Key Points
High-affinity tumor/self antigen-specific TCRs that surpass the threshold for normal thymic selection can be safe for TCR gene therapy.
T cells that express endogenous TCRs that are self-reactive can survive in the periphery with diminished TCR expression levels.
Abstract
Many of the most promising tumor antigens for T-cell-based cancer immunotherapies are unmodified self-antigens. Unfortunately, the avidity of T cells specific for these antigens is limited by central tolerance during T-cell development in the thymus, resulting in decreased anti-tumor efficacy of these T cells. One approach to overcoming this obstacle is to mutate T-cell receptor (TCR) genes from naturally occurring T cells to enhance the affinity for the target antigen. These enhanced-affinity TCRs can then be developed for use in TCR gene therapy. Although TCRs with significantly enhanced affinity have been generated using this approach, it is not clear whether these TCRs, which bypass the affinity limits imposed by negative selection, remain unresponsive to the low levels of self-antigen generally expressed by some normal tissues. Here we show that 2 variants of a high-affinity WT1-specific TCR with enhanced affinity for WT1 are safe and do not mediate autoimmune tissue infiltration or damage when transduced into peripheral CD8 T cells and transferred in vivo. However, if expressed in developing T cells and subjected to thymic selection, the same enhanced-affinity TCRs signal tolerance mechanisms in the thymus, resulting in T cells with attenuated antigen sensitivity in the periphery.
Introduction
T-cell receptor (TCR) gene transfer as a strategy to create tumor-reactive T cells is an emerging approach with the potential to overcome many of the obstacles associated with conventional T-cell adoptive immunotherapy.1 With TCR gene therapy, a single, well-characterized, high-affinity TCR can be used as an “off the shelf” reagent for treatment of all patients with tumors expressing the target antigen, so long as the patient expresses the appropriate HLA allele. However, many promising tumor antigens are overexpressed self proteins, and when targeting these antigens, even the highest-affinity naturally occurring TCRs may not possess adequate affinity to efficiently lyse target cells because of the elimination of self-reactive T cells by negative selection in the thymus.2 In these cases, the affinity of such naturally occurring TCRs can be enhanced through in vitro directed evolution strategies as a means to increase the anti-tumor efficacy of the gene-therapy treatment.3-5
Approaches to increase the affinity of tumor-reactive TCRs are predicated on the assumption that the thymus to some degree overprotects against self-reactivity, such that T cells expressing higher-affinity variants will be tolerated when transferred in vivo or that the extent of tissue injury or T-cell dysfunction resulting from recognition of normal tissues will be acceptable. Many tumor antigens that are candidates for therapeutic targeting are expressed at high levels during embryogenesis, but very low levels in adult tissues.6-8 In this study, we test the hypothesis that thymic selection may frequently be overprotective for such tumor antigens and that increasing the affinity of TCRs specific for naturally occurring tumor/self antigens beyond the affinity threshold necessary for negative selection or other tolerance mechanisms operative during development in the thymus does not necessarily lead to autoimmunity in the periphery. We assessed the safety and efficacy of a TCR specific for a self/tumor antigen currently being evaluated as a target in humans, WT1, in the context of H-2Db (Db), as well as 2 variants of this TCR that we modified to have an enhanced affinity for WT1-Db. Peripheral T cells transduced in vitro with any of the 3 TCRs failed to induce self-reactivity against their cognate self-antigen in vivo, even when the TCR gene-modified T cells were stimulated and expanded in vivo in response to infection with recombinant Listeria monocytogenes (LMWT1) to form effector cells. In contrast, T cells from retrogenic mice9 expressing the higher-affinity variants showed attenuation of antigen sensitivity when subjected to thymic selection. Similar findings were observed for a high-affinity Mesothelin (MSLN)-specific TCR. These data support the notion that T-cell selection events in the thymus may overprotect against responses to tumor/self-antigens that are expressed at low levels in normal adult tissues. If the extent of overprotection can be defined for an antigen, bypassing such thymic selection could provide a window of opportunity and allow the use of enhanced-affinity TCRs that safely enhance the anti-tumor response of TCR gene-modified T cells.
Material and methods
Mice
C57BL/6 (B6), Thy1.1 congenic B6, and B6 Rag2−/− mice were purchased from the Jackson Laboratory. P14 TCR transgenic mice bred on the B6 background were a kind gift from Dr Murali Krishna-Kaja and were bred with Thy1.1 B6 mice to generate P14/Thy1.1 mice. All animal research performed for this study was approved under University of Washington Institutional Animal Care and Use Committee protocol 2013-01.
Retroviral TCR expression construct
The retroviral expression constructs Mig-3Dαβ consist of TCRα and TCRβ genes cloned from the high-affinity WT1-specific T-cell clone 3D (supplemental Methods, available on the Blood Web site). These genes were codon-optimized, linked by a porcine teschovirus-1 2A element, and cloned into a variant of the retroviral vector MigR1 that lacks green fluorescent protein.10 Enhanced-affinity mutations (supplemental Methods) were incorporated into this codon-optimized construct, using the Quikchange II site-directed mutagenesis kit (Agilent Technologies).
Retroviral transduction
The retroviral packaging cell line Plat-E was transduced with retroviral vector, using effectene transduction reagent (Qiagen), and virus-containing supernatant was collected on days 2 and 3. Retroviral transduction of T cells was performed in 24-well plates precoated with retronectin (Takara). Virus was bound to the retronectin-coated plates by centrifuging the virus-containing supernatant for 90 minutes at 1000 g. The supernatant was then removed, the wells were washed once with PBS, and anti-CD3/CD28-stimulated P14 splenocytes (24 hours poststimulation) were added to the wells at 3 × 106 cells/well, with interleukin 2 (IL-2; 50 IU), followed by centrifugation for 10 minutes to facilitate contact with the retronectin-bound virus.
Transfer of TCR-transduced T cells into mice and LM challenge
On day 7 after retroviral transduction, T cells were restimulated with irradiated splenocytes pulsed with the WT1 peptide RMFPNAPYL (WT1126-134). On day 5 after this second stimulation, cells were injected into Thy-1 congenic B6 hosts. At this point, cell cultures generally consisted of more than 90% tetramer-positive T cells. Host mice were injected 1:1 (1 × 107 each) with TCR-transduced T cells and irradiated (3000 rad) peptide-pulsed splenocytes as stimulators and were given IL-2 (104 units/mouse) for 10 days postinjection. Cohorts of T-cell-treated mice were boosted with wild-type LM or LM expressing the WT1 RMFPNAPYL epitope within a piece of the ovalbumin gene (LMWT1; provided by Aduro BioTech) 2 to 3 weeks after T-cell injection. The blood of LM/LMWT1-treated animals was analyzed on day 6 postchallenge, and mice were monitored for signs of possible autoimmune disease. After 2 to 3 weeks, animals were killed and tissues, including bone marrow (BM) cells, kidney, and lung, were collected and analyzed for evidence of T-cell infiltration and/or autoimmune pathology. Microscopy was done using a Nikon E800 with a 10× 0.45 N.A. aperture Plan Apo objective, a Photometrics CF camera, and MetaMorph analysis software.
Generation of retrogenic mice
BM cells were isolated from the leg bones of B6/Rag2−/− mice, and hematopoietic stem and progenitor cells were enriched by magnetic cell sorting, using the mouse lineage cell depletion kit from Miltenyi. BM collected from a single donor mouse was used for 2 recipients. BM was cultured in media containing IL-3 (20 ng/mL), IL-6 (50 ng/mL), stem cell factor (50 ng/mL), and Fms-related tyrosine kinase 3 ligand (FLT-3L) (5 ng/mL) for 3 days. On the first 2 days after BM isolation, the purified progenitor cells were centrifuged for 10 minutes onto plates containing retronectin-bound retroviral particles, as described earlier. On the third day, the transduced hematopoietic stem and progenitor cells were injected into sublethally irradiated B6 host mice.
Results
Generation of high-affinity WT1-specific TCRs
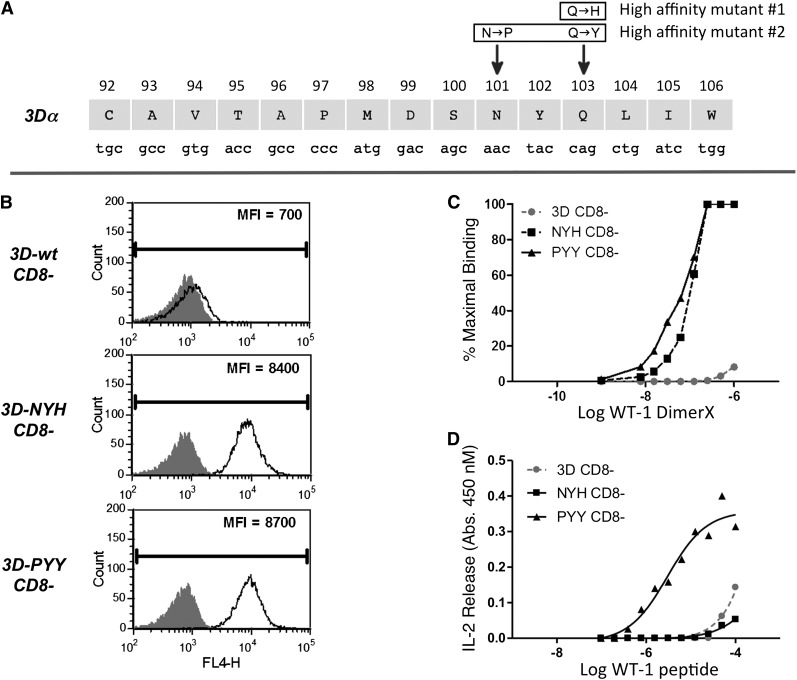
The 3D clone (3D-wt) was the highest-affinity clone isolated from wild-type B6 mice, responding to target cells pulsed with peptide at a concentration of 100 ng/mL or lower. Enhanced-affinity variants of the 3D TCR were generated using a T-cell display system, as has been previously described,3 and selection was made with WT1/Db Ig DimerX (BD Biosciences) (supplemental Methods). Two enhanced-affinity complementarity determining region (CDR) 3α mutants were identified that bound the peptide/major histocompatibility complex (MHC) dimer independent of CD8: the Q103H mutant (3D-NYH) and the N101P, Q103Y mutant (3D-PYY) (Figure 1A-B). The 3D-wt TCR and its higher-affinity variants were transduced into the TCRαβ-deficient, CD8-negative T-cell line 58−/−.11 Both the 3D-NYH and 3D-PYY mutants bound peptide/MHC dimer with enhanced equilibrium-binding kinetics compared with 3D-wt, and both mutants bound peptide/MHC dimer independent of CD8 (Figure 1C). In contrast, the wild-type TCR (3D-wt) in the absence of CD8 exhibited negligible binding of peptide/MHC dimer and displayed a significantly lower affinity for peptide/MHC dimer than the 2 enhanced affinity variants, even in the presence of CD8 (data not shown). The highest-affinity variant also exhibited enhanced functional avidity, as measured by cytokine release (IL-2) in response to peptide-pulsed target cells, with the 58−/− cells expressing the 3D-PYY variant showing ∼100-fold increase in antigen sensitivity (Figure 1D). The NYH mutant did not show an increase in functional avidity in the absence of CD8, likely reflecting the sharp affinity threshold previously observed for IL-2 production by 58−/− cells expressing MHC-I restricted TCRs.12 The affinity threshold for CD8-independent peptide/MHC multimer binding occurs at KD values less than ∼5 μM13,14; thus, the 3D-NYH and 3D-PYY TCRs likely have affinity values at least in this range.
Figure 1.
Generation of enhanced affinity variants of the 3D TCR. (A) Schematic of the 3D TCRα chain CDR3 region showing the location of the mutations that confer higher affinity for WT1 peptide/MHC. (B) 58−/− T cells (CD8-negative) transduced to express 3D-wt, 3D-NYH, or 3D-PYY TCRs (black line) and the 58−/− parental line as a control (gray histograms) stained with WT-1/Db DimerX at 125 nM. (C) WT-1/Db DimerX titration analysis by flow cytometry at 4°C and relative mean fluorescence intensity values were used to generate equilibrium-binding curves. (D) T-cell activation in the presence of WT-1 peptide titrated on T2-Db cells with 3D-wt, 3D-NYH, and 3D-PYY.
In vivo safety of peripheral T cells expressing high-affinity WT1-specific TCRs
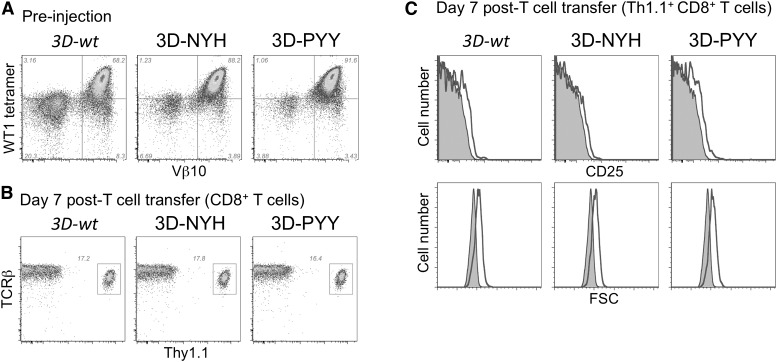
To test safety in vivo, P14 splenocytes were retrovirally transduced with either 3D-wt or the enhanced-affinity 3D mutants, and similar to how human TCR gene therapy is being pursued, the in vivo biology of transduced peripheral CD8 T cells was assessed. T cells were stimulated with anti-CD3 and anti-CD28 antibodies, transduced, and cultured with IL-2 for 7 days. On day 7, T cells were restimulated with irradiated RMFPNAPYL peptide-pulsed (10 μg/mL) splenocytes to expand WT1-specific T cells. On day 12, similar proportions of CD8+ T cells expressing the transduced TCR from each of the 3 constructs were detected by flow cytometry, and the cells exhibited similar levels of TCR expression (Figure 2A). Differences in affinity between the constructs could not be observed by tetramer staining because of the relatively high contribution of CD8 to tetramer staining on murine CD8+ T cells.15
Figure 2.
Analysis of TCR-transduced T cells in vivo. (A) P14 splenocytes were stimulated in vitro with gp33 peptide and transduced with the indicated 3D TCR construct, restimulated with splenocytes pulsed with the WT1 peptide after 7 days, and analyzed by flow cytometry on day 5 postrestimulation. (B and C) 10 × 106 TCR-transduced P14 T cells were transferred into irradiated B6 mice along with 10 × 106 irradiated B6 splenocytes pulsed with the WT1 peptide. Blood from treated mice (solid line) was analyzed by flow cytometry on day 7 after T-cell transfer for relative size (FSC) and CD25 expression compared with a Thy1.1+ control (shaded histogram).
Transduced T cells (1 × 107/mouse) were then injected into recipient Thy-1 congenic B6 mice and immunized with irradiated, WT1 peptide-pulsed (10 μg/mL) splenocytes to promote in vivo activation and expansion. After 7 days, blood was collected from recipient mice and assessed for donor cell engraftment and persistence, as well as for persistent activation of T cells expressing an enhanced-affinity TCR from recognition of normal host cells expressing low levels of the self-antigen (Figure 2B). A similar percentage of donor TCR-transduced T cells was detected (∼17%), regardless of the TCR construct expressed, and analysis of surface TCR expression levels did not reveal activation-induced downregulation of the TCR on the TCR-transduced T cells. Transferred T cells were also assessed for other evidence of persistent activation, including CD25 expression or the presence of larger blast cells that might be indicative of autoimmune activation. The 3D-wt as well as the PYY and NYH enhanced-affinity mutants were all CD25−/lo, and all had similar forward scatter (FSC) profiles. When T cells expressing 3D-wt and 3D-PYY TCRs were analyzed beyond day 7 post-T-cell transfer, both were found to contract to less than 1% of CD8+ T cells by day 14 posttransfer, but showed stable persistence and were detectable at similar levels at day 28, with CD44hi CD62L+ memory T cells present at similar frequencies (supplemental Figure 2A). The lack of preferential expansion or deletion of T cells expressing enhanced-affinity TCRs, the maintenance of TCR expression levels, and the lack of CD25 expression suggests the enhanced affinity TCRs are not responding to WT1 or other self-antigens in vivo.
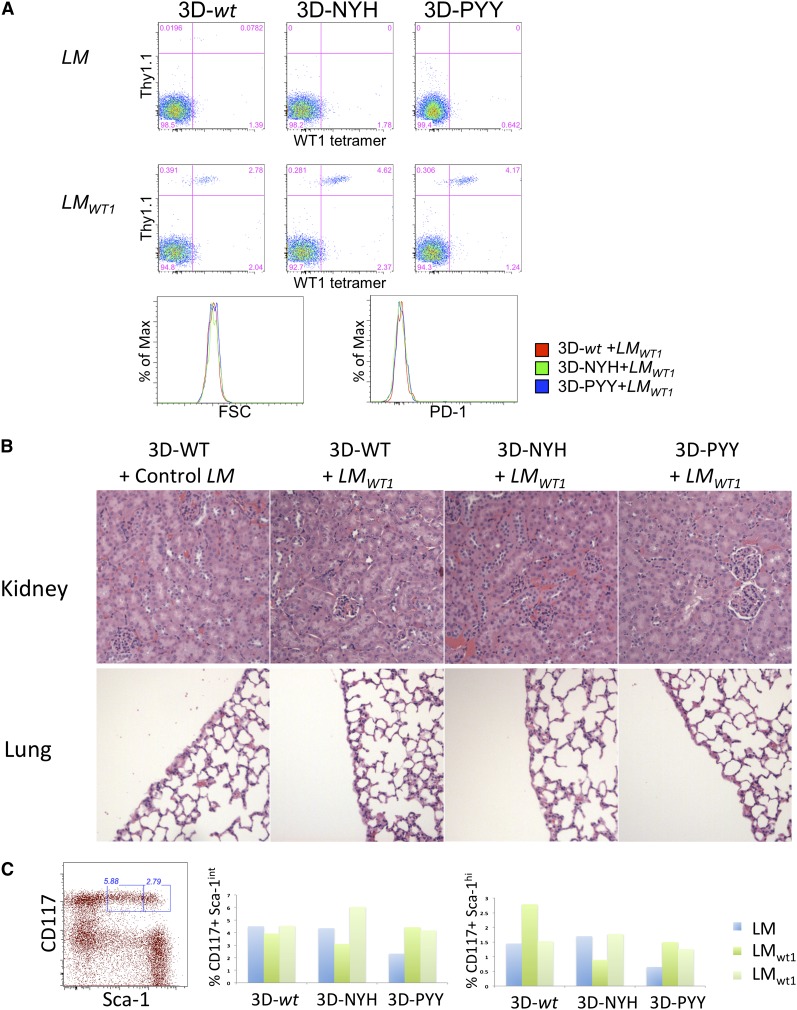
To further assess potential self-reactivity, mice that received either the 3D-wt TCR or its high-affinity variants were boosted on day 18 after cell transfer with either wild-type LM or LMWT1, as LM induces effector CD8 T cells in vivo. Six days after boosting with LM, analysis of blood revealed activation of all 3 groups of T cells expressing 3D TCRs, as indicated by the expansion of donor T cells after infection with LMWT1 but not LM (Figure 3A). Although expansion of donor T cells was observed for all TCR variants, the high-affinity variants exhibited somewhat greater expansion (4.6% and 4.2% of total CD8s in blood, as opposed to 2.8% for 3D-wt), potentially reflecting enhanced responsiveness to the LMWT1-encoded antigen. TCR-transferred T cells can be detected in the spleen of LMWT1-treated mice that received T cells expressing either the 3D-wt or 3D-PYY TCR on day 21 postchallenge, and ∼30% of persisting cells express high levels of CD44 and CD62L, indicative of persistent memory cells (supplemental Figure 3B). Importantly, all the mice remained healthy and did not show any evidence of autoimmunity, even when the mice were immune-depleted by sublethal irradiation before T-cell transfer (supplemental Figure 2B). Furthermore, chronically activated T cells could again not be detected by increased size or expression of PD-1 (Figure 3A), which is often associated with chronically stimulated T cells.
Figure 3.
Safety of T cells expressing enhanced affinity WT1-specific TCRs in mice infected with LMWT1. (A) TCR-transduced P14 T cells were transferred into B6 mice and, after 18 days, challenged with LM or LMWT1. After 6 days, blood from treated mice was analyzed by flow cytometry. Data are representative of 3 independent experiments with at least 1 LM and 2 LMWT1 mice per experiment. (B) BM cells were purified from treated mice, and the percentage of CD117+ Sca-1int and Sca-1hi cells was determined. Data shown are from an experiment in which a single mouse per TCR construct was immunized with control LM and 2 mice were challenged with recombinant LMWT1. (C) Kidney and lung were also collected from these animals and analyzed by hematoxylin and eosin staining for T-cell infiltration and/or tissue damage.
To more directly assess autoimmune pathology, mice receiving TCR gene-modified T cells were killed 2 to 3 weeks after LM challenge, and normal tissues known to express WT1 in mice and humans were analyzed for T-cell-mediated tissue injury (Figure 3B). WT1 is expressed at low levels in the kidney, lung pleura, and hematopoietic stem cells (HSCs).16 Tissue sections from lung and kidney of mice treated with transduced T cells were analyzed for T-cell infiltration and/or tissue damage after LM or LMWT1 challenge (Figure 3B). No evidence of pathologic injury or significant T-cell infiltration with the wild-type or higher-affinity 3D mutants was detected. As HSCs also express low levels of WT1, BM cells were purified from the challenged mice. The BM was assessed for depletion of early long-term reconstituting HSCs or more differentiated HSCs, which are enriched within the lineage marker negative CD117+ sca-1hi and CD117+ sca-1int populations, respectively.17 The percentage of CD117+ sca-1int and sca-1hi cells in the BM varied between different mice, but there was no statistical evidence of HSC depletion (Figure 3C). These data, in addition to the absence of any other evidence of autoimmunity, suggest that the 2 enhanced-affinity TCRs, despite having an affinity for WT1 that is likely in the low micromolar range, do not cause autoimmune pathology in this model system.
Thymic selection and peripheral function of enhanced-affinity WT1-specific TCRs in retrogenic mice
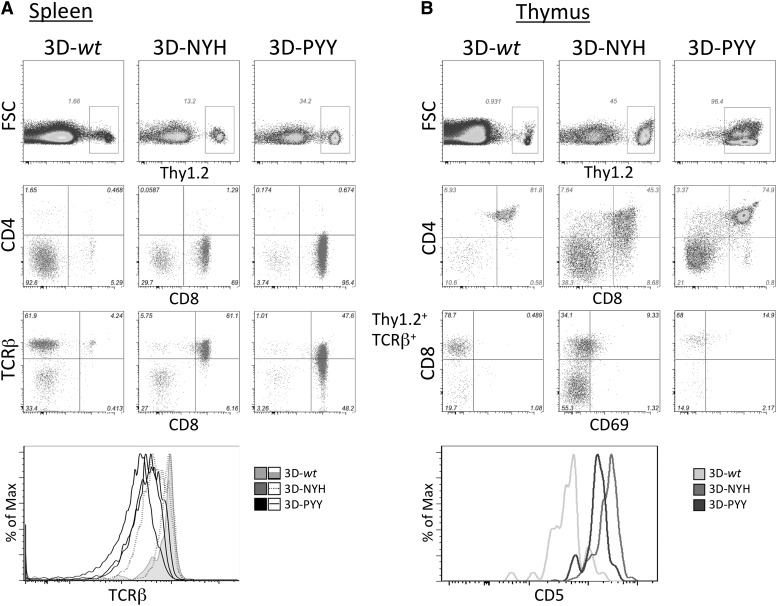
Because the NYH and PYY variants were substantially higher affinity than the highest-affinity clones we could isolate from the normal immune repertoire of B6 mice, we wanted to determine whether our inability to isolate such TCRs reflected deletion of T cells with such receptors by negative selection in the thymus. Therefore, retrogenic mice expressing the 3D TCR or its higher-affinity variants were generated by retroviral transduction of Rag2−/− BM-derived HSC with the TCR constructs followed by BM reconstitution of irradiated B6 host animals, as described by Holst et al.9 Six to 8 weeks after BM transfer, the spleen and thymus from these retrogenic mice were analyzed by flow cytometry (Figure 4).
Figure 4.
Analysis of retrogenic mice. 3D TCR-transduced Rag2−/− BM cells (Thy1.2) were transferred into irradiated Thy1.1 B6 recipients and analyzed more than 5 weeks posttransfer. (A) Splenocytes were analyzed for Thy1.2 expression by flow cytometry as a marker of donor-derived cells, which were gated and analyzed for CD4, CD8, and TCRβ expression. (B) Thymocytes from retrogenic mice were analyzed for Thy1.2 expression, and donor cells were then analyzed for CD4 and CD8 expression. Because the only marker of successful transduction was retrogenic TCR expression by the Rag2−/− cells, TCRβ+ cells were gated and analyzed for CD8, CD69, and CD5 expression.
Rag2−/− HSC transduced with each of the 3D TCR constructs all reconstituted peripheral T cells (Figure 4A). For each construct, TCR-transduced peripheral T cells were skewed to the CD8 lineage, with almost complete absence of CD4+ T cells. This is consistent with 3D TCR being a CD8-restricted receptor. Significantly fewer T cells were consistently observed in spleens of mice expressing the 3D-wt construct. This could be the result of either negative selection or inefficient positive selection. CD5 expression levels have been shown to correlate with the strength of the TCR signals thymocytes receive during thymic selection.18 Therefore, CD5 expression levels on thymocytes have been used to differentiate intrathymic events, with negative selection associated with high levels of CD5 and inefficient positive selection associated with low CD5 expression levels. We consistently observed low CD5 expression in 3D-wt retrogenic mice in both the thymic double-positive population (Figure 4B) and peripheral T cells (data not shown). This suggests that thymocytes developing in these retrogenic mice are receiving a suboptimal positive selection signal that contributes to the lower T-cell numbers detected in mice expressing the 3D-wt TCR. However, the mature CD8+ T cells that do develop appear to be otherwise phenotypically and functionally normal. A population of TCR+ CD4 CD8 double-negative cells is evident in both the thymus and spleen of all retrogenic mice: These cells develop in many TCR transgenic and retrogenic mice because of early expression of a mature αβTCR before β-selection.19 This population is likely more pronounced in 3D-wt retrogenic mice because conventional CD8 T cells are less abundant.
In male transgenic and retrogenic mice that express the male-reactive HY TCR in developing T cells, thymocyte selection was also altered because of self-reactivity of the TCR.9,20 A fraction of the self-reactive T cells was eliminated in males by negative selection, but a substantial proportion of thymocytes developed into CD8 single positive (SP) cells with decreased antigen sensitivity because of reduced TCR surface expression and downregulation of CD8 expression. To assess whether the enhanced-affinity TCRs exhibited similar aberrant maturation because of increased self-reactivity during selection, TCR and CD8 expression on retrogenic thymocytes was analyzed by flow cytometry (Figure 4A). Significantly decreased TCR expression was consistently observed in the CD8 SP population of 3D-PYY retrogenic mice, and variable reduction in TCR surface expression was observed in mice expressing the NYH variant, consistent with TCR signaling above the normal threshold during thymic selection. However, such TCR downregulation was not observed in CD8 SP thymocytes from mice expressing the 3D-wt TCR, suggesting that T cells that are successfully positively selected in these mice are able to then develop normally.
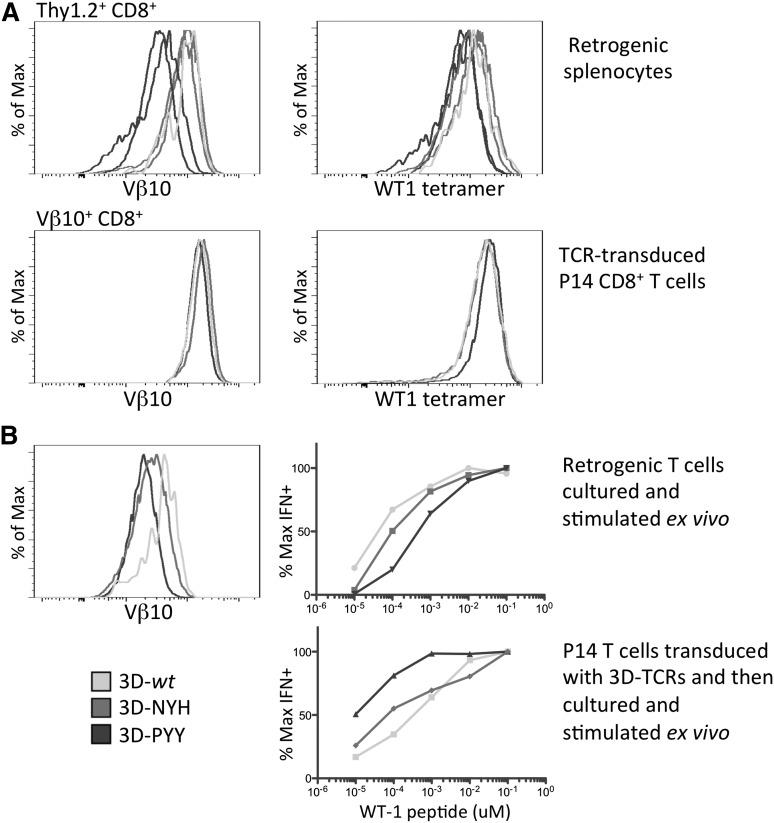
TCRβ variable region 10 (Vβ10) staining was used to compare TCR surface expression by the 3D TCR retrogenic T cells with WT1-specific TCR expression by mature P14 T cells transduced with the various 3D TCRs, as the latter is analogous to transduced patient T cells used for TCR gene therapy (Figure 5A). The level of Vβ10 staining was slightly higher on the transduced P14 T cells than on retrogenic T cells, perhaps reflecting activation during the transduction process. Although this difference in TCR levels was minimal between the 3D-wt retrogenics and transduced peripheral T cells, the enhanced affinity retrogenic T cells isolated from the periphery had substantially lower levels of TCR expression than transduced peripheral T cells. As a fraction of the TCRs expressed by the transduced peripheral T cells could constitute mispaired TCRs in which the introduced Vβ10 chain paired with the endogenous P14 TCRα chain, it was formally possible that transduced P14 T cells actually expressed similar or lower levels of the WT1-specific TCR than the retrogenic T cells. Therefore, both the retrogenic T cells and transduced peripheral T cells were stained with tetramer to assess the level of antigen-specific TCR on the surface of each. Tetramer staining matched the results with Vβ10 staining, with transduced peripheral T cells binding the highest level of tetramer, 3D-wt and -NYH retrogenic T cells binding only slightly less tetramer, and the highest-affinity 3D-PYY variant binding significantly lower tetramer.
Figure 5.
Functional analysis of retrogenic and transduced peripheral T cells. (A) Retrogenic splenocytes expressing each of the TCRs gated on donor Thy1.2+CD8+ cells, and TCR-transduced P14 splenocytes gated on transduced (Vβ10+) CD8+ T cells, were analyzed for expression levels of the retrogenic TCRβ chain, Vβ10, and for tetramer binding. Data are representative of at least 16 mice per TCR. Two samples for each enhanced affinity receptor are included to show the typical range of variation between mice. (B) Retrogenic T cells and TCR-transduced P14 T cells were stimulated with WT1 peptide and expanded for 1 week in vitro and then assayed for functional avidity by measuring interferon γ production in response to Ag-presenting cells pulsed with decreasing concentrations of WT1 peptide. TCR surface expression is shown for each T-cell population at the time the functional assay was performed.
The decreased level of TCR surface expression and tetramer staining by the highest-affinity 3D-PYY TCR variants suggested that T cells in retrogenic mice expressing this TCR might have a similar or lower avidity for APC/targets expressing the WT1 antigen than cells from mice expressing the wt TCR, even though the TCR itself has substantially higher affinity for WT1 antigen. To compare the functional avidity of the mature CD8+ T cells, antigen-specific splenic T cells were expanded by a single peptide stimulation in vitro and analyzed for responsiveness to APCs (splenocytes) pulsed with decreasing concentrations of WT1 peptide on day 6 (Figure 5B). Retrogenic T cells expressing the 3D-wt and 3D-NYH variants had similar functional avidities (a similar proportion of T cells produced cytokines in response to each concentration of peptide), whereas retrogenics expressing the 3D-PYY variant demonstrated a lower avidity for WT1 peptide-pulsed target cells, requiring higher peptide concentrations to induce similar responses. In contrast, P14 peripheral CD8+ T cells transduced with the 3D-PYY TCR displayed a ∼10-fold increase in antigen sensitivity compared with the 3D-wt or 3D-NYH TCR constructs. To affirm that peripheral TCR downregulation observed in retrogenic mice expressing the enhanced-affinity 3D-PYY TCR is not unique to the 3D TCR and its variants, but rather reflects a more general property of enhanced affinity TCRs to self-antigens, we repeated these analyses with wild-type and enhanced-affinity TCRs specific for MSLN, another promising tumor-associated antigen (supplemental Figure 1), and obtained similar results.
Discussion
Many of the most promising tumor-associated antigens currently being targeted by adoptive T-cell therapy or TCR gene therapy trials are overexpressed, unmodified self-antigens.21 Because the T cells/TCRs being evaluated are generally derived from the peripheral T-cell pool of individuals who express these target molecules as self-antigens in normal tissues and the thymus, many have a relatively low affinity for the target antigen. Because T cells that express higher-affinity TCRs are generally more effective at eliminating tumor cells than T cells expressing lower-affinity TCRs for the same antigen,12,22,23 strategies to enhance the affinity of tumor-antigen-specific TCRs are being pursued to increase the activity of transduced T cells for tumor cells expressing the target antigen.4,5,24,25
WT1 is expressed at high levels by many solid and hematological tumors and is detected at low levels by a few normal cell types.26,27 We isolated 2 variants of a murine WT1-specific TCR from a saturation mutagenesis screen that have enhanced affinity for WT1, resulting in CD8-independent binding of these TCRs to peptide/MHC multimers. These modified WT1-specific TCRs confer a significantly enhanced functional avidity to TCR-transduced peripheral T cells compared with the parental 3D TCR. However, when transferred in vivo into wt mice, T cells transduced with these enhanced-affinity TCRs did not undergo autoimmune activation and did not mediate overt autoimmune pathology.
Potentially autoreactive T cells may remain ignorant of peripheral tissue expression of the self-antigen and only became dangerous when activated in the more immunogenic context of a robust immune response against an external pathogen.28-30 However, we tested whether tissue damage could be induced by immunizing mice that received transferred high-affinity TCR-transduced T cells with a recombinant strain of LM expressing the target WT1 antigen and found that even after expanding in response to such inflammatory in vivo stimulation, treated mice did not display T-cell-mediated tissue damage or T-cell infiltration in organs known to normally express WT1. The lack of T-cell-mediated autoimmunity after transfer of T cells expressing a high-affinity WT1-specific TCR, even in the inflammatory setting of LMWT1, likely reflects the limited tissue distribution of WT1 in normal hosts and the relatively low-level expression of this self-antigen by normal tissues,27 particularly as compared with the transgenic antigens that have been studied.28-30
The finding that T cells expressing enhanced-affinity WT1-specific TCRs can respond to highly immunogenic infected targets in vivo without causing autoimmunity to normal tissue targets expands on observations that WT1-specific CD8+ T cells from the normal repertoire can differentiate between the low levels of WT1 expressed by normal self-tissue and the higher levels expressed by tumor cells.31-34 Importantly, T cells expressing enhanced-affinity WT1-specific TCRs in our studies did not mediate on-target autoimmune reactivity against normal tissues that express low levels of WT1, nor did the enhanced-affinity TCRs mediate any detectable off-target effects resulting from cross-reactivity with other self-antigens. The absence of cross-reactivity from either of the enhanced affinity variants may in part reflect our restriction of modifications to the α chain CDR3, precluding changes to the CDR1 and CDR2 domains that interact primarily with the MHC helices.35 These results support the potential safety of this approach for increasing the affinity of tumor-reactive TCRs.
The generation of retrogenic mice has proven useful for quickly analyzing the in vivo function and thymic selection of TCRs from antigen-specific T-cell clones.36,37 One caveat of this approach is that, as T cells are positively selected with different levels of efficiency in the thymus, it is difficult to predict how well any TCR will be selected, as this requires low-affinity interactions with unknown self-peptides in the thymic cortex. This may be particularly problematic for enhanced-affinity TCRs, which are not selected on the basis of secondary interactions with low-affinity self-peptides, and thus may not have sufficiently high affinity for any self-antigen in the thymus to adequately drive positive selection, despite having enhanced affinity for the cognate antigen.
We observed substantial variation in the efficiency of positive selection between the TCRs employed in this study, as also reported in other retrogenic mice.36,37 For the 3D TCR, the capacity of the parental 3D-wt to support positive selection appeared quite low. Such positive-selecting ligands are also responsible for maintenance, survival, and homeostatic proliferation of naive T cells in the periphery.38-40 In the case of the 3D-NYH and 3D-PYY TCRs, modifications to the 3D CDR3α appear to have increased the affinity for positive selecting ligand(s) compared with the 3D-wt TCR, However saturation mutagenesis of CDR3 regions is just as likely to decrease the efficacy of these interactions. This would represent another situation in which TCR gene therapy provides an opportunity to use a high-affinity TCR that might otherwise be unavailable in the normal repertoire not because of problematic self-reactivity but, rather, because of an inability to be positively selected and/or to persist in the periphery. Because only naive T cells are dependent on low-affinity interactions for survival, an affinity-enhanced TCR that does not have an adequate affinity for a positively selecting ligand could still mediate effective anti-tumor activity when transduced into activated peripheral T cells. These observations serve to highlight the fact that retrogenic mice generated with affinity-modified TCRs can provide unique insights into the biology of the TCR in the context of both positive and negative selection during normal T-cell development.
We have found that for both WT1- and MSLN-specific TCRs, high-affinity retrogenic T cells in the periphery can respond to antigen, but with a functional avidity reduced at or below the threshold level of the highest-avidity naturally occurring T cells in the normal repertoire (Figure 5 and supplemental Figure 1). This retention of function, and evidence that the cells retain a naive phenotype (data not shown), supports the notion that the TCR down-modulation observed with TCRs specific for WT1 occurs during antigen exposure in the thymus and is not the result of antigen recognition in the periphery. This finding has implications for clinical strategies based on TCR gene transfer to HSCs, suggesting that peripheral T cells generated through this approach could potentially be deleted or exhibit attenuated antigen sensitivity if the antigen being targeted is expressed at high levels in the thymus. Indeed, TCR down-modulation by surviving thymocytes that emigrate to the periphery appears to be a common feature in both transgenic and retrogenic mice expressing a TCR that surpasses an affinity threshold set during thymic selection.9,34,37,41,42 In this regard, it might prove useful to isolate naive T cells from the repertoire based on expression of lower surface levels of TCR, as generating antigen-reactive cells from this population might yield naturally occurring TCRs that have a higher affinity for tumor/self-antigens, which could then be harnessed for TCR gene therapy in transduced peripheral T cells. Such a strategy has the potential to circumvent the risks and technologies associated with generating modified TCRs for expression in T cells.
These studies suggest that TCR gene therapy with enhanced-affinity TCRs can be a safe and effective approach for increasing the efficacy of anti-tumor adoptive T-cell therapies that are currently being developed to treat human malignancies. TCRs that are affinity-enhanced sufficiently to become CD8-independent may also be used to recruit the activity of CD4+ T cells against class 1 HLA-restricted tumor antigens. This approach can further increase anti-tumor activity by enhancing the survival and long-term persistence of tumor-reactive effector T cells.43 These murine studies provide insights into the biology of enhanced-affinity TCRs targeting antigens such as WT1. However, human clinical trials will need to be done to fully define the safety and efficacy of this approach.
Supplementary Material
Acknowledgments
The authors thank Tom Dubensky and Pete Lauer from Aduro BioTech for kindly providing us with the LMwt1 strain of Listeria monocytogenes.
This work was supported by grants from the National Institutes of Health National Cancer Institute (P01 CA18029 and R01 CA33084 to P.D.G. and P01 CA97296 to D.M.K.), a grant from the Korean Research Institute of Bioscience and Biotechnology (to P.D.G.), and a grant from the Melanoma Research Alliance (to D.M.K.). T.M.S. is supported by the Doug and Maggie Walker Postdoctoral Fellowship.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.M.S. designed and performed the research and wrote the manuscript; D.H.A., I.M.S., S.A.R., and M.L.D. designed and performed experiments; D.M.K. designed experiments and interpreted results; and P.D.G. designed the experiments, interpreted results, and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Phil Greenberg, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, Seattle, WA 98109, Mail Stop D3-100.
References
- 1.Schmitt TM, Ragnarsson GB, Greenberg PD. T cell receptor gene therapy for cancer. Hum Gene Ther. 2009;20(11):1240–1248. doi: 10.1089/hum.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falkenburg WJJW, Melenhorst JJJ, van de Meent MM, et al. Allogeneic HLA-A*02-restricted WT1-specific T cells from mismatched donors are highly reactive but show off-target promiscuity. J Immunol. 2011;187(5):2824–2833. doi: 10.4049/jimmunol.1100852. [DOI] [PubMed] [Google Scholar]
- 3.Chervin AS, Aggen DH, Raseman JM, Kranz DM. Engineering higher affinity T cell receptors using a T cell display system. J Immunol Methods. 2008;339(2):175–184. doi: 10.1016/j.jim.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins PF, Li YF, El-Gamil M, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180(9):6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Moysey R, Molloy PE, et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23(3):349–354. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 6.Ariyaratana S, Loeb DM. The role of the Wilms tumour gene (WT1) in normal and malignant haematopoiesis. Expert Rev Mol Med. 2007;9(14):1–17. doi: 10.1017/S1462399407000336. [DOI] [PubMed] [Google Scholar]
- 7.Scharnhorst V, van der Eb AJ, Jochemsen AG. WT1 proteins: functions in growth and differentiation. Gene. 2001;273(2):141–161. doi: 10.1016/s0378-1119(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 8.Simpson AJG, Caballero OL, Jungbluth A, Chen Y-T, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5(8):615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 9.Holst J, Vignali KM, Burton AR, Vignali DA. Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods. 2006;3(3):191–197. doi: 10.1038/nmeth858. [DOI] [PubMed] [Google Scholar]
- 10.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11(3):299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 11.Letourneur F, Malissen B. Derivation of a T cell hybridoma variant deprived of functional T cell receptor α and β chain transcripts reveals a nonfunctional α-mRNA of BW5147 origin. Eur J Immunol. 1989;19(12):2269–2274. doi: 10.1002/eji.1830191214. [DOI] [PubMed] [Google Scholar]
- 12.Chervin AS, Stone JD, Holler PD, Bai A, Chen J, Eisen HN, Kranz DM. The impact of TCR-binding properties and antigen presentation format on T cell responsiveness. J Immunol. 2009;183(2):1166–1178. doi: 10.4049/jimmunol.0900054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18(2):255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 14.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126(2):165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191(2):335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue K, Ogawa H, Sonoda Y, et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997;89(4):1405–1412. [PubMed] [Google Scholar]
- 17.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 18.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrence K, Pavlovich CP, Matechak EO, Fowlkes BJ. Premature expression of T cell receptor (TCR)alphabeta suppresses TCRgammadelta gene rearrangement but permits development of gammadelta lineage T cells. J Exp Med. 2000;192(4):537–548. doi: 10.1084/jem.192.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egawa T, Kreslavsky T, Littman DR, von Boehmer H. Lineage diversion of T cell receptor transgenic thymocytes revealed by lineage fate mapping. PLoS ONE. 2008;3(1):e1512. doi: 10.1371/journal.pone.0001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid DA, Irving MB, Posevitz V, et al. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol. 2010;184(9):4936–4946. doi: 10.4049/jimmunol.1000173. [DOI] [PubMed] [Google Scholar]
- 23.Johnson LA, Heemskerk B, Powell DJ, Jr, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177(9):6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Bennett AD, Zheng Z, et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol. 2007;179(9):5845–5854. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holler PD, Holman PO, Shusta EV, O’Herrin S, Wittrup KD, Kranz DM. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc Natl Acad Sci USA. 2000;97(10):5387–5392. doi: 10.1073/pnas.080078297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Schalling M, Bernard A, et al. The Wilms tumour gene WT1 is expressed in murine mesoderm-derived tissues and mutated in a human mesothelioma. Nat Genet. 1993;4(4):415–420. doi: 10.1038/ng0893-415. [DOI] [PubMed] [Google Scholar]
- 27.Lee SB, Haber DA. Wilms tumor and the WT1 gene. Exp Cell Res. 2001;264(1):74–99. doi: 10.1006/excr.2000.5131. [DOI] [PubMed] [Google Scholar]
- 28.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25(2):261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi PS, Oehen S, Buerki K, et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 31.Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96(4):1480–1489. [PubMed] [Google Scholar]
- 32.Oka Y, Udaka K, Tsuboi A, et al. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol. 2000;164(4):1873–1880. doi: 10.4049/jimmunol.164.4.1873. [DOI] [PubMed] [Google Scholar]
- 33.Gao L, Xue SA, Hasserjian R, et al. Human cytotoxic T lymphocytes specific for Wilms’ tumor antigen-1 inhibit engraftment of leukemia-initiating stem cells in non-obese diabetic-severe combined immunodeficient recipients. Transplantation. 2003;75(9):1429–1436. doi: 10.1097/01.TP.0000061516.57346.E8. [DOI] [PubMed] [Google Scholar]
- 34.Pospori C, Xue S-A, Holler A, et al. Specificity for the tumor-associated self-antigen WT1 drives the development of fully functional memory T cells in the absence of vaccination. Blood. 2011;117(25):6813–6824. doi: 10.1182/blood-2010-08-304568. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 36.Alli R, Nguyen P, Geiger TL. Retrogenic modeling of experimental allergic encephalomyelitis associates T cell frequency but not TCR functional affinity with pathogenicity. J Immunol. 2008;181(1):136–145. doi: 10.4049/jimmunol.181.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton AR, Vincent E, Arnold PY, et al. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes. 2008;57(5):1321–1330. doi: 10.2337/db07-1129. [DOI] [PubMed] [Google Scholar]
- 38.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11(2):183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11(2):173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 40.Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10(5):559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 41.Ohlén C, Kalos M, Cheng LE, et al. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195(11):1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kisielow P, Blüthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 43.Soto CM, Stone JD, Chervin AS, Engels B, Schreiber H, Roy EJ, Kranz DM. MHC-class I-restricted CD4 T cells: a nanomolar affinity TCR has improved anti-tumor efficacy in vivo compared to the micromolar wild-type TCR. Cancer Immunol Immunother. 2013;62(2):359–369. doi: 10.1007/s00262-012-1336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.