funds. A.K. received support from Amphiaraion Hellenic Institute, Hellenic Society of Infectious Diseases, European Society for Pediatric Infectious Diseases, and European Commission Framework Program 7. P.P. was supported by the European Commission Erasmus Program 2011.
E.R. has received research grant support from Pfizer, Gilead, Enzon, Schering, and Wyeth, has served as a consultant to Schering, Gilead, Astellas Gilead, Cephalon, and Pfizer, and has been on the speakers' bureaus of Wyeth, Schering, Merck, Aventis, and Astellas. T.J.W. is a scholar of the Henry Schueler Foundation and a scholar of pediatric infectious diseases of the Sharpe Family Foundation; he receives support from the SOS Kids Foundation, as well as research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Novartis, Merck, ContraFect, and Pfizer, and has served as a consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. The remaining authors have no relevant disclosures.
Footnotes
Published ahead of print 25 March 2013
REFERENCES
- 1. Cauda R. 2009. Candidaemia in patients with an inserted medical device. Drugs 69(Suppl 1):33–38 [DOI] [PubMed] [Google Scholar]
- 2. Rueping MJ, Vehreschild JJ, Cornely OA. 2009. Invasive candidiasis and candidemia: from current opinions to future perspectives. Expert Opin. Invest. Drugs 18:735–748 [DOI] [PubMed] [Google Scholar]
- 3. Cuellar-Cruz M, Lopez-Romero E, Villagomez-Castro JC, Ruiz-Baca E. 2012. Candida species: new insights into biofilm formation. Future Microbiol. 7:755–771 [DOI] [PubMed] [Google Scholar]
- 4. Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. 2009. Characterization of mucosal Candida albicans biofilms. PLoS One 4:e7967 doi:10.1371/journal.pone.0007967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr, Noverr MC. 2010. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156:3635–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blankenship JR, Mitchell AP. 2006. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9:588–594 [DOI] [PubMed] [Google Scholar]
- 7. Chandra J, Mukherjee PK, Ghannoum MA. 2008. In vitro growth and analysis of Candida biofilms. Nat. Protoc. 3:1909–1924 [DOI] [PubMed] [Google Scholar]
- 8. Steinbach WJ, Roilides E, Berman D, Hoffman JA, Groll AH, Bin-Hussain I, Palazzi DL, Castagnola E, Halasa N, Velegraki A, Dvorak CC, Charkabarti A, Sung L, Danziger-Isakov L, Lachenauer C, Arrieta A, Knapp K, Abzug MJ, Ziebold C, Lehrnbecher T, Klingspor L, Warris A, Leckerman K, Martling T, Walsh TJ, Benjamin DK, Jr, Zaoutis TE. 2012. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr. Infect. Dis. J. 31:1252–1257 [DOI] [PubMed] [Google Scholar]
- 9. Trofa D, Gacser A, Nosanchuk JD. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 21:606–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mikulska M, Bassetti M, Ratto S, Viscoli C. 2011. Invasive candidiasis in non-hematological patients. Mediterr. J. Hematol. Infect. Dis. 3:e2011007 doi:10.4084/MJHID.2011.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, Suh SP, Ryang DW. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 40:1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chow JK, Golan Y, Ruthazer R, Karchmer AW, Carmeli Y, Lichtenberg D, Chawla V, Young J, Hadley S. 2008. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin. Infect. Dis. 46:1206–1213 [DOI] [PubMed] [Google Scholar]
- 13. Kuhn DM, Mikherjee PK, Clark TA, Pujol C, Chandra J, Hajjeh RA, Warnock DW, Soil DR, Ghannoum MA. 2004. Candida parapsilosis characterization in an outbreak setting. Emerg. Infect. Dis. 10:1074–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukherjee PK, Chandra J. 2004. Candida biofilm resistance. Drug Resist. Updat. 7:301–309 [DOI] [PubMed] [Google Scholar]
- 15. Sucher AJ, Chahine EB, Balcer HE. 2009. Echinocandins: the newest class of antifungals. Ann. Pharmacother. 43:1647–1657 [DOI] [PubMed] [Google Scholar]
- 16. Perlin DS. 2011. Current perspectives on echinocandin class drugs. Future Microbiol. 6:441–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katragkou A, Chatzimoschou A, Simitsopoulou M, Dalakiouridou M, Diza-Mataftsi E, Tsantali C, Roilides E. 2008. Differential activities of newer antifungal agents against Candida albicans and Candida parapsilosis biofilms. Antimicrob. Agents Chemother. 52:357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi HW, Shin JH, Jung SI, Park KH, Cho D, Kee SJ, Shin MG, Suh SP, Ryang DW. 2007. Species-specific differences in the susceptibilities of biofilms formed by Candida bloodstream isolates to echinocandin antifungals. Antimicrob. Agents Chemother. 51:1520–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferreira JA, Carr JH, Starling CE, de Resende MA, Donlan RM. 2009. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob. Agents Chemother. 53:4377–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiori B, Posteraro B, Torelli R, Tumbarello M, Perlin DS, Fadda G, Sanguinetti M. 2011. In vitro activities of anidulafungin and other antifungal agents against biofilms formed by clinical isolates of different Candida and Aspergillus species. Antimicrob. Agents Chemother. 55:3031–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miceli MH, Bernardo SM, Lee SA. 2009. In vitro analysis of the occurrence of a paradoxical effect with different echinocandins and Candida albicans biofilms. Int. J. Antimicrob. Agents 34:500–502 [DOI] [PubMed] [Google Scholar]
- 23. Ku TS, Bernardo SM, Lee SA. 2011. In vitro assessment of the antifungal and paradoxical activity of different echinocandins against Candida tropicalis biofilms. J. Med. Microbiol. 60:1708–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cateau E, Berjeaud JM, Imbert C. 2011. Possible role of azole and echinocandin lock solutions in the control of Candida biofilms associated with silicone. Int. J. Antimicrob. Agents 37:380–384 [DOI] [PubMed] [Google Scholar]
- 25. Bassetti M, Taramasso L, Nicco E, Molinari MP, Mussap M, Viscoli C. 2011. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One 6:e24198 doi:10.1371/journal.pone.0024198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das I, Nightingale P, Patel M, Jumaa P. 2011. Epidemiology, clinical characteristics, and outcome of candidemia: experience in a tertiary referral center in the UK. Int. J. Infect. Dis. 15:e759–e763 [DOI] [PubMed] [Google Scholar]
- 27. Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M. 2012. The changing epidemiology of healthcare-associated candidemia over three decades. Diagn. Microbiol. Infect. Dis. 73:45–48 [DOI] [PubMed] [Google Scholar]
- 28. Velegraki A, Kambouris ME, Skiniotis G, Savala M, Mitroussia-Ziouva A, Legakis NJ. 1999. Identification of medically significant fungal genera by polymerase chain reaction followed by restriction enzyme analysis. FEMS Immunol. Med. Microbiol. 23:303–312 [DOI] [PubMed] [Google Scholar]
- 29. Mannarelli BM, Kurtzman CP. 1998. Rapid identification of Candida albicans and other human pathogenic yeasts by using short oligonucleotides in a PCR. J. Clin. Microbiol. 36:1634–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Jr, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3:1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seidler M, Salvenmoser S, Muller FM. 2006. In vitro effects of micafungin against Candida biofilms on polystyrene and central venous catheter sections. Int. J. Antimicrob. Agents 28:568–573 [DOI] [PubMed] [Google Scholar]
- 32. Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatzimoschou A, Katragkou A, Simitsopoulou M, Antachopoulos C, Georgiadou E, Walsh TJ, Roilides E. 2011. Activities of triazole-echinocandin combinations against Candida species in biofilms and as planktonic cells. Antimicrob. Agents Chemother. 55:1968–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tournu H, Van Dijck P. 2012. Candida biofilms and the host: models and new concepts for eradication. Int. J. Microbiol. 2012:845352 doi:10.1155/2012/845352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katragkou A, Simitsopoulou M, Chatzimoschou A, Georgiadou E, Walsh TJ, Roilides E. 2011. Effects of interferon-gamma and granulocyte colony-stimulating factor on antifungal activity of human polymorphonuclear neutrophils against Candida albicans grown as biofilms or planktonic cells. Cytokine 55:330–334 [DOI] [PubMed] [Google Scholar]
- 38. Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, de Gaetano Donati K, La Sorda M, Spanu T, Fadda G, Cauda R, Sanguinetti M. 2007. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. 2009. Our current understanding of fungal biofilms. Crit. Rev. Microbiol. 35:340–355 [DOI] [PubMed] [Google Scholar]
- 40. Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. 2009. Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med. Mycol. 47:681–689 [DOI] [PubMed] [Google Scholar]
- 41. Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164–176 [DOI] [PubMed] [Google Scholar]
- 42. Hasan F, Xess I, Wang X, Jain N, Fries BC. 2009. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 11:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Estivill D, Arias A, Torres-Lana A, Carrillo-Munoz AJ, Arevalo MP. 2011. Biofilm formation by five species of Candida on three clinical materials. J. Microbiol. Methods 86:238–242 [DOI] [PubMed] [Google Scholar]
- 44. Axner-Elings M, Botero-Kleiven S, Jensen RH, Arendrup MC. 2011. Echinocandin susceptibility testing of Candida isolates collected during a 1-year period in Sweden. J. Clin. Microbiol. 49:2516–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jacobson MJ, Steckelberg KE, Piper KE, Steckelberg JM, Patel R. 2009. In vitro activity of micafungin against planktonic and sessile Candida albicans isolates. Antimicrob. Agents Chemother. 53:2638–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ostrosky-Zeichner L, Rex JH, Pappas PG, Hamill RJ, Larsen RA, Horowitz HW, Powderly WG, Hyslop N, Kauffman CA, Cleary J, Mangino JE, Lee J. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bernhardt H, Knoke M, Bernhardt J. 2011. Efficacy of anidulafungin against biofilms of different Candida species in long-term trials of continuous flow cultivation. Mycoses 54:e821–e827 [DOI] [PubMed] [Google Scholar]
- 48. Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, Graybill JR, Lopez-Ribot JL. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cateau E, Rodier MH, Imbert C. 2008. In vitro efficacies of caspofungin or micafungin catheter lock solutions on Candida albicans biofilm growth. J. Antimicrob. Chemother. 62:153–155 [DOI] [PubMed] [Google Scholar]
- 50. Cocuaud C, Rodier MH, Daniault G, Imbert C. 2005. Anti-metabolic activity of caspofungin against Candida albicans and Candida parapsilosis biofilms. J. Antimicrob. Chemother. 56:507–512 [DOI] [PubMed] [Google Scholar]
- 51. Song JW, Shin JH, Shint DH, Jung SI, Cho D, Kee SJ, Shin MG, Suh SP, Ryang DW. 2005. Differences in biofilm production by three genotypes of Candida parapsilosis from clinical sources. Med. Mycol. 43:657–661 [DOI] [PubMed] [Google Scholar]
- 52. Lattif AA, Mukherjee PK, Chandra J, Swindell K, Lockhart SR, Diekema DJ, Pfaller MA, Ghannoum MA. 2010. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. Int. J. Med. Microbiol. 300:265–270 [DOI] [PubMed] [Google Scholar]
- 53. Pannanusorn S, Fernandez V, Romling U. 1 November 2012. Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses. [Epub ahead of print.] doi:10.1111/myc.12014 [DOI] [PubMed] [Google Scholar]
- 54. Zhang J, Silao FG, Bigol UG, Bungay AA, Nicolas MG, Heitman J, Chen YL. 2012. Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS One 7:e44192 doi:10.1371/journal.pone.0044192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walker LA, Gow NA, Munro CA. 2013. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob. Agents Chemother. 57:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barchiesi F, Spreghini E, Tomassetti S, Della Vittoria A, Arzeni D, Manso E, Scalise G. 2006. Effects of caspofungin against Candida guilliermondii and Candida parapsilosis. Antimicrob. Agents Chemother. 50:2719–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Melo AS, Colombo AL, Arthington-Skaggs BA. 2007. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 51:3081–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bizerra FC, Melo AS, Katchburian E, Freymuller E, Straus AH, Takahashi HK, Colombo AL. 2011. Changes in cell wall synthesis and ultrastructure during paradoxical growth effect of caspofungin on four different Candida species. Antimicrob. Agents Chemother. 55:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moriyama B, Henning SA, Penzak SR, Walsh TJ. 2012. The postantifungal and paradoxical effects of echinocandins against Candida spp. Future Microbiol. 7:565–569 [DOI] [PubMed] [Google Scholar]
- 61. Shields RK, Nguyen MH, Du C, Press E, Cheng S, Clancy CJ. 2011. Paradoxical effect of caspofungin against Candida bloodstream isolates is mediated by multiple pathways but eliminated in human serum. Antimicrob. Agents Chemother. 55:2641–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]