Abstract
Volunteer peer leaders (PLs) benefit from their involvement in health interventions but we know little about how they compare with other non-PL volunteers or with the intervention recipients themselves. We randomized 58 veterans’ service organizations’ posts (e.g. VFW) to peer- versus professionally led self-management support interventions. Our primary research questions were whether hypertensive PLs changed over the course of the project, whether they changed more than hypertensive volunteers who were not randomized to such a role [i.e. post representatives (PRs)] and whether they changed more than the intervention recipients with respect to health knowledge, health beliefs and health outcomes from baseline to 12 months. After the intervention, PLs provided open-ended feedback and participated in focus groups designed to explore intervention impact. Hypertensive PLs improved their systolic blood pressure and hypertension knowledge and increased their fruit/vegetable intake and pedometer use. We found no differences between PLs and PRs. PLs improved knowledge and increased fruit/vegetable intake more than intervention recipients did; they provided specific examples of personal health behavior change and knowledge acquisition. Individuals who volunteer to be peer health leaders are likely to receive important benefits even if they do not actually take on such a role.
Introduction
Peer-led health promotion models have been used to address a variety of chronic health conditions, such as diabetes, heart disease and human immunodeficiency virus [1–3]. Most published studies have found that peer-led interventions improve health knowledge, self-efficacy, health behaviors and health-related quality of life among populations at risk for or living with various chronic health conditions [4–8]. For example, in a randomized trial among persons with diabetes, Lorig et al. [5] demonstrated that those assigned to the Chronic Disease Self-Management Program improved several self-management skills, including reading nutrition labels, self-monitoring glucose control, engaging in aerobic exercise and communicating with physicians, more than controls.
In contrast, few studies explore how peer-led health interventions affect the health and well-being of the peer leaders (PLs) themselves. There is evidence that being a PL increases health knowledge, skills, self-efficacy and intention to improve health behaviors [9–11]. For example, Birnbaum et al. [12] found that PLs increased fruit and vegetable consumption by almost a full serving with the context of a nutrition intervention. Nine months after having completed their participation in a peer-led HIV prevention and education intervention, PLs demonstrated significant improvements in HIV knowledge and increases in self-reported HIV testing [13]. Although intervention recipients also experienced improved knowledge and behavioral change outcomes, no comparisons were made between PLs and the recipients on these measures. Our review of the literature suggests that PLs experience important cognitive and behavioral benefits. However, few studies report whether PLs benefit more than intervention participants. Moreover, since PLs are typically self-selected, it is unclear if their improvement simply reflects their intrinsic motivation to address their health. Therefore, we used data from a large community-based randomized peer-led trial to determine whether hypertensive PLs changed over the course of the project, whether they changed more than hypertensive individuals who volunteered to be PLs but who were randomly assigned to a non-PL role (whom we call post representatives [PRs]), and whether they changed more than the intervention recipients (whom we call post members [PMs]) with respect to health, health knowledge, patient activation, self-efficacy and health behavior.
Materials and methods
Study setting
We conducted the present cluster randomized controlled trial among local Veterans Service Organization posts (e.g. American Legion, Veterans of Foreign Wars) in Southeastern Wisconsin to compare two approaches to encourage hypertension self-management: (i) a peer-led intervention that occurred in the context of monthly post meetings and (ii) an educational seminar intervention covering similar information in stand-alone 90-min presentations by health professionals. Posts have formal monthly meetings led by elected PMs (i.e. commanders). Post membership varies widely in number and the proportion of members who regularly attend meetings is typically small (10–25%). For example, the number of veterans attending the monthly meetings of posts participating in our peer-led hypertension intervention ranged from 5 to 65 (mean = 22.5, SD = 13.5). We recruited posts within a 60-mile radius of our VA medical center. Given our large catchment area, we grouped posts into five geographic regions to make their attendance at meetings more convenient. We used computer-generated random numbers to assign posts to the two interventions, ensuring that half of the posts in each of five regions were assigned to each arm.
The trial was reviewed and approved by the Human Studies Subcommittee (institutional review board) at the Clement J. Zablocki VA Medical Center. We invited 218 posts to participate in a randomized clinical trial of two approaches to encourage effective self-management. In total, 58 posts agreed to participate and they identified appropriate volunteer leaders (VLs; see Fig. 1). VLs were PMs with or without a history of hypertension who agreed to serve the project but did not know the role to which they would be assigned until posts were later randomized to one of two conditions (described below). We met with VLs in small groups to review the research project and describe their potential roles. VLs recruited PMs with hypertension for an evaluation of the effect of the interventions on hypertension self-management and control. When all PMs had been recruited, we randomized the posts (n = 58) and thereby the VLs (n = 114) to either a peer-led or professional education approach. As a result of randomization, 58 volunteers became PLs (representing 30 posts) and 56 volunteers became PRs (representing 28 posts); these roles are described below. PLs and PRs could also participate in this study if hypertensive; 77.2% of PLs and 64.3% of PRs did so. Their data were used for outcome comparison purposes in the present study.
Fig. 1.
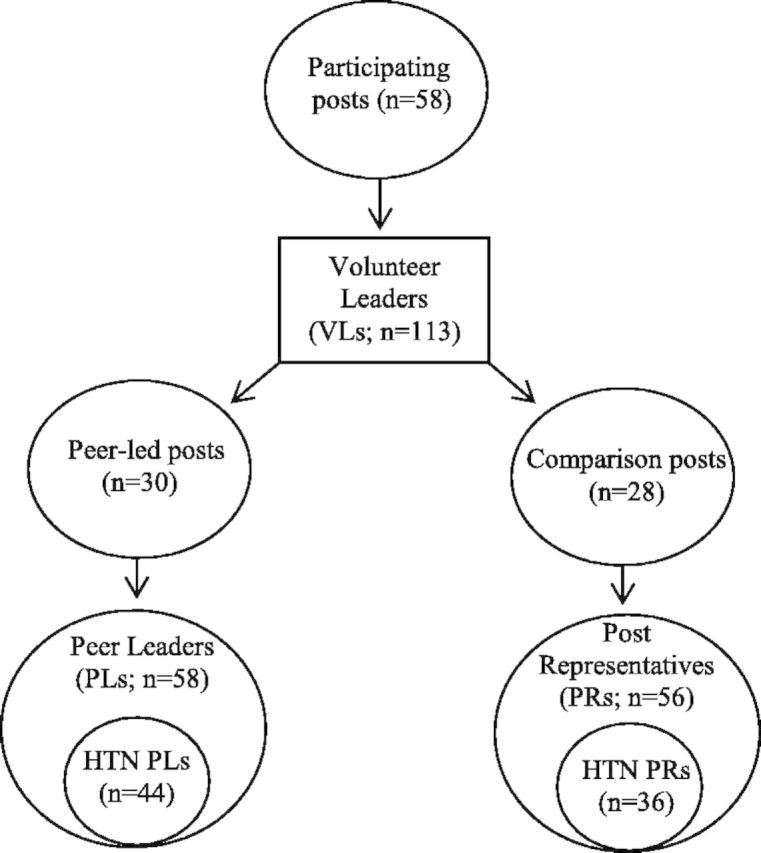
Randomization of posts and volunteer leaders.
Interventions
All posts participating in the study received a digital bathroom scale, six pedometers and two automated sphygmomanometers to facilitate self-monitoring. At the small group meetings during which time their potential study roles were explained (i.e. before they had been randomized to PL versus PR status), study staff demonstrated use of the self-monitoring equipment. The VLs were asked to look after the equipment and encourage its use at post meetings or in the case of pedometers between post meetings. After this point, the activities of PRs and PLs differed in ways that will be described below.
Educational seminar intervention arm
Shortly after randomization, the PRs met in small groups with the study team to plan three educational sessions regarding hypertension and related issues. PRs were then asked to advertise the date, time and locations of these sessions at post meetings. The three sessions, which each lasted ∼90 min and were led by the principal investigator, were repeated six times around southeastern Wisconsin so that at least one was convenient to members of all participating posts. Despite these efforts, sessions were poorly attended, except by PRs (∼5% of those in attendance were PMs). PRs in attendance were encouraged to bring educational materials from the sessions to subsequent post meetings so that members who did not attend could review these materials. Thus, total PR contact time with study staff was ∼8 h, and many PRs spent additional time conveying information about the program to their PMs.
Peer-led intervention arm
Two underlying theories guided the implementation of our peer-led intervention arm. The Chronic Disease Model [14] suggests that an informed activated patient is needed for optimal chronic disease management. The Health Belief Model [15] suggests that people’s willingness to make healthy behavioral choices is dependent on appropriate beliefs about illness severity and individuality susceptibility; their assessment of the relative costs and benefits of making the choice; and cues to action. In our peer-led intervention, we focused on the importance of peer support, a key reinforcing factor that can act as a facilitator or a barrier to behavior change. Moreover, PLs addressed deficits in knowledge (e.g. risks associated with poor hypertension control, information about how behavior can affect blood pressure [BP] control) to influence beliefs about severity and susceptibility. We provided tools to reduce the costs associated with self-management practices, such as providing the posts with BP monitors, weight scales and pedometers. Thus, PL presentations and self-management tools provided by the project (e.g. BP monitors, weight scales and pedometers) which were used in ‘health corners’ and which will be described shortly served as important cues to action.
PLs went through extensive training and took a more active role in delivering information to their fellow PMs than PRs did. Groups of PLs from geographically clustered posts first attended an 8-h orientation session during which time study staff introduced information about hypertension and hypertension self-management and described the PL role. Specifically, we provided information about effective presentation strategies, methods for working effectively with co-PLs and the post commander, and how study staff would support them. During the monthly post meeting following this session, PLs educated PMs about the importance of self-monitoring. They also created post ‘health corners’ (i.e. tables or bulletin boards displaying health-related posters and brochures). To that end, PMs who were in the PL group received 12 topics (∼1 topic/month for 1 year). The average length of each presentation was 12 min. Including the time that PMs spent using health corner equipment and/or asking health-related questions of their PLs, we estimate the PMs experienced ∼3 h of direct project contact time.
PLs attended a series of eight 90- to 120-min ‘mini-training sessions’ held monthly for the first 4 months and then bimonthly for an additional 8 months, resulting in 20–24 h of training (specific components of the training program can be found at http://www.milwaukee.va.gov/Power/Power.asp). During each mini-training sessions, we provided PLs with information and materials related to a specific health topic, such as physical activity, medication adherence or the importance of knowing one’s medications. For each topic, we gave them a presentation script, pertinent handouts and related tools (e.g. exercise bands, pill minders, wallet medication cards) to be delivered to PMs at a subsequent post meeting. We used a variety of educational techniques in the mini-training sessions (e.g. didactic, think–pair–share and role-playing exercises) and the PI responded directly to health and medical questions PLs had. We asked PLs to deliver the presentations at their posts’ monthly meetings and to encourage all PMs to participate in study activities, regardless of whether they were in the evaluation study. PLs who were unable to attend a given training session were provided the same detailed script to enable them to deliver the prescribed topic at a subsequent post meeting. Individual PLs attended between 22% and 100% of the training sessions (mean = 87.6%; mode = 100%). We lost n = 7 PLs throughout the program (due to a lack of available time, health problems, relocation and death).
Data collection
At study entry, research assistants measured hypertensive participants’ (N = 404, including n = 44 PLs, n = 36 PRs and n = 153 PMs) BP and weight using calibrated equipment and standard techniques. They used validated instruments to measure sodium intake, fruit and vegetable intake, self-reported patient activation, social support, self-efficacy and hypertension knowledge [16–21]. This procedure was repeated 6 and 12 months after the intervention began. Given our interest in capturing longer term change, only baseline and 12-month survey data (which represented the end of the study) were used for this study. All survey data were managed using REDCap electronic data capturing tools [22] and analyzed in SAS. Data were reviewed every 6 months for missing values and shifted responses, and after correction, 10% of the data were randomly selected for re-entry to verify data entry accuracy. The error rate at each step was estimated to be no more than 0.5% per item entered before additional errors found on double entry were corrected.
PLs and PRs contributed additional data. First, at the time volunteers consented to participate as either PLs or PRs, they completed a brief survey regarding demographics, predetermined factors we thought might affect their success (e.g. prior medical training) and open-ended responses related to reasons they offered to serve as PLs. These data were then coded for content and five key reasons for volunteering were identified. Second, near the conclusion of the last MTS, PLs were asked to provide written feedback about what they learned through the project and one behavior they might either start or continue to do in the 6 months subsequent to the end of the POWER project. Third, PL performance was evaluated by PL self-reports of presentation activities and project evaluator inspections of post health corners and observations of two post meetings where PLs presented a health topic. Fourth, after the intervention had concluded, we organized and conducted four focus groups each consisting of 8–13 PLs. Within each focus group, we purposively selected members that were diverse in terms of military background, gender, and positive and negative experiences with the program as determined by study staff observations and participant interactions. Focus group participants (n = 38, 84.2% male) were asked about the relative value of the program, strategies that worked best for the PLs to engage PMs and to recommend suggestions to improve the program. Focus group audio recordings were transcribed for data analysis. For the purposes of this study, we present data related to PLs’ reflections on the impact the program had on themselves.
Data analyses
We first calculated descriptive statistics for PLs and PRs at baseline. We then calculated descriptive statistics for weight, BP, self-reported fruit and vegetable intake, pedometer use and sodium intake; and self-reported patient activation, social support, self-efficacy and hypertension knowledge for the following: (i) hypertensive PLs (n = 44); (ii) hypertensive PRs (n = 36) and (iii) PMs who were study participants at a PL post at which one or more PL was hypertensive (n = 153) at both baseline and 12 months. Although there was some study attrition with respect to PLs during the course of the study (as described earlier), there was no attrition among hypertensive PLs or PRs during the study period and thus data analyses comparing these groups or comparing PLs with PMs included data from all original VLs.
To determine whether hypertensive PLs changed over the course of the project, whether they changed more than hypertensive PRs and whether they changed more than hypertensive PMs with respect to health, health knowledge, patient activation, self-efficacy and health behavior, we used Wilcoxon sign controlling for baseline values for each variable. A generalized mixed model with random effect for post and fixed effect for VL was used to account for the cluster-sampled data in the study. An independence covariance structure was used, which assumes that posts are statistically independent. Least-square means estimates were used to estimate mean changes for PLs, PRs and PMs and for the differences in changes between PLs and PRs and PLs and PMs. A significance level of 0.05 was used for all comparisons.
We calculated descriptive statistics in order to describe focus group participants. With respect to our evaluation open-ended survey and focus group data, we used standard qualitative content analysis procedures to identify themes. For the present study, we conceptually organized themes related to the effects the intervention had on the PLs. Relevant and representative quotations were extracted for illustrative purposes.
Results
Table I illustrates comparisons between PLs and PRs with respect to sociodemographics at baseline. A more detailed description of PMs has been reported elsewhere [23]. There was no difference between PLs and PRs in terms of gender, medical training, occupational status (i.e. retired or working), age or involvement in the evaluation study, which served as a proxy for a diagnosis of hypertension. PRs differed from PLs with respect to their reasons for volunteering. Specifically, PRs were more likely to report having an interest in the topic of hypertension than were PLs. Health and health behavior at baseline for hypertensive PLs, PRs and PMs are shown in Table II. In addition to the relative homogeneity of sociodemographic characteristics comparing PLs to PRs reported in Table I, we found no significant differences in baseline BP, weight, health behaviors or psychological characteristics between PLs and PRs who participated in the study.
Table I.
Baseline comparisons of PLs and PRs
| PLs (n = 58) | PRs (n = 56) | P-value* | |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 48 (82.8) | 51 (91.1) | 0.19 |
| Female | 10 (17.2) | 5 (8.9) | |
| Age in years, mean (SD); ranged from 36 to 84 years | 62.8 (11.7) | 62.9 (11.06) | 0.96 |
| Years of Veterans Service Organization involvement, mean (SD) | 17.5 (12.2) | 16.5 (13.3) | 0.69 |
| Diagnosed with hypertension, n (%) | 44 (74.1) | 36 (62.5) | 0.18 |
| Medical background (e.g. paramedic, physician), n (%) | 21 (36.2) | 15 (26.8) | 0.28 |
| Occupation status, n (%) | |||
| Retired | 35 (60.3) | 37 (66.1) | 0.33 |
| Working for pay | 20 (34.5) | 14 (25.0) | |
| Education, n (%) | |||
| High school diploma, GED or less | 8 (14.1) | 14 (25.0) | 0.12 |
| Some college | 21 (36.8) | 23 (41.1) | |
| Earned college degree | 18 (31.6) | 14 (25.0) | |
| Some graduate training or degree | 8 (14.1) | 2 (3.6) | |
| Reasons for volunteering, n (%) | |||
| Was asked to volunteer | 5 (9.3) | 2 (3.8) | 0.25 |
| To help the post | 35 (64.8) | 25 (47.2) | 0.07 |
| Personal health reasons | 13 (24.1) | 15 (28.3) | 0.62 |
| To share my expertise | 2 (3.7) | 2 (3.8) | 0.99 |
| Interest in the topic of hypertension | 10 (18.5) | 21 (39.6) | 0.02 |
*P-values based on the appropriate statistical test (e.g. chi-square tests of independence of independent samples t-tests). Significance at P < 0.05 level. The value in bold is significant.
Table II.
Baseline health and health behavior statuses for hypertensive PLs, PRs and PMsa
| PLs (n = 44) | PRs (n = 36) | PMs (n = 153) | |
|---|---|---|---|
| Weight | 223.6 (50) | 207.9 (36) | 200.7 (39) |
| Systolic BP | 132.1 (13.5) | 128.1 (14.2) | 131.8 (14) |
| Diastolic BP | 74.6 (8.6) | 73.3 (9.7) | 70.0 (11) |
| Fruit and vegetablesb (servings/day) | 3.6 (1.6) | 3.1 (1.4) | 3.6 (1.6) |
| Pedometer useb | 1.4 (1.1) | 0.9 (1.0) | 0.6 (0.8) |
| Sodium intakeb | 5.1 (2.2) | 5.4 (2.0) | 5.2 (2.0) |
| Patient activationb | 62.2 (14.5) | 55.4 (10.9) | 57.8 (14) |
| Self-efficacyb | 32.7 (3.9) | 30.8 (4.1) | 31.5 (3.5) |
| Hypertension knowledgeb | 9.6 (1.9) | 9.2 (2.4) | 8.5 (2.2) |
aData for the subset of PLs and PRs who were hypertensive and completed study measures and the post members who received the intervention and whose PLs were hypertensive and completed study measures. Mean scores are reported; SDs are in parentheses. bHigher scores reflect better outcomes.
With respect to the question of PL health, health knowledge, patient activation, self-efficacy and health behavior changes from baseline to 12 months (i.e. the conclusion of the intervention), hypertensive PLs lowered their systolic BP by 3.93 mmHg (P = 0.04), increased their daily intake of fruits and vegetables by 0.55 servings (P = 0.02), reported more frequent use of a pedometer to monitor daily physical activity (P = 0.01) and improved their knowledge about hypertension (P < 0.001, see Table III). With respect to changes across groups, no significant differences were observed when comparing hypertensive PLs and hypertensive PRs (Table IV). Hypertensive PLs reported greater improvement in daily fruit and vegetable consumption by over one-half serving per day when compared with their hypertensive PMs (mean = 0.57, SE = 0.25, P = 0.03). Hypertensive PLs also improved hypertension knowledge more than their hypertensive PMs (mean = 0.82, SE = 0.32, P = 0.01; see Table V).
Table III.
PL health and health behavior change over duration of POWER projecta
| Baseline | 12 Month | Δ12 Month* | |
|---|---|---|---|
| Weight | 223.6 (50) | 221.7 (44) | −1.90 |
| Systolic BP | 132.1 (13.5) | 128.1 (4.2) | −3.90 |
| Diastolic BP | 74.6 (8.6) | 73.3 (9.7) | −1.30 |
| Fruit and vegetablesb (servings per day) | 3.60 (1.6) | 4.10 (1.9) | +0.55 |
| Pedometer useb | 1.40 (1.1) | 1.80 (1.1) | +0.48 |
| Sodium intake | 5.10 (2.2) | 5.10 (1.7) | 0.00 |
| Patient activationb | 62.2 (14.5) | 63.6 (15.2) | +1.32 |
| Self-efficacyb | 32.7 (3.9) | 33.4 (4.3) | +0.67 |
| Hypertension knowledgeb | 9.60 (1.9) | 10.8 (1.9) | +1.17 |
aData are only available for the subset of PLs who participated in the study (n = 44). Mean scores are reported; SDs are in parentheses. bHigher scores reflect better outcomes.
*P-value based on Wilcoxon–Mann–Whitney tests; significance criterion was P < 0.05. Values in bold are significant.
Table IV.
Comparison of 12-month differences in health and health behavior change between PLs and PRs
| PLs (n = 44) | PRs (n = 36) | P-value* | |
|---|---|---|---|
| Weight | −1.90 | +1.83 | 0.18 |
| Systolic BP (mmHg) | −3.93 | −3.79 | 0.60 |
| Diastolic BP (mmHg) | −1.30 | −0.82 | 0.95 |
| Fruit and vegetablesa | +0.55 | +0.32 | 0.36 |
| Pedometer usea | +0.48 | +0.72 | 0.34 |
| Sodium intakea | 0.00 | +0.03 | 0.81 |
| Patient activationa | +1.3 | +4.5 | 0.37 |
| Self-efficacya | +0.67 | +1.45 | 0.35 |
| Hypertension knowledgea | +1.17 | +0.42 | 0.18 |
aHigher scores reflect better outcomes.
*P-value based on Wilcoxon–Mann–Whitney tests; significance criterion was P < 0.05.
Table V.
Comparison of 12-month differences in health and health behavior change between PLs and PMs
| PLs (n = 44) | PMs (n = 153) | P-value* | |
|---|---|---|---|
| Weight | −1.90 | −0.19 | 0.34 |
| Systolic BP (mmHg) | −3.93 | −2.85 | 0.66 |
| Diastolic BP (mmHg) | −1.30 | −1.57 | 0.79 |
| Fruit and vegetablesa | +0.55 | −0.02 | 0.03 |
| Pedometer usea | +0.48 | +0.60 | 0.51 |
| Sodium intakea | 0.00 | −0.22 | 0.52 |
| Patient activationa | +1.32 | +1.17 | 0.95 |
| Self-efficacya | +0.67 | +0.50 | 0.82 |
| Hypertension knowledgea | +1.17 | +0.35 | 0.01 |
aHigher scores reflect better outcomes.
*P-value based on Wilcoxon–Mann–Whitney tests; significance criterion was P < 0.05. Values in bold are significant.
In terms of the qualitative data, PLs reported learning how to control stress, eat the right foods and the right amounts of foods, how to read food labels, how to order healthy food at restaurants, which foods are unhealthy, how to use BP monitors and how to prepare for doctors’ visits. More generally, they also reported learning about the effect of lifestyle changes on BP and their general health and how to get and stay healthy. For example, they reported having joined a gym and engaging in more regular exercise. In response to our question of which behaviors they would personally continue after the conclusion of the project, the majority reported wanting to make good diet choices (39.2%) and engaging in regular exercise (35.3%). Other behaviors included the regular monitoring of steps (via pedometers), BP and weight (19.6%); practicing stress reduction techniques (3.9%) and preparing questions for doctors’ visits (2.0%).
Focus group participants (n = 38) were primarily male (84.2%) and ranged in age from 36 to 79 years (mean = 62.5, SD = 10.4). Focus group data corroborated the responses to open-ended survey prompts. PLs reported engaging in regular exercise, having lost a substantial amount of weight and having lowered their cholesterol and BP. For example, one PL discussed how he had changed his behavior after a presumably long period of inactivity:
This program put me on my bike. You were joking when I came here but that’s the truth. And I gotta tell you. I hadn’t even—that bike was dusty and dirty and flat tires a year ago and I’ve already got 30 miles which isn’t much but the weather isn’t quite there yet. But this year I already got 30 miles.
Others also attributed their health behavior change (and those in their immediate social networks) to their involvement as PLs in the project. Indeed, the language they used sometimes sounded as if they had themselves been the target of the intervention:
Now, that’s something that you know doesn’t really fall in measuring any of this but it’s motivation to move and I know for myself and my husband, I don’t know if I would’ve gotten my husband motivated to—we’re trying to lose weight and exercise more. I had tried before and I didn’t have any luck getting my husband motivated and if he’s not motivated then I don’t do it. So we’re both working on it. And I don’t think without the program I would have been successful. So the motivation part I think has been helpful.
For some (and in this case, a PL who enjoys relatively good health), involvement in the program served as a wake-up call of sorts:
What I’m getting at is that my health is better than 95% of the people in our post … But in any case, I did not understand that high blood pressure is as toxic to our organs as diabetes is and I did not know that until I sat in the [mini training session]. And I’ve said that a half a dozen times to people … I understood I need to manage my blood pressure but I didn’t realize how bad it would be if I did not manage it.
Discussion
PLs increased their knowledge about health behaviors, checked their BP more often, reported more physical activity and became healthier at the end of the intervention when compared with baseline. Although all groups improved with respect to BP and pedometer use, PLs were more knowledgeable about hypertension and reported higher fruit and vegetable consumption than were PMs. These findings are consistent with what others have found with respect to PL improvements in health knowledge and behavior [9, 11–13].
In addition to the analysis comparing intervention recipients (i.e. PMs) with intervention leaders (i.e. PLs), our study extends the extant literature by systematically comparing PLs with other VLs who were randomized to a non-PL role. Indeed, such analyses revealed that PLs did not differ from PRs in substantive ways. That is to say, PRs also demonstrated improvements above and beyond those the PMs demonstrated. Such a finding is notable given that PMs included in this study belonged to intervention posts, whereas PRs belonged to comparison posts. We must conclude, then, that being a PL did not confer any benefits above and beyond what benefit may have come (or may have existed) from volunteering to take a leadership role in a project aimed at improving PMs’ behaviors and hypertension-related health outcomes.
Similarities between PLs and PRs on key outcomes suggest that there may be something about those who volunteer for leadership positions that make them more amenable to behavioral change. Indeed, we found that the only difference between the two groups at project commencement was their interest in the topic of hypertension. It may be that any gains that PLs received from having had exposure to the materials twice (i.e. once when learning about the material and the second time when delivering it) or from adopting the peer-leader role may have been attenuated by PRs greater interest, as a group, in the topic of hypertension. Further research is needed to understand why this occurs and how to maximize the benefits of the intervention for all participants.
Similarities among PLs and PRs might be due to congruous personal and psychological characteristics that would result in greater success or greater investment in the program even if they had not been trained as PLs. For example, some may have had leadership roles prior to becoming a VL (and/or became a VL because of such leadership). Alternatively, they may have greater health-related self-efficacy (i.e. the belief that one has the ability to successfully enact health behaviors [24]) or a greater health-related internal locus of control (i.e. the belief that the control one has over one’s health is due to personal factors other than to luck, chance or powerful others’ influence [25]), both of which have been widely associated with one’s ability to improve health behavior [26–28].
Limitations
Although we have provided evidence to suggest that PLs changed over the course of the intervention and that they demonstrated greater changes in terms of self-care behaviors and health status in comparison to the intervention recipients, we are limited in two key ways in terms of the conclusions that we can draw. First, we could not randomize PMs to take on a leadership role. Although randomization at the post level and the homogeneity of the VLs at the point of entry into the study gives us confidence that those who eventually became PLs and PRs were likely quite similar in terms of their motivation, PMs who chose to become VLs—whether they participated because of extrinsic or intrinsic motivational factors—were not (and frankly, cannot) be randomly assigned. Second, the degree of contact that we had with PLs was substantially different from the degree of contact that we had with either PRs or PMs. For example, although we maintained regular contact with the PRs for the duration of the study (e.g. before post randomization, we met with all VLs for 2 h to educate them on the research process, provide them with some basic information about hypertension, ask them to help with recruiting PMs to the study and give them information about how to use the BP cuffs and pedometers; after randomization, we continued to work closely with the PRs to develop the PI-led lecture series and encourage PMs to attend). Indeed, we had greater number of average contact hours with PLs compared with PRs and PMs. Therefore, we cannot definitively conclude whether PLs’ improvements can be attributed to their role, the special and prolonged attention they received from study staff or a combination of these factors.
Conclusions and future research directions
Although we are limited in our ability to infer causality, PLs demonstrated improvement above and beyond their peers who were the intervention recipients. Given the relative similarity in outcomes between PRs and PLs in conjunction with the greater improvements among PLs versus their own PMs, it is possible that whatever factors predispose a person to volunteer for such a leadership position are critical for health behavior change. Further research is needed to determine whether PLs benefit more than the recipients of their intervention by virtue of their role in the intervention, other predisposing (e.g. personality) factors or because of the additional attention they received from research staff. Researchers will need to develop more sophisticated methods of eliciting a truly random sample of individuals from the target population who might serve as leaders. This task will not be easy as researchers and interventionists alike are interested in using PLs who have the skill set, personality and motivation to effectively engage their peers’ in the intervention [29]. We do in fact intend to measure personality and leadership variables in our future peer-led intervention work with veterans to examine correlates between PL-level variables and outcome success.
Once we have developed meaningful ways of ensuring that the PLs are not already apt to experience improvements prior to their involvement as PLs, it will be important to understand whether the assigned PL role influences PL outcomes and whether we can maximize intervention effects for all participants. For example, some research suggests that identity may play an important role in behavioral enactment [30, 31] and the principle of cognitive dissonance would suggest that PLs brought motivation for health behavior change in line with their new identities as peer health leaders [32–34]. Unfortunately, we could find no literature on identity specific to PLs or the effects such an identity might have on behavior change. Given the specific roles that PLs have or develop as a result of being a PL in health interventions, it may be useful to examine how such roles affect PL identities and whether or not those identities are related to the degree to which these individuals engage in health-related behavioral change.
Funding
Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development [IAB 06-086-2]. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Conflict of interest statement
None declared.
Acknowledgements
We gratefully acknowledge all the participating veterans service organizations and their posts that worked extensively and collaboratively to make the POWER Program possible.
References
- 1.Heisler M. Different models to mobilize peer support to improve diabetes self-management and clinical outcomes: evidence, logistics, evaluation considerations and needs for future research. Fam Pract. 2010;27(Suppl. 1):i23–32. doi: 10.1093/fampra/cmp003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Parry M, Watt-Watson J. Peer support intervention trials for individuals with heart disease: a systemic review. Eur J of Cardiovasc Nurs. 2010;9:57–67. doi: 10.1016/j.ejcnurse.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Weeks MR, Li J, Dickson-Gomez J, et al. Outcomes of a peer HIV prevention program with injection drug and crack users: the Risk Avoidance Partnership. Subst Use Misuse. 2009;44:253–81. doi: 10.1080/10826080802347677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auslander W, Haire-Joshu D, Houston C, et al. A controlled evaluation of staging dietary patterns to reduce the risk of diabetes in African-American women. Diabetes Care. 2002;25:809–14. doi: 10.2337/diacare.25.5.809. [DOI] [PubMed] [Google Scholar]
- 5.Lorig K, Ritter PL, Villa FJ, et al. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ. 2009;35:641–51. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- 6.Parikh P, Simon EP, Fei K, et al. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health. 2010;100:S232–9. doi: 10.2105/AJPH.2009.170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, et al. Peer-led diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial. Diabetes Care. 2011;34:1926–31. doi: 10.2337/dc10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22:1029–40. doi: 10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker C, Bull S, Smith LM, et al. Effects of being a peer-leader in an eating disorder prevention program: can we further reduce eating disorder risk factors? Eat Disord. 2008;16:444–59. doi: 10.1080/10640260802371596. [DOI] [PubMed] [Google Scholar]
- 10.Goto K, Pelto GH, Pelletier D, et al. ‘It really opened my eyes:’ The effects on youth peer educators participating in an action research project. Hum Organ. 2010;69:192–9. [Google Scholar]
- 11.Taylor T, Serrano E, Anderson J, et al. Knowledge, skills, and behavioral improvements on peer educators and low-income Hispanic participants after a stage of change-based bilingual nutrition education program. J Community Health. 2000;25:241–62. doi: 10.1023/a:1005160216289. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaum AS, Lytle LA, Story M, et al. Are differences in exposure to a multicomponent school-based intervention associated with varying dietary outcomes in adolescents? Health Educ Behav. 2002;29:427–43. doi: 10.1177/109019810202900404. [DOI] [PubMed] [Google Scholar]
- 13.Ross MW, Harzke AJ, Scott DP, et al. Outcomes of Project Wall Talk: an HIV/AIDS peer education program implemented within the Texas State Prison system. AIDS Educ Prev. 2006;18:504–17. doi: 10.1521/aeap.2006.18.6.504. [DOI] [PubMed] [Google Scholar]
- 14.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–44. [PubMed] [Google Scholar]
- 15.Becker MH, Maiman LA. Sociobehavioral determinants of compliance with health and medical care recommendations. Med Care. 1975;13:10–24. doi: 10.1097/00005650-197501000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–26. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins PN, Williams RR, Kuida H, et al. Predictive value of a short dietary questionnaire for changes in serum lipids in high-risk Utah families. Am J Clin Nutr. 1989;50:292–300. doi: 10.1093/ajcn/50.2.292. [DOI] [PubMed] [Google Scholar]
- 18.Resnicow K, Campbell MK, Carr C, et al. Body and soul. A dietary intervention conducted through African-American churches. Am J Prev Med. 2004;27:97–105. doi: 10.1016/j.amepre.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Schapira M, Fletcher K, Hayes A, et al. The development and validation of the hypertension evaluation lifestyle and management (HELM) knowledge scale. J Clin Hypertens. 2012;14:461–6. doi: 10.1111/j.1751-7176.2012.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzer R, Jerusalem M. Generalized Self-Efficacy Scale. Measure in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. Windsor: NFER-Nelson; 1995. pp. 35–7. [Google Scholar]
- 21.Sherbourne CD, Stewar AL. The MOS social support survey. Soc Sci Med. 1991;32:705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whittle J, Schapira MM, Fletcher K et al. Impact of a peer support intervention delivered in veterans service organizations on blood pressure control. Poster presented at the National VA HSRD Meeting in Washington D.C., February 2011.
- 24.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 25.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) scales. Health Edu Monogr. 1978;6:160–70. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 26.Hainsworth J, Barlow J. Volunteers’ experiences of becoming arthritis self-management lay leaders: “It’s almost as if I’ve stopped aging and started to get younger!”. Arthritis Rheum. 2001;45:378–83. doi: 10.1002/1529-0131(200108)45:4<378::AID-ART351>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Morrow-Howell N, Hong SL, McCrary S, et al. Changes in activity among older volunteers. Res Aging. 2012;34:174–96. [Google Scholar]
- 28.Oman D, Thoresen CE, McMahon K. Volunteerism and mortality among the community-dwelling elderly. J Health Psychol. 1999;4:301–16. doi: 10.1177/135910539900400301. [DOI] [PubMed] [Google Scholar]
- 29.Mosack KE, Wendorf AR, Brouwer A, et al. Veterans service organization engagement in ‘POWER,’ a peer-led hypertension intervention. Chronic Illn. 2012;8:252–64. doi: 10.1177/1742395312437978. [DOI] [PubMed] [Google Scholar]
- 30.Stets JE, Burke PJ. Identity theory and social identity theory. Soc Psychol Q. 2000;63:224–37. [Google Scholar]
- 31.Stryker S, Burke PJ. The past, present, and future of identity theory. Soc Psychol Q. 2000;63:284–97. [Google Scholar]
- 32.Stone J, Focella E. Hypocrisy, dissonance and the self-regulation processes that improve health. Self Identity. 2011;10:295–303. [Google Scholar]
- 33.Festinger L. A Theory of Cognitive Dissonance. Stanford: Stanford University Press; 1962. [Google Scholar]
- 34.Stone J, Fernandez NC. To practice what we preach: the use of hypocrisy and cognitive dissonance to motivate behavior change. Soc Personal Psychol Compass. 2008;2:1024–51. [Google Scholar]


