Key Points
Recombinant HPA-1a antibody B2G1Δnab protects platelets from destruction by anti–HPA-1a in the circulation of HPA-1a1b human volunteers.
B2G1Δnab is a potential therapeutic agent for antenatal treatment of fetomaternal alloimmune thrombocytopenia due to HPA-1a antibodies.
Abstract
Fetomaternal alloimmune thrombocytopenia, caused by the maternal generation of antibodies against fetal human platelet antigen-1a (HPA-1a), can result in intracranial hemorrhage and intrauterine death. We have developed a therapeutic human recombinant high-affinity HPA-1a antibody (B2G1Δnab) that competes for binding to the HPA-1a epitope but carries a modified constant region that does not bind to Fcγ receptors. In vitro studies with a range of clinical anti–HPA-1a sera have shown that B2G1Δnab blocks monocyte chemiluminescence by >75%. In this first-in-man study, we demonstrate that HPA-1a1b autologous platelets (matching fetal phenotype) sensitized with B2G1Δnab have the same intravascular survival as unsensitized platelets (190 hours), while platelets sensitized with a destructive immunoglobulin G1 version of the antibody (B2G1) are cleared from the circulation in 2 hours. Mimicking the situation in fetuses receiving B2G1Δnab as therapy, we show that platelets sensitized with a combination of B2G1 (representing destructive HPA-1a antibody) and B2G1Δnab survive 3 times as long in circulation compared with platelets sensitized with B2G1 alone. This confirms the therapeutic potential of B2G1Δnab. The efficient clearance of platelets sensitized with B2G1 also opens up the opportunity to carry out studies of prophylaxis to prevent alloimmunization in HPA-1a–negative mothers.
Introduction
Fetomaternal alloimmune thrombocytopenia (FMAIT), caused by alloimmunization of pregnant women against human platelet antigens (HPAs), is the commonest cause of severe neonatal thrombocytopenia, with a reported incidence of 1 in 1000 live births.1-4 The antigen HPA-1a is implicated in 75% of cases.5-8 Severe fetal thrombocytopenia occurs in a quarter of HPA-1a alloimmunized pregnancies and the most severe complication, fetal intracranial hemorrhage (ICH), occurs in 10% to 20% of these latter cases.9-11 Treatment in the neonatal period is based on early recognition of the condition, and transfusion of antigen-negative platelets.12,13 Antenatal treatment is somewhat controversial.14 Many authors recommend the use of immunomodulatory therapy to the mother with IV immunoglobulin (IVIg) possibly in combination with steroids.8,15,16 These treatments are expensive, limited by access to IVIg, and not without side effects, and therefore some authors recommend the use of a stratified treatment approach based on the severity of previously affected pregnancies (the only clear predictor of disease severity).16-18 Although the rate of fetal ICH in pregnancies undergoing immunomodulatory treatment appears low, it is clear that this is not accompanied by a consistent rise in platelet count in the fetus.19,20 It may be that IVIg somehow lessens the risk of bleeding even in the absence of a rise in platelet count but it is also possible that the reduction of ICH comes with the increased care provided to the pregnant woman. This hypothesis is supported by screening studies showing reduction in fetal/neonatal morbidity through prior identification of HPA-1a alloimmunization and increased antenatal/neonatal care.4 The use of intrauterine transfusion of antigen-negative platelets for antenatal treatment of fetal thrombocytopenia is limited by the significant risk of fetal loss associated with the procedure15,21-23 and is now seen as a second-choice rescue therapy option by many clinicians.
It has been shown that the binding site for polyclonal HPA-1a antibodies is limited to a finite number of epitopes on the β3 integrin, with leucine-33 being a critical residue.24 We hypothesized that it would therefore be possible to generate an HPA-1a–specific therapeutic IgG antibody of sufficient affinity to block maternal antibodies to the HPA-1a epitope. Modifications would be made to the constant region to render the antibody nondestructive but preserve its half-life and transport across the placenta via the FcRn receptor, thereby removing the need for risky intrauterine procedures. In essence, women who are alloimmunized and at high risk of FMAIT could be treated by regular IV injections of a recombinant antibody that would cross the placenta and compete with maternal HPA-1a antibodies in binding to the fetal platelets. Sufficient protection of the platelets would raise the fetal platelet count to a level that would prevent serious in utero and perinatal bleeding events.
A human single-chain variable domain antibody fragment of nanomolar affinity (Kd = 6 × 10−8 M) for HPA-1a was generated from the maternal B cells of an FMAIT case by phage display.25 The recombinant human immunoglobulin G1 (IgG1) antibody (B2G1) derived from this fragment was shown to be sufficiently specific for HPA-1a to permit its use as a routine phenotyping reagent.26 Crucially, in in vitro studies, we showed that B2G1 was of sufficient affinity to block binding of maternal polyclonal HPA-1a antibodies from 18 cases of FMAIT to fetal HPA-1a1b platelets by 70% to 95%.27 Importantly, we also demonstrated that binding of B2G1 to HPA-1a1b platelets did not affect their functionality in a range of in vitro assays.28
To generate the nondestructive constant region while preserving the half-life and transplacental transport capabilities of a human IgG1 antibody, residues from human IgG2 and IgG4 were substituted into regions of the IgG1 Constant region of the Immunoglobulin Heavy Chain domain 2 (CH2) involved in binding to human FcγRI-III.29 In vitro studies have shown that the monocyte chemiluminescence (MCL) response to HPA-1a1b platelets sensitized with this antibody, B2G1Δnab, is only 15% of that observed with B2G1. Crucially, in competition assays, B2G1Δnab reduced MCL response to platelets sensitized with 18 different maternal sera containing HPA-1a antibodies by >75%.
In a final step of prehuman studies, we used a chimeric mouse model expressing the human HPA-1a epitope on their platelets to confirm that platelet destruction of HPA-1a–positive platelets by human polyclonal anti–HPA-1a could be prevented by the use of this recombinant antibody.27
The study reported here describes first-in-man human studies with B2G1Δnab to answer 2 critical questions ahead of potential clinical studies. First, does the reduced in vitro MCL response observed with B2G1Δnab-sensitized platelets translate into increased in vivo platelet survival in the circulation of healthy volunteers, confirming the potential protective effect of B2G1Δnab on platelets in patients? Second, does the reduced MCL response observed in vitro in competition studies with B2G1Δnab and clinical anti–HPA-1as translate into a potentially beneficial gain in platelet survival in vivo? As patient-derived polyclonal anti–HPA-1a sera cannot be used for platelet survival studies in human volunteers, we measured intravascular survival of platelets sensitized with different ratios of B2G1 (as an exemplar of destructive anti–HPA-1a) and B2G1Δnab.
Materials and methods
Study design
Radiolabeling platelet survival studies were performed in HPA-1a1b healthy blood donors, each with 2 aliquots of autologous platelets. The first phase investigated whether binding of B2G1Δnab to platelets affected platelet survival in vivo. Here, 1 platelet aliquot was sensitized ex vivo with either B2G1 or B2G1Δnab and the other was left unsensitized. This was followed by radiolabeling of each aliquot with either 111In or 51Cr in a crossover design prior to re-injection into the volunteer. Regular sampling post–re-injection and γ counting of each sample allowed calculation of intravascular survival of each platelet population separately.
The second phase of the study was designed to ascertain whether platelets sensitized with a combination of destructive IgG1 anti–HPA-1a (in this case, B2G1) and B2G1Δnab show a gain of survival when compared with platelets sensitized with B2G1 alone. The mixtures of antibodies used were 75% B2G1Δnab/25% B2G1 and 90% B2G1Δnab/10% B2G1, which have been shown to lead to a 65% and 80% reduction in in vitro MCL response, respectively.27 For reference, B2G1Δnab reduced MCL by over 75% when competing with 18 different clinical polyclonal anti–HPA-1a sera.27
Recruitment of HPA-1a1b volunteers
Eighteen healthy HPA-1a1b subjects were recruited from the genotyped donor panel at the NHS Blood and Transplant Cambridge Centre, with written informed consent and permission from the Local Research Ethics Committee, the Administration of Radioactive Substances Advisory Committee, and the Medicines and Healthcare Devices Regulatory Agency. Volunteers who had had a splenectomy or used medications which affect platelet function were excluded. A follow-up medical check and repeat blood sampling were performed 4 to 6 weeks after the study.
Production of clinical-grade HPA-1a antibodies
The structure and generation of B2G125,26 and B2G1Δnab29 have been described.
Clinical-grade antibodies were produced under European Union good manufacturing practice conditions30 in the NHSBT’s Clinical Biotechnology Centre (Bristol, United Kingdom) and tested in accordance with the European Union guidelines for the production and quality control of monoclonal antibodies31 (see supplemental Materials for details, available on the Blood website).
Platelet sensitization, radiolabeling, and blood sampling
Platelet sensitization and radiolabeling was carried out, adapting the procedure of the Biomedical Excellence for Safer Transfusion (BEST) collaborative.32 In short, on the day of study, 100 mL of blood was collected into 2 sterile plastic syringes each containing 9 mL of sterile acid citrate dextrose solution A (ACDA; Ipswich Hospital Pharmacy) alongside a 7-mL blood sample into an EDTA tube for determination of elution. The 2 aliquots were centrifuged at 200g for 15 minutes to produce platelet-rich plasma to which 15% v/v ACD was added before centrifuging 15 minutes at 2000g. Platelet-poor plasma (PPP) was removed, centrifuged at 2000g for 15 minutes, and stored for later use. The 2 harvested platelet pellets were each resuspended into 2 mL of ACDA-saline (1 part ACDA, 7 parts 0.9% saline; Ipswich Hospital Pharmacy). Each was sensitized for 20 minutes at room temperature by addition of 4 mL of antibody or left unsensitized (4 mL of sterile phosphate-buffered saline [PBS]). Antibody samples were 0.2 mg/mL B2G1, B2G1Δnab, a mixture of 90% B2G1Δnab/10% B2G1, or a mixture of 75% B2G1Δnab/25% B2G1. Each platelet aliquot was spun at 2000g for 15 minutes and the platelets were resuspended into 2 mL of ACDA-saline. Platelet radiolabeling with 111In oxine and Na251Cr2O4 was carried out as published,32 each pellet resuspended in 8 mL of the reserved PPP, and samples taken for standard preparation.32 Each platelet aliquot was re-infused into the volunteer under clinical supervision with vital signs recorded at regular intervals for the next 5 hours. Blood samples (9 mL) were taken in EDTA pre-infusion and at the following time points post-infusion: 5, 10, 15, 20, 30, 60 minutes, then 1.5, 2, 2.5, 3, 4, and 5 hours then on day 1, 2, 3, 6, 7, and 10 thereafter. Each sample, including the elution control, was aliquoted and centrifuged into whole blood, cellular, and plasma fractions and read on the automatic scintillation counter (Compugamma 1282; LKB/Wallac).32
Measurement of platelet recovery and survival
Measured counts were fully adjusted for background, cross-talk, radionucleide decay, volume, elution, and non–platelet-bound residual activity at day 10; expected time (0) count was determined using the calculated blood volume for each volunteer and standard as described.32 The platelet recovery for each time point was calculated by the following equation: recovery (time t) = fully adjusted count (time t)/expected time (0) count. The recommended way to assess platelet survival relies on the multiple hit calculation model, which fits a theoretical survival curve to the measurements of platelet recovery at the various time points. The COST program32 was used to infer platelet survival and recovery at time 0 from the fitted curve and assess whether the data “fits” the theoretical curve, that is, whether recovery and survival can be reliably calculated from the data provided.
The recommendation is that the first time point used for curve fitting should be taken after equilibrium is reached and in particular after the effect of splenic trapping has reached a plateau (usually >20 hours after re-injection).33,34 For samples with slow platelet destruction, which showed a plateau during the first 24 hours, time point 24 hours was used as the first point for the curve fit. For samples where rapid destruction was observed without a plateau phase, time point 5 minutes was used.
Flow cytometric analysis
Flow cytometry was used to confirm that donor platelets were HPA-1a–positive and equally sensitized with either form of recombinant anti–HPA-1a. Briefly, aliquots of platelets were washed with phosphate-buffered saline containing 10 mmol/L EDTA and 0.25% bovine serum albumin (PBS/EDTA/BSA). After incubation with fluorescein isothiocyanate (FITC)–labeled anti–HPA-1a (Bristol Institute For Transfusion Sciences) for HPA-1a typing or FITC anti-human IgG (DAKO), the platelets were washed with PBS/BSA/EDTA and analyzed by flow cytometry (EPICS XL-MCL; Beckman Coulter). In addition, to ascertain that ex vivo manipulation was not introducing a platelet activation bias in the final re-injectate, P-selectin surface expression levels on the platelets at baseline in whole blood and at the time of re-injection were measured following incubation with either FITC anti-CD62P (Beckman Coulter) or IgG1 isotype control (Beckman Coulter). Data from 5000 platelets were acquired using logarithmic settings for forward and side scatter, and the median fluorescence for the FITC signal recorded. Finally, tracking of the sensitized platelets in the volunteers’ circulation was carried out with FITC anti-human IgG as described at the beginning of this paragraph at various time points after re-injection. Data from 3 × 106 platelets were acquired and antibody sensitized platelets were identified from a plot of logarithmic forward scatter against logarithmic FITC signal.
Results
Volunteer details
Thirteen male and 5 female HPA-1a1b heterozygous volunteers were recruited and given a unique participant number (UPN). The volunteers’ baseline platelet count, HPA-1a surface expression levels, antibodies, and radiolabel combinations are described in Table 1. In 9 volunteers, paired platelet survival data compared either B2G1Δnab- (n = 7) or B2G1-sensitized (n = 2) platelets with unsensitized platelets. In a further group of 9 volunteers, paired platelet survival data compared platelets sensitized with a combination of 75% B2G1Δnab/25% B2G1 (n = 3) or 90% B2G1Δnab/10% B2G1 (n = 6) with either unsensitized (n = 3) or with B2G1-sensitized platelets (n = 6), respectively. Although all volunteers were confirmed as HPA-1a1b by genotyping, volunteer 18 had much higher HPA-1a surface antigen expression levels. Results acquired with this volunteer were treated separately for analysis purposes (see section “Intravascular survival of B2G1δnab-sensitised platelets is the same as that of unsensitized platelets”). Levels of platelet-bound human antibodies after sensitization were similar (except for volunteer 18), attesting to comparable levels of sensitization between volunteers and between antibodies. Of note, P-selectin surface expression levels on the platelets within the re-injectate measured in 8 of the 18 volunteers (5 B2G1-, 3 B2G1Δnab-, and 3 90% B2G1Δnab/10% B2G1-sensitized and 5 unsensitized samples) showed no statistically significant difference between volunteers or any of the antibody combinations/unsensitized platelets. No adverse events were recorded during the study.
Table 1.
Volunteer details, radiolabeling, and sensitization combinations and individual platelet recovery and survival data using the COST program
| UPN | Platelet count | HPA-1a expression levels, MFI | Radiolabel and antibody combination | Five-minute recovery, % | COST data | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unsensitized | B2G1Δnab | Unsensitized | B2G1Δnab | Unsensitized | B2G1Δnab | |||||
| Recovery, % | Survival, h | Recovery, % | Survival, h | |||||||
| 1 | 172 | 16.3 | Cr51 | 111In | 66 | 94 | 48 | 83 | 73 | 98 |
| 2 | 270 | 15.8 | 111In | Cr51 | 72 | 94 | 59 | 191 | 71 | 177 |
| 3 | 412 | 15.6 | Cr51 | 111In | 47 | 36 | 36 | 151 | 30 | 283 |
| 4 | 260 | 18.2 | Cr51 | 111In | 54 | 68 | 54 | 214 | 65 | 238 |
| 5 | 270 | No data | 111In | Cr51 | 51 | 42 | 51 | 254 | 33 | 215 |
| 6 | 299 | 17.1 | Cr51 | 111In | 38 | 61 | 32 | 212 | 57 | 216 |
| 7 | 324 | 16.9 | 111In | Cr51 | 69 | 77 | 61 | 212 | 65 | 243 |
|
Unsensitized |
B2G1 |
|||||||||
|
Unsensitized |
B2G1 |
Unsensitized |
B2G1 |
Recovery, % |
Survival, h |
Recovery, % |
Survival, min |
|||
| 8 | 249 | 19.2 | 111In | Cr51 | 93 | 53 | 62 | 198 | 38 | 14 |
| 9 | 311 | 14.3 | Cr51 | 111In | 57 | 63 | 42 | 223 | 39 | 18 |
|
Unsensitized |
B2G1Δnab75* |
|||||||||
|
Unsensitized |
B2G1Δnab75 |
Unsensitized |
B2G1Δnab75 |
Recovery, % |
Survival, h |
Recovery, % |
Survival, min |
|||
| 10 | 190 | No data | Cr51 | 111In | 70 | 82 | 58 | 152 | 56 | 48 |
| 11 | 257 | 17.7 | 111In | Cr51 | 70 | 75 | 60 | 219 | 43 | 40 |
| 12 | 230 | 18.2 | 111In | Cr51 | 60 | 50 | 54 | 162 | 59 | 45 |
|
B2G1 |
B2G1Δnab90* |
|||||||||
|
B2G1 |
B2G1Δnab90 |
B2G1 |
B2G1Δnab90 |
Recovery, % |
Survival, min |
Recovery, % |
Survival, min |
|||
| 13 | 234 | 14.7 | 111In | Cr51 | 95 | 83 | 70 | 28 | 50 | 45 |
| 14 | 238 | 15.2 | Cr51 | 111In | 51 | 77 | 27 | 14 | 30 | 50 |
| 15 | 175 | 14.0 | 111In | Cr51 | 65 | 60 | 63 | 15 | 44 | 77 |
| 16 | 239 | 18.0 | 111In | Cr51 | 60 | 79 | 65 | 18 | 86 | 62 |
| 17 | 198 | 16.6 | 111In | Cr51 | 78 | 79 | 36 | 17 | 61 | 56 |
| 18 | 276 | 26.3† | Cr51 | 111In | 57 | 69 | 81 | 19 | 96 | 25 |
The COST program is a mathematical tool that fits a theoretical survival curve to the measurements of platelet recovery at the various time points32 and infers platelet survival and recovery at time 0 from the fitted curve using the multiple hit calculation model. The first time point used for the curve fitting should be taken after equilibrium.33,34 For unsensitized and B2G1Δnab-sensitized platelets which showed a plateau phase during the first 24 hours (see Figure 1), the 24-hour time point was used as the first point for the curve fit. For B2G1-sensitized platelets and platelets sensitized with a combination of 75% B2G1Δnab/25% B2G1 and 90% B2G1Δnab/10% B2G1B2G1Δnab 90%/ B2G1 10% (*B2G1Δnab75 and -90, respectively) where rapid destruction was observed without a plateau phase (see Figure 1), the 5-minute time point was used (see “Results”).
UPN 18 had a much higher HPA-1a expression level (MFI 26.3 vs mean ± SD MFI of 16.5 ± 1.6 for the rest of the volunteers), and platelet survival data were very different from other volunteers (see supplemental Figure 1). Data from this volunteer were therefore excluded from subsequent analysis.
Platelet recovery at 5 minutes is comparable regardless of the sensitizing antibody and radiolabel used
Initial analysis of the data showed variable levels of platelet recovery at 5 minutes for each volunteer compared with the calculated dose injected, but this initial percentage of recovery was comparable within the same volunteer regardless of the antibody combination and the radiolabel used (P > .2, paired t test) (see Table 1). Five-minute recoveries were 62% ± 14%, 67% ± 23%, 74% ± 9%, 69% ± 17%, and 66% ± 15% (mean ± SD) for unsensitized, B2G1Δnab-, 90% B2G1Δnab/10% B2G1-, 75% B2G1Δnab/25% B2G1- and B2G1-sensitized platelets, respectively.
Intravascular survival of B2G1Δnab-sensitized platelets is the same as that of unsensitized platelets
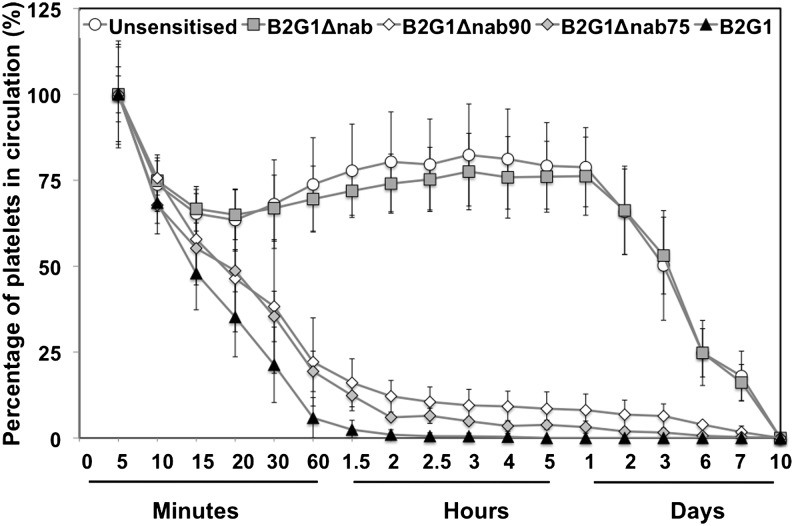
Figure 1 shows the percentage of platelets remaining in circulation comparing unsensitized platelets (n = 12) with platelets sensitized with either B2G1Δnab (n = 7), B2G1 (n = 8), or a combination of 75% B2G1Δnab/25% B2G1 (n = 3) or 90% B2G1Δnab/10% B2G1 (n = 5). The data acquired from all volunteers were normalized so that platelet recovery at 5 minutes was taken as 100% and data were pooled for each of the antibody combinations. Two patterns emerged: on the one hand, rapid exponential destruction for platelets sensitized with B2G1, 90% B2G1Δnab/10% B2G1, and 75% B2G1Δnab/25% B2G1. On the other hand, unsensitized and B2G1Δnab-sensitized platelets showed parallel kinetics with a first phase of rapid clearance down to 68% ± 13% and 67% ± 10% (mean ± SD), respectively, in the first 30 minutes, followed by a rebound to reach a plateau at 2 hours (79% ± 11% and 76% ± 11%, respectively) that was maintained up to the 24-hour point before a steady disappearance over the next 10 days. For unsensitized and B2G1Δnab-sensitized platelets, survival and recovery at time 0 were therefore inferred using the multiple hits method with the 24-hour time point as the first time point of the decay kinetic curve (see “Materials and methods”). The calculated recovery at time point 0 was 55% ± 12% (mean ± SD) for the unsensitized platelets and 56% ± 17% for the B2G1Δnab-sensitized platelets. Platelet survival was 189 ± 45 hours and 196 ± 50 hours, respectively. These differences did not reach statistical significance (P > .25, t test, Table 2). When data were considered for the volunteers in whom a direct comparison between unsensitized and B2G1Δnab-sensitized platelets was performed (n = 7, volunteers 1-7), recoveries were 49% ± 11% and 56% ± 17% (P > .1, paired t test) and platelet survivals were 188 ± 55 hours and 196 ± 50 hours (P > .2, paired t test), respectively.
Figure 1.
Platelet recovery (%) after re-injection. The graph shows the percentage of platelets remaining in circulation post-reinjection after normalizing at 100% for the first sample (5 minutes, see “Results”) for each condition: unsensitized platelets (n = 12) and platelets sensitized with either B2G1Δnab (n = 7), B2G1 (n = 8), or a combination of 75% B2G1Δnab/25% B2G1 (n = 3) or 90% B2G1Δnab/10% B2G1 (n = 5). Error bars represent the SD.
Table 2.
Platelet recovery and survival data for each antibody combination using COST software
| Antibody combination | |||||
|---|---|---|---|---|---|
| Unsensitized | B2G1Δnab | B2G1Δnab90* | B2G1Δnab75* | B2G1 | |
| N | 12 | 7 | 3 | 5 | 8 |
| COST data, mean ± SD | |||||
| Recovery | 55% ± 12% | 56% ± 17% | 54% ± 20% | 53% ± 8% | 52% ± 16% |
| Survival | 189 ± 45 h | 196 ± 50 h | 58 ± 12 min | 44 ± 4 min | 18 ± 5 min |
| P > .25 | P = .06 | ||||
| P < .01 | |||||
| P < .01 | |||||
B2G1Δnab90 and -75 denote platelet sensitized with a combination of 90% B2G1Δnab/10% B2G1 and 75% B2G1Δnab/25% B2G1, respectively. For unsensitized and B2G1Δnab-sensitized platelets which showed identical kinetics with a plateau phase during the first 24 hours (see Figure 1), the 24-hour time point was used as the first point for the curve fitting in the COST program. For B2G1-sensitized platelets and platelets sensitized with a combination of 75% B2G1Δnab/25% B2G1 and 90% B2G1Δnab/10% B2G1, where rapid destruction was observed without a plateau phase (see Figure 1), the 5-minute time point was used (see “Results”).
The rate of disappearance of platelets from the circulation is dependent on the number of IgG1 molecules on the surface of the platelets
In contrast to the unsensitized and B2G1Δnab-sensitized platelets, platelets coated with B2G1 were rapidly cleared from circulation, with no sensitized platelets detected 2 hours after re-injection (Figure 1). Although platelets coated with 75% B2G1Δnab/25% B2G1 and 90% B2G1Δnab/10% B2G1 also showed a sharp decrease in the first hour, the clearance rate was slower than that for platelets coated with B2G1 alone. Notably, clearance was also incomplete, with 5% ± 1% and 8% ± 2% (mean ± SD) of platelets remaining in circulation after 24 hours with these 2 antibody combinations, respectively. Given the rapid platelet destruction, the time point 5 minutes was used to fit the kinetic curve in the multiple hits model. Calculated recoveries at time point 0 were not significantly different for the 3 types of platelet (Table 2). Calculated platelet survival was 18 ± 5 minutes, 44 ± 4 minutes, and 58 ± 12 minutes (mean ± SD) for the 3 antibody combinations, respectively. The difference in survival between B2G1-sensitized and either 75% B2G1Δnab/25% B2G1- or 90% B2G1Δnab/10% B2G1-sensitized platelets reached significance (P < .01, t test) in both cases. The difference between the survival of 75% B2G1Δnab/25% B2G1- and 90% B2G1Δnab/10% B2G1-sensitized platelets did not reach statistical significance (P = .06, t test). For the 5 volunteers who had received the combination of B2G1- and 90% B2G1Δnab/10% B2G1-sensitized platelets (volunteers 13-17, excluding 18, see next paragraph), the direct comparison showed recoveries of 52% ± 19% and 54% ± 20%, respectively (not significant), and survival of 19 ± 5 minutes and 58 ± 12 minutes, respectively (P < .005, paired t test).
Volunteer 18, who had a much higher level of HPA-1a expression than the others (Table 1), received platelets coated with B2G1 and 90% B2G1Δnab/10% B2G1. Clearance of B2G1-sensitized platelets was similar to that seen in other volunteers (recovery 81%, survival 19 minutes), but platelets sensitized with 90% B2G1Δnab/10% B2G1 showed a much higher rate of clearance (recovery 96%, survival 25 minutes) than in volunteers 13 to 17 who received platelets sensitized with the same antibody combination (Table 1 and supplemental Figure 1).
Flow cytometry shows that data accumulated in the first 24 hours post–re-injection are the most relevant to assess the effect of the antibodies on platelet survival
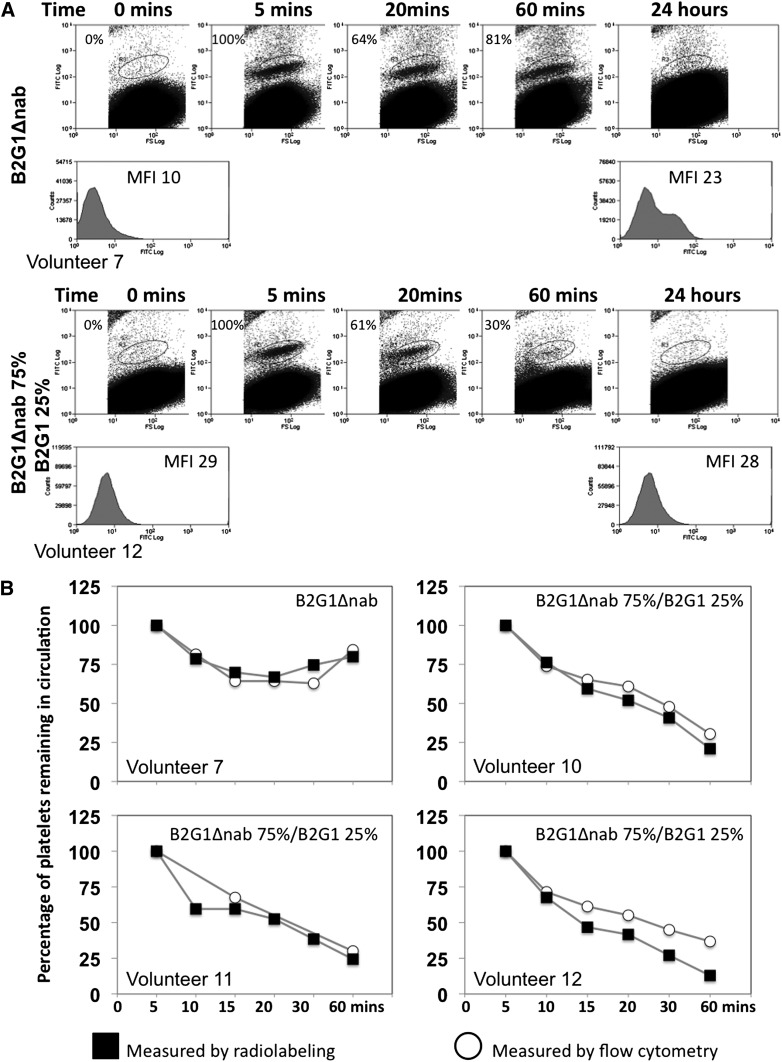
No free antibodies were administered to the volunteers in this study, only antibodies bound to platelets. This raised the question of whether the antibody stayed bound to the radiolabeled platelets after re-injection. We therefore sought to identify the sensitized platelets in blood samples of the volunteer post-injection by flow cytometry using a FITC-labeled anti-human IgG secondary antibody. The data are presented in Figure 2A for volunteer 7 who received a combination of unsensitized and B2G1Δnab-sensitized platelets, and for volunteer 12 who received a combination of unsensitized and 75% B2G1Δnab/25% B2G1-sensitized platelets.
Figure 2.
In vivo platelet tracking using flow cytometry. (A) Flow cytometric data showing sensitized platelets in peripheral blood. Sensitized platelets can be detected with an FITC-labeled anti-human IgG antibody in the peripheral blood after re-injection. Platelet sensitized with B2G1Δnab (top panel) are detected up to 1 hour post-reinjection but after 24 hours the antibody has redistributed to the whole platelet population as evidenced by the shoulder on the histogram and increased MFI for the whole platelet population. In contrast, when platelets are sensitized with a combination of 75% B2G1Δnab/25% B2G1, the majority of platelets are rapidly cleared from circulation after 60 minutes. (B) Correlation between flow cytometry and radiolabeling studies data in 4 volunteers. The percentage of platelets (normalized at 100% at the 5-minute time point) remaining in circulation in the first hour, measured either by flow cytometry or radiolabeling, is consistent between the 2 methods. Over 75% of platelets sensitized with B2G1Δnab only remain in circulation (n = 1) while platelets sensitized with 75% B2G1Δnab/25% B2G1 show a sharp decrease in the first hour down to below 25%.
The data show that a population of B2G1Δnab-sensitized platelets can be detected in the circulation up to 24 hours, although by that point the antibody has clearly eluted from the sensitized platelets and redistributed to the whole population of platelets, as indicated by the right shift and increased mean fluorescence intensity (MFI) of the whole platelet population. A full blood count for this volunteer at 24 hours showed no difference in platelet count from the baseline sample.
The data from 75% B2G1Δnab/25% B2G1 platelets show, in contrast, a rapid disappearance of the sensitized platelets from circulation in the first hour, in keeping with the radiolabeling data. Figure 2B shows the similarity of data between flow cytometry and radiolabeling measurements in the first hour for volunteers 7 and 12 as well as volunteers 10 and 11 who received the combination of unsensitized and 75% B2G1Δnab/25% B2G1 platelets and for whom flow cytometry data were available. These data show that, in the first 24 hours, the platelet radiolabeling survival data truly reflects the survival of the population of platelets sensitized with the various antibody combinations.
Discussion
This first phase of this study examined whether the introduction of modifications to the constant region of a recombinant high-affinity human IgG1 HPA-1a antibody (B2G1) to render it nondestructive translated into increased survival of sensitized platelets in the circulation of human volunteers when compared with platelets sensitized with the native IgG1 antibody. This was the natural progression from in vitro studies that showed that platelet sensitized with this modified antibody (B2G1Δnab) only elicited 15% of the MCL response seen with B2G1.27 Platelets sensitized with B2G1 were cleared rapidly from the volunteer’s circulation, with no intravascular platelets detectable 2 hours post–re-injection. In contrast, B2G1Δnab-sensitized platelets had a survival in the circulation similar to unsensitized platelets.
To use “micro-doses” of antibody in the volunteers, platelets had to be sensitized ex vivo, and re-injected. Therefore, and as expected, flow cytometry studies showed that after 24 hours B2G1Δnab had redistributed to the whole platelet population. Therefore, only the first 24 hours of the study data truly reflect the survival of platelets that are sensitized. During that time period, both the percentages of platelets remaining in circulation and the kinetics were identical for unsensitized and B2G1Δnab-sensitized samples. This is in keeping with the in vitro data showing that this antibody does not cause FcR-mediated effector cell activation.29,35 In a previous study to assess survival of red cells sensitized with an anti-D (Fog-1) carrying the same G1Δnab constant region,36 Fog-1G1Δnab–sensitized red cells pooled in the spleen before reappearing in the circulation after 3 to 4 hours. We did not have the opportunity to use a γ camera to confirm where the platelets went following re-injection but the curve obtained in the first few hours post-injection suggests this may happen for platelets too, with a trough reached at 20 minutes and subsequent rise reaching a plateau at 2 hours. Notably, this phenomenon was observed for both unsensitized and B2G1Δnab-sensitized platelets whereas, previously, no data were available for unsensitized red cells. Because the COST analysis to assess platelet recovery and survival of unsensitized and B2G1Δnab-sensitized platelets was run using the 24-hour time point as the first point of the kinetic curve, the similarity of the platelet survivals derived from this latter analysis only reflects the fact that prior sensitization with B2G1Δnab does not affect subsequent platelet survival after elution of the antibody.
In the second phase of this study, we examined the effect on platelet survival of sensitization with different ratios of B2G1/B2G1Δnab, consistent with what could theoretically be achieved in an affected fetus, using B2G1Δnab as a therapeutic blocking antibody in the presence of maternal anti-HPA-1a. Even with only 10% B2G1/90% B2G1Δnab, we observed that sensitized platelets were cleared from the circulation along an exponential curve similar to that observed for B2G1 alone. Crucially, however, there was a slower rate of clearance. COST analysis of the data (this time using the 5-minute time point as the first point of the curve given the absence of an “equilibrium” plateau phase) indicated that platelet survival when sensitized with a combination of B2G1Δnab and B2G1 was 2 to 3 times longer than that of platelets sensitized with B2G1 only. It is as yet unclear whether this would have a relevant clinical effect in the treated fetus. However, the most severe complication of FMAIT, ICH, virtually always occurs when the platelet count is below 20 × 109/L,8 so that even a small increase in platelet count to >20-30 × 109/L could have a profound effect on the risk of ICH. Assuming that platelet production is already maximal in the marrow of an affected fetus, a 2 to 3 times prolongation of platelet survival could achieve the desired clinical effect.
In conclusion, the data acquired with B2G1Δnab, and in particular with the combination of B2G1/B2G1Δnab, indicates real therapeutic potential of B2G1Δnab as a blocking antibody. Obviously, the work described here would have to be followed by pharmacodynamic studies and clinical studies covering safety, efficacy, and dosage. These were discussed extensively in a previous publication on B2G1Δnab.27
Just as FcRn-mediated transport is essential for the transfer of maternal HPA-1a antibodies to the fetus and the development of FMAIT, as has been shown in an animal model,37 this mechanism of transport is also crucial in the case of the therapeutic mechanism of B2G1Δnab. Although studies to assess the binding of B2G1Δnab to FcRn and efficiency of transport across the placenta are ongoing, data are available from a study in rats showing appropriate transplacental transport of mutated IgG2 antibodies that carry both the Δb residues (corresponding to the natural IgG2 residues) and Δa modified residues (corresponding to IgG4) found in B2G1Δnab.38
FMAIT differs from hemolytic disease of the newborn (HDN; caused by maternal alloantibodies against fetal red cell antigens) in several crucial aspects. First of all, red cell alloimmunization usually results from a sensitizing event, in most cases at the time of birth. HDN usually becomes a clinical issue in subsequent pregnancies, while FMAIT can affect primigravidae.3 Second, clinical severity of HDN can also be predicted from the quantification of alloantibodies in the maternal serum. Some studies have shown the levels of maternal HPA-1a antibodies to be predictive of outcome,39,40 while others have not,8 further complicating the identification of the patients that would most benefit from therapy. From the large prospective screening studies in Norway, data have emerged showing that a significant proportion of HPA-1a negative mothers develop HPA-1a alloimmunity postdelivery rather than in the first pregnancy itself.40,41 It is therefore possible that fetomaternal alloimmunization against HPA-1a is more akin to the pattern of red cell immunization than was suggested from previous retrospective data. This observation has raised the question of the feasibility and potential clinical benefit of prophylactic measures to prevent HPA-1a immunization. The use of anti-D prophylaxis for prevention of hemolytic disease of the newborn is one of immunotherapy’s most successful stories. Although the mechanisms behind this process remain largely unknown, a mouse model of fetomaternal platelet alloimmunization demonstrated that administration of platelet anti-β3 antibodies prevented alloimmunization.42 Here, we have shown that B2G1 very quickly clears HPA-1a–positive platelets from the circulation and would therefore constitute an ideal candidate to progress on to clinical studies for prophylaxis after delivery in HPA-1a–negative women. If this proved to be effective, one could consider follow-on studies to assess effectiveness of this prophylactic approach during pregnancy, in particular including primigravidae where alloimmunization can lead to significant fetal thrombocytopenia. This is made possible by the knowledge that among HPA-1a–negative women, 80% of those who develop an antibody carry the HLA-type DRB3*0101 and therefore we could target the group of women most at risk of immunization including primigravidae.
Supplementary Material
Acknowledgments
We are extremely grateful to all volunteers who took part in this study. We thank the staff from the Wellcome Trust Clinical Research Facility and the Nuclear Medicine Department at the Addenbrooke’s Hospital where sampling, nuclear labeling, and re-injections were carried out. A special thank you to Dr Larry J Dumont (Cell Labeling Laboratory, Dartmouth-Hitchcock Medical Center, Lebanon, NH) for his input into the manuscript.
This work was supported by grants from the National Health Service (NHS) Blood and Transplant (to C.G., N.H., N.G., L.H., W.H.O., and L.M.W.) and the National Institute for Health Research (NIHR) (to A.C.-H. and S.F.G.). C.G. is also supported by a personal Intermediate Clinical Fellowship from the British Heart Foundation (FS/09/039). K.L.A. and M.R.C. gratefully acknowledge support from the Department of Pathology’s income derived from commercial exploitation of patented antibodies.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.G. designed and performed the experiments and wrote the manuscript; N.H., L.H., and N.G. recruited the volunteers and performed experiments and samplings; P.C. performed data analysis; S.F.G., A.C.-H., and A.E. performed flow cytometry; P.L.-E. oversaw the antibody production to clinical grade; K.B. oversaw the radiolabeling procedure; and W.H.O., K.L.A., M.R.C., and L.M.W. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: K.L.A., M.R.C., and L.M.W. have filed patent applications (WO 99/58572), owned by the University of Cambridge and covering use of the mutant IgG constant region studied in this work. The remaining authors declare no competing financial interests.
Correspondence: Cedric Ghevaert, Division of Transfusion Medicine, Department of Haematology, University of Cambridge/NHS Blood and Transplant, Long Rd, Cambridge CB2 2PT, United Kingdom; e-mail: cg348@cam.ac.uk.
References
- 1.Hohlfeld P, Forestier F, Kaplan C, Tissot JD, Daffos F. Fetal thrombocytopenia: a retrospective survey of 5,194 fetal blood samplings. Blood. 1994;84(6):1851–1856. [PubMed] [Google Scholar]
- 2.Bussel JB, Zabusky MR, Berkowitz RL, McFarland JG. Fetal alloimmune thrombocytopenia. N Engl J Med. 1997;337(1):22–26. doi: 10.1056/NEJM199707033370104. [DOI] [PubMed] [Google Scholar]
- 3.Williamson LM, Hackett G, Rennie J, et al. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92(7):2280–2287. [PubMed] [Google Scholar]
- 4.Kjeldsen-Kragh J, Killie MK, Tomter G, et al. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110(3):833–839. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]
- 5.Mueller-Eckhardt C, Kiefel V, Grubert A, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989;1(8634):363–366. doi: 10.1016/s0140-6736(89)91733-9. [DOI] [PubMed] [Google Scholar]
- 6.Berry JE, Murphy CM, Smith GA, et al. Detection of Gov system antibodies by MAIPA reveals an immunogenicity similar to the HPA-5 alloantigens. Br J Haematol. 2000;110(3):735–742. doi: 10.1046/j.1365-2141.2000.02170.x. [DOI] [PubMed] [Google Scholar]
- 7.Davoren A, Curtis BR, Aster RH, McFarland JG. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion. 2004;44(8):1220–1225. doi: 10.1111/j.1537-2995.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 8.Ghevaert C, Campbell K, Walton J, et al. Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion. 2007;47(5):901–910. doi: 10.1111/j.1537-2995.2007.01208.x. [DOI] [PubMed] [Google Scholar]
- 9.Kamphuis MM, Paridaans N, Porcelijn L, et al. Screening in pregnancy for fetal or neonatal alloimmune thrombocytopenia: systematic review. BJOG. 2010;117(11):1335–1343. doi: 10.1111/j.1471-0528.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 10.Bussel JB, Zacharoulis S, Kramer K, McFarland JG, Pauliny J, Kaplan C. Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatr Blood Cancer. 2005;45(2):176–183. doi: 10.1002/pbc.20282. [DOI] [PubMed] [Google Scholar]
- 11.Dreyfus M, Kaplan C, Verdy E, Schlegel N, Durand-Zaleski I, Tchernia G Immune Thrombocytopenia Working Group. Frequency of immune thrombocytopenia in newborns: a prospective study. Blood. 1997;89(12):4402–4406. [PubMed] [Google Scholar]
- 12.Allen D, Verjee S, Rees S, Murphy MF, Roberts DJ. Platelet transfusion in neonatal alloimmune thrombocytopenia. Blood. 2007;109(1):388–389. doi: 10.1182/blood-2006-05-026419. [DOI] [PubMed] [Google Scholar]
- 13.Allen DL, Samol J, Benjamin S, Verjee S, Tusold A, Murphy MF. Survey of the use and clinical effectiveness of HPA-1a/5b-negative platelet concentrates in proven or suspected platelet alloimmunization. Transfus Med. 2004;14(6):409–417. doi: 10.1111/j.1365-3148.2004.00536.x. [DOI] [PubMed] [Google Scholar]
- 14.Bussel JB, Berkowitz RL, Lynch L, et al. Antenatal management of alloimmune thrombocytopenia with intravenous gamma-globulin: a randomized trial of the addition of low-dose steroid to intravenous gamma-globulin. Am J Obstet Gynecol. 1996;174(5):1414–1423. doi: 10.1016/s0002-9378(96)70582-3. [DOI] [PubMed] [Google Scholar]
- 15.van den Akker ES, Oepkes D, Lopriore E, Brand A, Kanhai HH. Noninvasive antenatal management of fetal and neonatal alloimmune thrombocytopenia: safe and effective. BJOG. 2007;114(4):469–473. doi: 10.1111/j.1471-0528.2007.01244.x. [DOI] [PubMed] [Google Scholar]
- 16.Bussel JB, Berkowitz RL, Hung C, et al. Intracranial hemorrhage in alloimmune thrombocytopenia: stratified management to prevent recurrence in the subsequent affected fetus. Am J Obstet Gynecol. 2010;203(2):135.e1–135.e14. doi: 10.1016/j.ajog.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Vinograd CA, Bussel JB. Antenatal treatment of fetal alloimmune thrombocytopenia: a current perspective. Haematologica. 2010;95(11):1807–1811. doi: 10.3324/haematol.2010.030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamphuis MM, Oepkes D. Fetal and neonatal alloimmune thrombocytopenia: prenatal interventions. Prenat Diagn. 2011;31(7):712–719. doi: 10.1002/pd.2779. [DOI] [PubMed] [Google Scholar]
- 19.Giers G, Wenzel F, Riethmacher R, Lorenz H, Tutschek B. Repeated intrauterine IgG infusions in foetal alloimmune thrombocytopenia do not increase foetal platelet counts. Vox Sang. 2010;99(4):348–353. doi: 10.1111/j.1423-0410.2010.01367.x. [DOI] [PubMed] [Google Scholar]
- 20.Giers G, Wenzel F, Stockschläder M, Riethmacher R, Lorenz H, Tutschek B. Fetal alloimmune thrombocytopenia and maternal intravenous immunoglobulin infusion. Haematologica. 2010;95(11):1921–1926. doi: 10.3324/haematol.2010.025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overton TG, Duncan KR, Jolly M, Letsky E, Fisk NM. Serial aggressive platelet transfusion for fetal alloimmune thrombocytopenia: platelet dynamics and perinatal outcome. Am J Obstet Gynecol. 2002;186(4):826–831. doi: 10.1067/mob.2002.122140. [DOI] [PubMed] [Google Scholar]
- 22.Berkowitz RL, Lesser ML, McFarland JG, et al. Antepartum treatment without early cordocentesis for standard-risk alloimmune thrombocytopenia: a randomized controlled trial. Obstet Gynecol. 2007;110(2 Pt 1):249–255. doi: 10.1097/01.AOG.0000270302.80336.dd. [DOI] [PubMed] [Google Scholar]
- 23.Birchall JE, Murphy MF, Kaplan C, Kroll H European Fetomaternal Alloimmune Thrombocytopenia Study Group. European collaborative study of the antenatal management of feto-maternal alloimmune thrombocytopenia. Br J Haematol. 2003;122(2):275–288. doi: 10.1046/j.1365-2141.2003.04408.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldberger A, Kolodziej M, Poncz M, Bennett JS, Newman PJ. Effect of single amino acid substitutions on the formation of the PlA and Bak alloantigenic epitopes. Blood. 1991;78(3):681–687. [PubMed] [Google Scholar]
- 25.Griffin HM, Ouwehand WH. A human monoclonal antibody specific for the leucine-33 (P1A1, HPA-1a) form of platelet glycoprotein IIIa from a V gene phage display library. Blood. 1995;86(12):4430–4436. [PubMed] [Google Scholar]
- 26.Garner SF, Smethurst PA, Merieux Y, et al. A rapid one-stage whole-blood HPA-1a phenotyping assay using a recombinant monoclonal IgG1 anti-HPA-1a. Br J Haematol. 2000;108(2):440–447. doi: 10.1046/j.1365-2141.2000.01839.x. [DOI] [PubMed] [Google Scholar]
- 27.Ghevaert C, Wilcox DA, Fang J, et al. Developing recombinant HPA-1a-specific antibodies with abrogated Fcgamma receptor binding for the treatment of fetomaternal alloimmune thrombocytopenia. J Clin Invest. 2008;118(8):2929–2938. doi: 10.1172/JCI34708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joutsi-Korhonen L, Preston S, Smethurst PA, et al. The effect of recombinant IgG antibodies against the leucine-33 form of the platelet beta3 integrin (HPA-1a) on platelet function. Thromb Haemost. 2004;91(4):743–754. doi: 10.1160/TH03-07-0484. [DOI] [PubMed] [Google Scholar]
- 29.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcgamma receptor I binding and monocyte triggering activities. Eur J Immunol. 1999;29(8):2613–2624. doi: 10.1002/(SICI)1521-4141(199908)29:08<2613::AID-IMMU2613>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.MHRA. Rules and Guidance for Pharmaceutical Manufacturers and Distributors. London, UK: Pharmaceutical Press; 2007. [Google Scholar]
- 31.European Union. Production and quality control of monoclonal antibodies. In: The Rules Governing Medicinal Products in the European Union: Guidelines: Medicinal Products for Human Use. Vol 3. Commission of the European Communities: Luxembourg; 1994:237-262. [Google Scholar]
- 32.The Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Platelet radiolabeling procedure. Transfusion. 2006;46(suppl s3):59S–66S. [Google Scholar]
- 33.Lötter MG, Heyns AD, Badenhorst PN, et al. Evaluation of mathematic models to assess platelet kinetics. J Nucl Med. 1986;27(7):1192–1201. [PubMed] [Google Scholar]
- 34.Dumont LJ. Analysis and reporting of platelet kinetic studies. Transfusion. 2006;46(suppl s3):67S–73S. [Google Scholar]
- 35.Armour KL, van de Winkel JG, Williamson LM, Clark MR. Differential binding to human FcgammaRIIa and FcgammaRIIb receptors by human IgG wildtype and mutant antibodies. Mol Immunol. 2003;40(9):585–593. doi: 10.1016/j.molimm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Armour KL, Parry-Jones DR, Beharry N, et al. Intravascular survival of red cells coated with a mutated human anti-D antibody engineered to lack destructive activity. Blood. 2006;107(7):2619–2626. doi: 10.1182/blood-2005-03-0989. [DOI] [PubMed] [Google Scholar]
- 37.Chen P, Li C, Lang S, et al. Animal model of fetal and neonatal immune thrombocytopenia: role of neonatal Fc receptor in the pathogenesis and therapy. Blood. 2010;116(18):3660–3668. doi: 10.1182/blood-2010-05-284919. [DOI] [PubMed] [Google Scholar]
- 38.Coder PS, Thomas JA, Stedman DB, Bowman CJ. Placental transfer of 125Iodinated humanized immunoglobulin G2Δa in the Sprague Dawley rat. Reprod Toxicol. 2013;38C:37–46. doi: 10.1016/j.reprotox.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand G, Drame M, Martageix C, Kaplan C. Prediction of the fetal status in noninvasive management of alloimmune thrombocytopenia. Blood. 2011;117(11):3209–3213. doi: 10.1182/blood-2010-08-302463. [DOI] [PubMed] [Google Scholar]
- 40.Killie MK, Husebekk A, Kjeldsen-Kragh J, Skogen B. A prospective study of maternal anti-HPA 1a antibody level as a potential predictor of alloimmune thrombocytopenia in the newborn. Haematologica. 2008;93(6):870–877. doi: 10.3324/haematol.12515. [DOI] [PubMed] [Google Scholar]
- 41.Killie MK, Husebekk A, Kaplan C, Taaning E, Skogen B. Maternal human platelet antigen-1a antibody level correlates with the platelet count in the newborns: a retrospective study. Transfusion. 2007;47(1):55–58. doi: 10.1111/j.1537-2995.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 42.Tiller H, Killie MK, Chen P, et al. Toward a prophylaxis against fetal and neonatal alloimmune thrombocytopenia: induction of antibody-mediated immune suppression and prevention of severe clinical complications in a murine model. Transfusion. 2012;52(7):1446–1457. doi: 10.1111/j.1537-2995.2011.03480.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.