Abstract
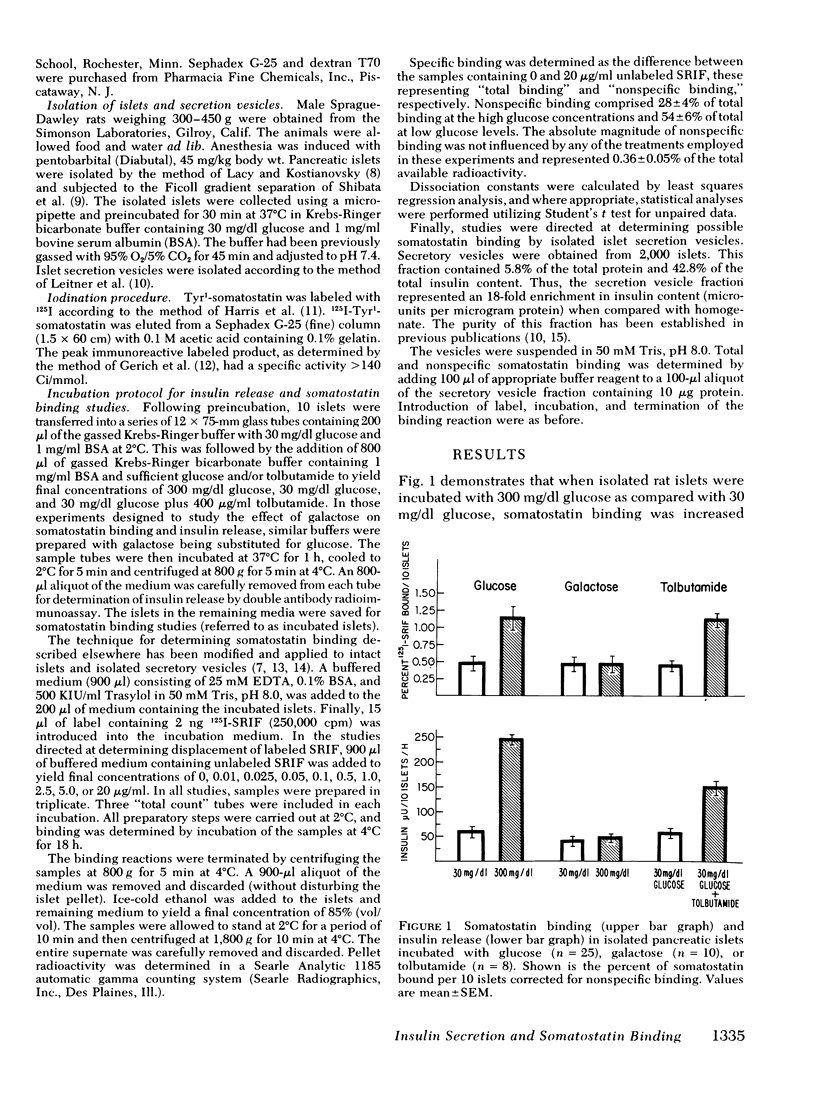
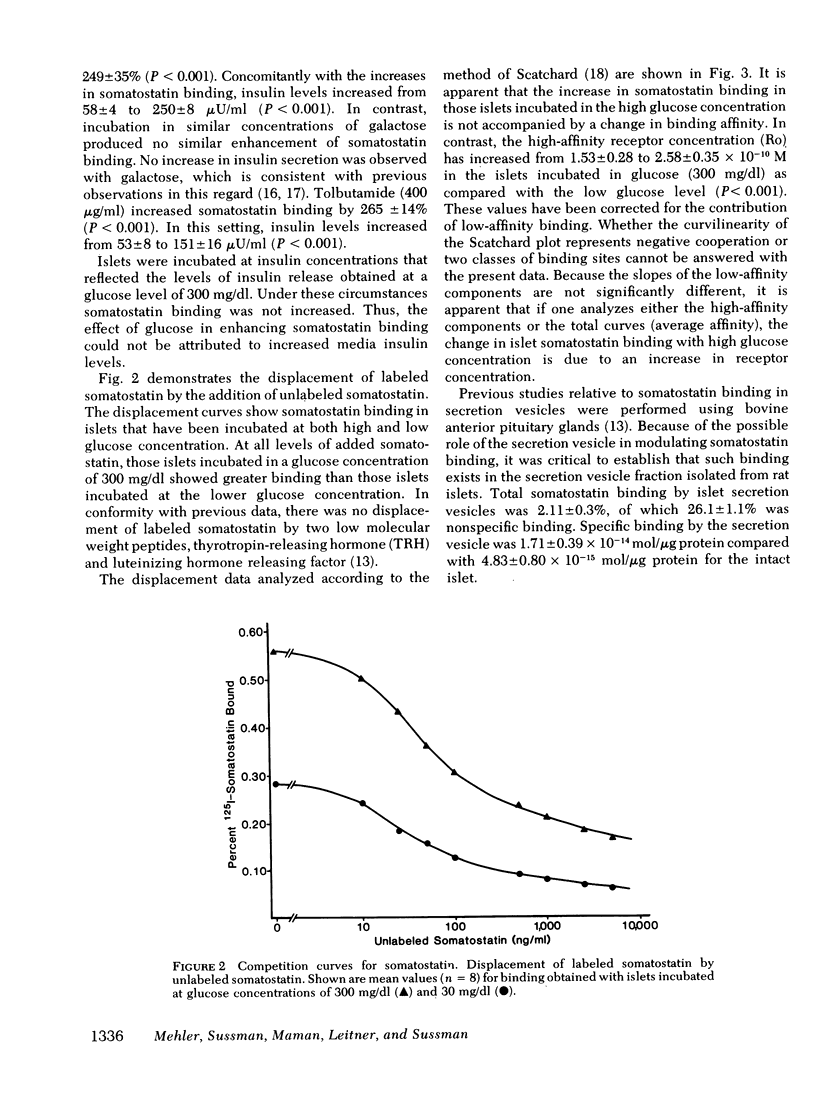
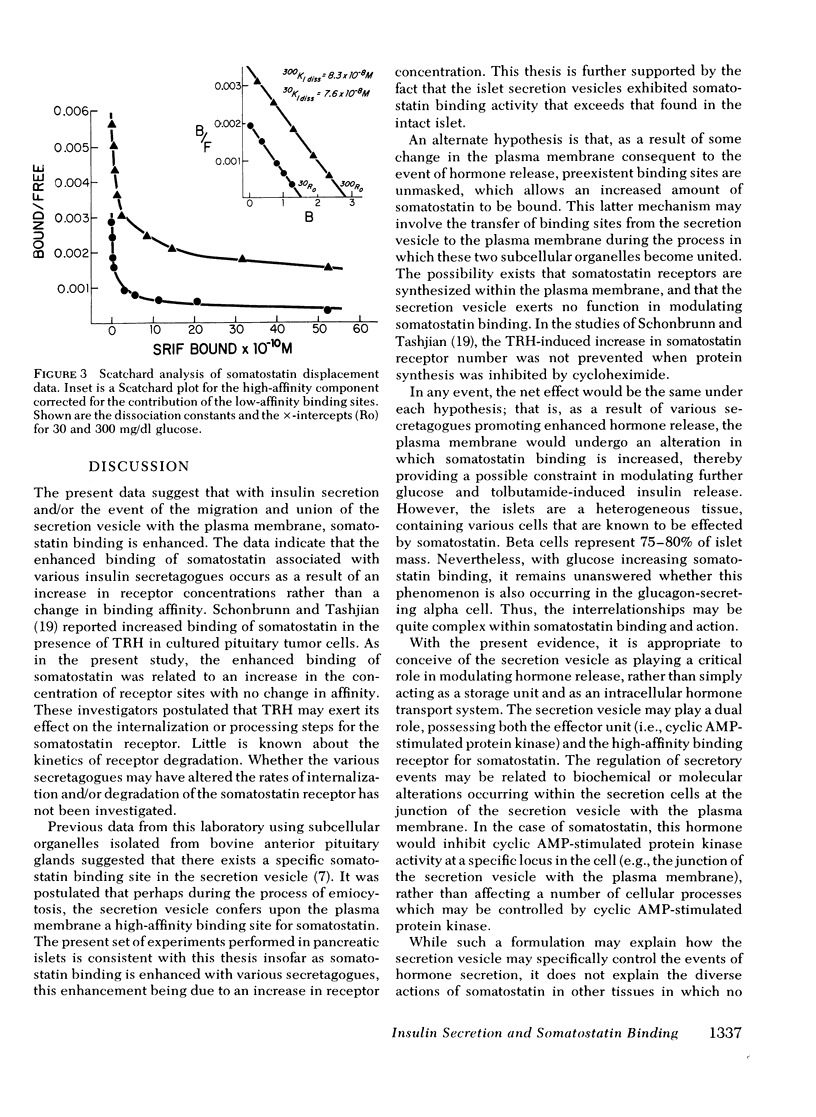
To study the possible role of the secretion vesicle inligant-receptor interaction, somatostatin binding was measured in islets in the presence of various substances known to promote secretion vesicle migration and fusion with the plasma membrane and insulin release. Rat islets were incubated with glucose, 30 and 300 mg/dl, for 60 min. After inculation, somatostatin binding was measured. In islets preincubated with glucose, 300 mg/dl, somatostatin binding was increased 250% when compared with glucose, 30 mg/dl (P < 0.001). Concomitant with enhanced somatostatin binding, insulin secretion was increased. Galactose, 300 mg/dl, did not stimulate insulin release, and somatostatin binding was unchanged from control levels. The increase in somatostatin binding with glucose was accounted for by a 186% increase in receptor concentration with no change in receptor affinity. Tolbutamide increased somatostatin binding by more than twofold, accompanied by a similar increase in insulin release. Secretion vesicles isolated from the islet exhibited somatostatin binding. We conclude that, first, somatostatin binding is increased concomitantly with the migration and fusion of the secretion vesicle with the plasma membrane and/or the release of insulin; second, enhanced somatostatin binding occurs as a consequence of an increased receptor concentration; and third, augmented somatostatin binding occurring with hormone release may provide a critical constraint in the regulation of secretory events.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- Gerich J., Greene K., Hara M., Rizza R., Patton G. Radioimmunoassay of somatostatin and its application in the study of pancreatic somatostatin secretion in vitro. J Lab Clin Med. 1979 Jun;93(6):1009–1017. [PubMed] [Google Scholar]
- Harris V., Conlon J. M., Srikant C. B., McCorkle K., Schusdziarra V., Ipp E., Unger R. H. Measurements of somatostatin-like immunoreactivity in plasma. Clin Chim Acta. 1978 Jul 15;87(2):275–283. doi: 10.1016/0009-8981(78)90348-0. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Condensing vacuole conversion and zymogen granule discharge in pancreatic exocrine cells: metabolic studies. J Cell Biol. 1971 Mar;48(3):503–522. doi: 10.1083/jcb.48.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F., Lemaire S., Poirier G., Pelletier G., Boucher R. Adenohypophyseal secretory granules. I. Their phosphorylation and association with protein kinase. J Biol Chem. 1971 Dec 10;246(23):7311–7317. [PubMed] [Google Scholar]
- Lacy P. E. Endocrine secretory mechanisms. A review. Am J Pathol. 1975 Apr;79(1):170–188. [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Leitner J. W., Rifkin R. M., Maman A., Sussman K. E. Somatostatin binding to pituitary plasma membranes. Biochem Biophys Res Commun. 1979 Apr 13;87(3):919–927. doi: 10.1016/0006-291x(79)92045-x. [DOI] [PubMed] [Google Scholar]
- Leitner J. W., Sussman K. E., Vatter A. E., Schneider F. H. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975 Mar;96(3):662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Schonbrunn A., Tashjian A. H., Jr Modulation of somatostatin receptors by thyrotropin-releasing hormone in a clonal pituitary cell strain. J Biol Chem. 1980 Jan 10;255(1):190–198. [PubMed] [Google Scholar]
- Schonbrunn A., Tashjian H., Jr Characterization of functional receptors for somatostatin in rat pituitary cells in culture. J Biol Chem. 1978 Sep 25;253(18):6473–6483. [PubMed] [Google Scholar]
- Shibata A., Ludvigsen C. W., Jr, Naber S. P., McDaniel M. L., Lacy P. E. Standardization fo a digestion-filtration method for isolation of pancreatic islets. Diabetes. 1976 Aug;25(8):667–672. doi: 10.2337/diab.25.8.667. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Leitner J. W. Conversion of ATP into other adenine nucleotides within isolated islet secretory vesicles. Effect of cyclic AMP on phosphorus translocation. Endocrinology. 1977 Sep;101(3):694–701. doi: 10.1210/endo-101-3-694. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Leitner J. W. Cyclic AMP stimulated protein kinase activity within the secretory vesicle fraction of rat islets. Biochem Biophys Res Commun. 1977 Nov 21;79(2):429–437. doi: 10.1016/0006-291x(77)90176-0. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Leitner J. W., Rifkin R. M. Somatostatin: selective inhibition of cyclic AMP stimulated protein kinase. Trans Assoc Am Physicians. 1978;91:129–143. [PubMed] [Google Scholar]
- Sussman K. E., Vaughan G. D., Timmer R. F. An in vitro method for studying insulin secretion in the perfused isolated rat pancreas. Metabolism. 1966 May;15(5):466–476. doi: 10.1016/0026-0495(66)90089-8. [DOI] [PubMed] [Google Scholar]


