Abstract
Background
We evaluated AGS-1C4D4, a fully human monoclonal antibody to prostate stem cell antigen (PSCA), with gemcitabine in a randomized, phase II study of metastatic pancreatic cancer.
Patients and methods
Patients with Eastern Cooperative Oncology Group (ECOG) performance status 0/1 and previously untreated, metastatic pancreatic adenocarcinoma were randomly assigned 1:2 to gemcitabine (1000 mg/m2 weekly seven times, 1 week rest, weekly three times q4weeks) or gemcitabine plus AGS-1C4D4 (48 mg/kg loading dose, then 24 mg/kg q3weeks IV). The primary end point was 6-month survival rate (SR). Archived tumor samples were collected for pre-planned analyses by PSCA expression.
Results
Between April 2009 and May 2010, 196 patients were randomly assigned to gemcitabine (n = 63) or gemcitabine plus AGS-1C4D4 (n = 133). The 6-month SR was 44.4% (95% CI, 31.9–57.5) in the gemcitabine arm and 60.9% (95% CI, 52.1–69.2) in the gemcitabine plus AGS-1C4D4 arm (P = 0.03), while the median survival was 5.5 versus 7.6 months and the response rate was 13.1% versus 21.6% in the two arms, respectively. The 6-month SR was 57.1% in the gemcitabine arm versus 79.5% in the gemcitabine plus AGS-1C4D4 arm among the PSCA-positive subgroup and 31.6% versus 46.2% among the PSCA-negative subgroup.
Conclusions
This randomized, phase II study achieved its primary end point, demonstrating an improved 6-month SR with addition of AGS-1C4D4 to gemcitabine among patients with previously untreated, metastatic pancreatic adenocarcinoma.
ClinicalTrials.gov identifier: NCT00902291.
Keywords: chemotherapy, clinical trial, gemcitabine, metastatic disease, pancreatic cancer, prostate stem cell antigen
introduction
Pancreatic cancer is a major cause of cancer-related death worldwide and 5-year overall survival (OS) is <5% [1, 2]. For patients with metastatic disease, treatment with weekly gemcitabine results in median OS of ∼6 months [3]. Multiple studies have attempted to improve upon the efficacy of gemcitabine by the addition of a second cytotoxic chemotherapy or targeted therapy, but these studies have largely failed to improve survival in these patients [4–12]. Recently, a combination regimen of fluorouracil, folinic acid, irinotecan, and oxaliplatin (FOLFIRINOX) has demonstrated improved efficacy in comparison to gemcitabine [13]; however, this regimen has greater toxicity and can be difficult to administer in several populations, including those with poorer performance status, older age, and elevated liver function tests [14, 15]. Therefore, the delineation of further treatment options remains critical to improving quality of life and survival of patients with pancreatic adenocarcinoma.
Prostate stem cell antigen (PSCA) is a glycosylphosphatidylinositol (GPI)-linked cell surface protein initially identified due to overexpression in prostate cancer [16]. Although it shares 30% homology with stem cell antigen type-2, a surface marker on immature lymphocytes, it is predominantly expressed on differentiated tissues of the gastrointestinal and genitourinary tracts [17]. Furthermore, PSCA is expressed on several cancers, including those arising from the prostate, bladder, and pancreas [18–20], with 60%–80% of pancreatic tumors expressing PSCA in prior studies [19, 21, 22]. Although the cellular function of PSCA remains poorly understood, preclinical studies have shown that targeting PSCA can be a successful anti-tumor strategy, including for pancreatic cancer [21–25]. Interestingly, a germline missense single-nucleotide polymorphism (SNP) in the first exon of PSCA has been identified as a predisposing factor to development of stomach and bladder cancers in large genome wide association studies (GWASs) [26–29].
AGS-1C4D4 is a fully human IgG1k anti-PSCA monoclonal antibody that has undergone phase I testing in patients with prostate cancer [30, 31]. No dose-limiting toxicity was noted in these studies. Therefore, AGS-1C4D4 at 48 mg/kg loading dose followed by 24 mg/kg every 3 weeks intravenously was selected for further investigation based on pharmacokinetic properties, rather than toxicity. To evaluate efficacy and toxicity of AGS-1C4D4 in patients with previously untreated, metastatic pancreatic cancer, we conducted an open-label, randomized, two-arm phase II study of gemcitabine alone and gemcitabine plus AGS-1C4D4, with primary end point of 6-month survival rate (SR).
methods
patients
This multicenter, open-label, randomized, phase II trial included patients aged ≥18 years with Eastern Cooperative Oncology Group (ECOG) performance status 0/1 and pathologically confirmed metastatic pancreatic adenocarcinoma. Patients with unresectable, locally advanced disease were ineligible. Measureable and non-measureable diseases by RECIST v1.1 were allowed. Prior chemotherapy for metastatic disease was not permitted. Prior treatment with gemcitabine for local or locally advanced disease was allowed if treatment was completed >6 months before enrollment. Prior chemotherapy other than gemcitabine and/or radiotherapy for local or locally advanced disease was allowed if treatment was completed >4 weeks before enrollment.
Further eligibility criteria included: adequate bone marrow (neutrophils ≥1500/μl, platelets ≥100 000/μl), renal (creatinine ≤2.0 mg/dl), and hepatic function [total bilirubin ≤2 × upper limit of normal (ULN), ALT/AST ≤2.5×ULN or ≤5×ULN if known liver metastases]; and INR <1.3 or ≤3 if on warfarin for therapeutic anticoagulation. The exclusion criteria included: known brain or leptomeningeal disease; major surgery within 28 days of enrollment; clinically significant cardiovascular disease; known chronic infection with HIV, HBV or HCV; and women pregnant or lactating. The protocol was reviewed by institutional review boards of each participating center and all patients provided written informed consent.
randomization and treatment
Patients were randomly assigned 1:2 to gemcitabine alone or gemcitabine plus AGS-1C4D4 (Agensys, Inc.). Randomization was stratified by geographic region (study centers in the United States/Canada versus Europe/Russia). Gemcitabine 1000 mg/m2 was administered over 30 min by intravenous infusion weekly for 7 weeks, followed by a 1-week rest, followed by weekly infusions for 3 of every 4 weeks. In the gemcitabine plus AGS-1C4D4 arm, the same schedule of gemcitabine was used with the addition of AGS-1C4D4 48 mg/kg loading dose, then 24 mg/kg q3weeks administered over 60 min intravenously. Dose modifications in AGS-1C4D4 were not allowed. If a dose of AGS-1C4D4 was skipped, it could be administered 2 weeks later with resumption of a new every 3-week dosing schedule. Dose modifications of gemcitabine were as per the package insert and institutional practice. Treatment was discontinued for progressive disease, unacceptable toxicity, or withdrawal of consent.
assessments
Pretreatment evaluations included medical history and physical examination, complete blood count (CBC), chemistry panel, INR, carbohydrate antigen 19-9 (CA19-9), pregnancy test (in women of childbearing potential), evaluation of serum anti-AGS-1C4D4 antibodies, electrocardiogram, and computed tomography scan or magnetic resonance imaging of the chest, abdomen, and pelvis. Physical examination, CBC, INR, chemistry panel, and serum CA19-9 were mandated at least every 3 weeks during treatment. Evaluation of serum anti-AGS-1C4D4 antibodies and imaging studies were carried out every 8 weeks. From patients who provided informed consent, archival tumor tissue was requested for analysis of PSCA cell surface staining.
Patients were evaluated for response according to RECIST criteria v1.1 every 8 weeks. Toxicity was assessed throughout the treatment period and until 4 weeks after the last treatment administration according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Investigators assessed the causal relationship between adverse events and study treatment by the yes/no question, ‘Is there a reasonable possibility that the event may have been caused by the investigational product?’
tumor PSCA immunohistochemistry (IHC) and scoring
Archival formalin-fixed, paraffin-embedded tumor tissue was received for 140 of 196 (71%) patients. Among these 140 patients, 123 had adequate tissue for IHC analysis. Four micron thick sections were dewaxed and hydrated for antigen retrieval in proteinase K (Dako, Carpenteria, California), and then treated with 3% hydrogen peroxide. Sections were incubated in either MC1-4.117 (specific anti-PSCA antibody comprised of human variable domains linked to murine IgG1 Fc domain, generated at Agensys, Inc.) at 1.5 µg/ml, or murine IgG1 isotype control antibody (Dako) at the same concentration, for 1 h using an automated instrument (Dako Autostainer, Carpenteria, California). Samples were incubated in the Envision + System-HRP Dual Link reagent (Dako) and then in DAB (Dako) as the chromogen. Sections were counterstained with hematoxylin, dehydrated, and coverslipped. The results of tumor staining for PSCA were expressed using a semi-quantitative H-score [32] by a certified pathologist blinded to patient identifiers and treatment assignment (Quest Laboratories, Van Nuys, CA). The intensity of IHC staining (0, negative; 1+, weak; 2+, moderate; 3+, strong) was multiplied by the percentage of stained cells with that intensity [H-score = (% at 1+) × 1 + (% at 2+) × 2 + (% at 3+)×3], resulting in an H-score ranging from 0 to 300. A priori, PSCA positivity was defined as an H-score of ≥100 [32].
statistical analyses and study design
The primary end point was 6-month SR, defined as a proportion of patients alive at 6 months from the date of randomization. Based on a comparison of two proportions with the chi-square test and 1:2 randomization scheme, a sample size of 185 patients was needed to detect a 20% increase in 6-month SR from 45% to 65% with 90% power and one-sided alpha of 0.10. Therefore, target enrollment was 60 patients in the gemcitabine arm and 125 patients in the gemcitabine plus AGS-1C4D4 arm. The primary efficacy and safety analysis population was defined as all patients who received ≥1 dose of treatment on study.
The secondary end points included OS (time between randomization and death), progression-free survival (PFS; time between randomization and death or disease progression), response rate [RR; proportion of patients with complete response (CR) or partial response (PR) by RECIST v1.1], disease control rate [proportion of patients with CR, PR, or stable disease (SD) by RECIST v1.1], and tolerability. Exploratory analysis was planned for efficacy end points by tumor PSCA expression and for incidence of anti-AGS-1C4D4 antibodies. Data analysis was planned for after 165 deaths. Although statistical hypothesis tests were pre-planned as one-sided tests, they are reported as two-sided in this paper.
Interim safety evaluations were carried out by an independent Data Safety Monitoring Committee (DSMC) established for this study. No formal statistical stopping rules were predetermined; however, the DSMC was chartered to recommend stopping the trial at any time if a concerning safety signal was identified.
The comparison of 6-month SR between treatment groups is reported with a two-sided Cochran–Mantel–Haenszel (CMH) chi-square test stratified for geographic region [33], and the Clopper–Pearson method for 95% confidence intervals [34]. Stratified Cox proportional hazards models were used to compare treatment arms for OS and PFS; survival probability estimates were calculated using the Kaplan–Meier method and compared with two-sided log-rank tests [35]. Similar analyses were carried out in subgroups defined by PSCA staining. The data-cut off was 8 August 2011.
To investigate whether a possible imbalance in patient characteristics between arms may have influenced the survival results, a Cox regression model was fitted with covariates whose individual univariate log-rank P values were ≤0.10. Covariates evaluated in univariate models included age (continuous), sex (male, female), ECOG performance status (0, 1), geographic region (North America, Europe/Russia), location of primary tumor (head, other site), liver metastases (yes, no), prior chemotherapy with gemcitabine (yes, no), prior receipt of radiotherapy (yes, no), and baseline serum CA19-9 (above/below the median). All analyses were carried out with SAS v9.1.3 (SAS Institute, Cary, NC).
results
patients
Between April 2009 and May 2010, 205 patients with metastatic pancreatic adenocarcinoma from 43 clinical centers were randomly assigned to gemcitabine (68 patients) or gemcitabine plus AGS-1C4D4 (137 patients). Five patients randomly assigned to gemcitabine and four patients randomly assigned to gemcitabine plus AGS-1C4D4 did not receive the allocated intervention. Therefore, efficacy and safety analyses included 63 patients randomly assigned to gemcitabine and 133 patients randomly assigned to gemcitabine plus AGS-1C4D4 (CONSORT Diagram). Patient characteristics are described in Table 1.
Table 1.
Patient demographic and clinical characteristics
| Characteristic | Gemcitabine (n = 63) |
Gemcitabine + AGS-1C4D4 (n = 133) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age (years) | ||||
| Median | 63 | 62 | ||
| Range | 37–89 | 40–88 | ||
| Female sex | 38 | 60.3 | 59 | 44.4 |
| ECOG PS at screening | ||||
| 0 | 11 | 17.5 | 35 | 26.5 |
| 1 | 52 | 82.5 | 97 | 73.5 |
| White race/ethnicity | 59 | 93.7 | 130 | 97.7 |
| Geographic region | ||||
| United States/Canada | 38 | 60.3 | 82 | 61.7 |
| France/Russia/Spain | 25 | 39.7 | 51 | 38.3 |
| Location of primary tumor | ||||
| Head | 36 | 57.1 | 66 | 49.6 |
| Body | 10 | 15.9 | 30 | 22.6 |
| Tail | 8 | 12.7 | 20 | 15.0 |
| Other/Unknown | 9 | 14.3 | 17 | 12.8 |
| Location of metastases | ||||
| Lung | 17 | 27.0 | 30 | 22.6 |
| Liver | 48 | 76.2 | 93 | 69.9 |
| Lymph nodes | 25 | 39.7 | 62 | 46.6 |
| Prior treatments | ||||
| Pancreatectomy | 12 | 19.0 | 22 | 16.5 |
| Chemotherapy | 7 | 11.1 | 18 | 13.5 |
| 5-Fluorouracil | 3 | 4.8 | 9 | 6.8 |
| Gemcitabine | 3 | 4.8 | 12 | 9.0 |
| Radiotherapy | 6 | 9.5 | 17 | 12.8 |
| Baseline serum CA19–9 (U/ml) | ||||
| Median range | 2445.0 (4–266 984) | 2771.5 (4–1 750 000) | ||
ECOG PS, Eastern Cooperative Oncology Group performance status
efficacy
At the time of analysis, 167 of 196 (85.2%) patients had died, and 5 patients remained on treatment (1 patient on the gemcitabine arm and 4 patients on the gemcitabine plus AGS-1C4D4 arm). The study met its primary end point of improvement in 6-month SR. The 6-month SR was 44.4% (95% CI, 31.9–57.5) in the gemcitabine arm and 60.9% (95% CI, 52.1–69.2) in the gemcitabine plus AGS-1C4D4 arm (CMH P = 0.03; Table 2).
Table 2.
Six-month survival rate by treatment arm and tumor PSCA expression
| Population | Gemcitabine | Gemcitabine + AGS-1C4D4 | P valueb |
|---|---|---|---|
| Primary end point | |||
| All patients | |||
| No. of patients | 63 | 133 | |
| Six-month SR (95% CI) | 44.4% (31.9–57.5) | 60.9% (52.1–69.2) | 0.03 |
| Tumor PSCA expressiona | |||
| PSCA-positive | |||
| No. of patients | 21 | 44 | |
| Six-month SR (95% CI) | 57.1% (34.0–78.2) | 79.5% (64.7–90.2) | 0.06 |
| PSCA-negative | |||
| No. of patients | 19 | 39 | |
| Six-month SR (95% CI) | 31.6% (12.6–56.6) | 46.2% (30.1–62.8) | 0.29 |
| PSCA unknown | |||
| No. of patients | 23 | 50 | |
| Six-month SR (95% CI) | 43.5% (23.2–65.5) | 56.0% (41.3–70.0) | 0.32 |
6-month SR (95% CI), 6-month survival rate (95% confidence intervals).
aTumor staining for PSCA evaluated by immunohistochemistry (IHC) and described using a semi-quantitative H-score. Tumors with an H-score of ≥100 were defined as PSCA-positive.
bTwo-sided P value by a Cochran–Mantel–Haenszel chi-square test stratified by geographic region.
PSCA, prostate stem cell antigen.
Median OS was 5.5 months for patients receiving gemcitabine versus 7.6 months for those receiving gemcitabine plus AGS-1C4D4 [hazard ratio (HR), 0.78; 95% CI, 0.56–1.07; log-rank P value, 0.12; Table 3 and Figure 1A]. Median PFS was 3.2 months versus 3.8 months (HR, 0.84; 95% CI, 0.61–1.15; log-rank P value, 0.27), while RR was 13.1% versus 21.6% (Table 3 and Figure 1B). Our multivariable-adjusted Cox model for OS additionally included ECOG performance status, baseline serum CA19-9 and presence of liver metastases. After inclusion of these factors in the model, the HR for OS comparing the two treatment arms was 0.82 (95% CI, 0.62–1.08), favoring gemcitabine plus AGS-1C4D4. Patients remained on study treatment for a median of 3.6 months in the gemcitabine arm and 4.3 months in the gemcitabine plus AGS-1C4D4 arm.
Table 3.
Summary of efficacy results for secondary end points
| Efficacy end point | Gemcitabine (n = 63) |
Gemcitabine + AGS-1C4D4 (n = 133) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Overall survival (OS) | ||||
| Median, months | 5.5 | 7.6 | ||
| Hazard ratio (95% CI) | 0.78 (0.56–1.07) | |||
| Log-rank P value | 0.12 | |||
| Progression-free survival (PFS) | ||||
| Median, months | 3.2 | 3.8 | ||
| Hazard ratio (95% CI) | 0.84 (0.61–1.15) | |||
| Log-rank P value | 0.27 | |||
| Best overall responsea | (n = 61) | (n = 125) | ||
| CR | 0 | 0 | 0 | 0 |
| PR | 8 | 13.1 | 27 | 21.6 |
| SD | 28 | 45.9 | 53 | 42.4 |
| PD | 15 | 24.6 | 25 | 20.0 |
| Clinical progression/death | 6 | 9.8 | 12 | 9.6 |
| Inadequate assessmentb | 4 | 6.6 | 8 | 6.4 |
| Response rate (CR + PR) | 13.1 | 21.6 | ||
| Disease control rate (CR + PR + SD) | 59.0 | 64.0 | ||
aAmong patients with measurable disease at baseline.
bIncludes patients who did not have objective tumor assessments and withdrew for reasons other than disease progression or death.
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
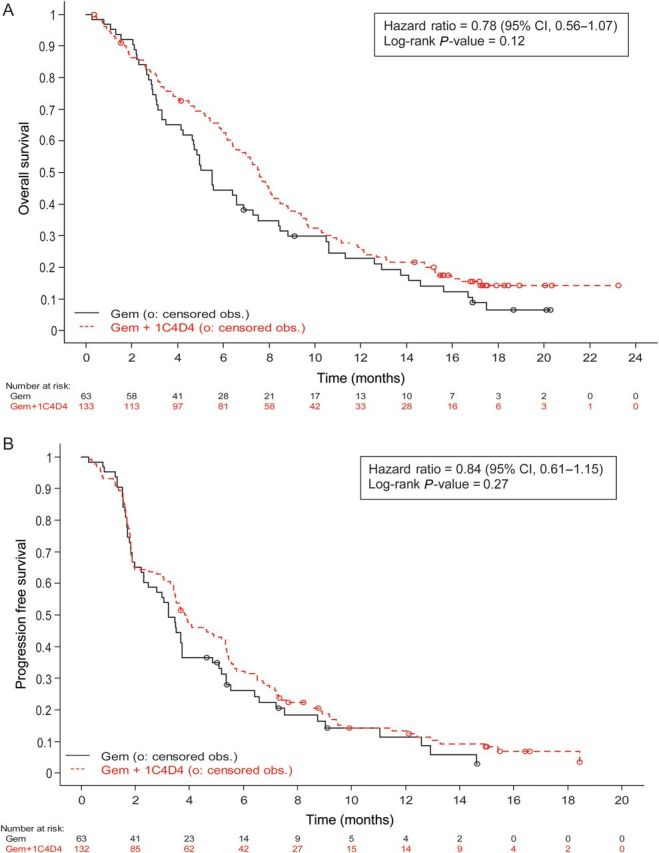
Duration of (A) overall survival (OS) and (B) progression-free survival (PFS) by treatment arm. (A) OS. (B) PFS.
tumor PSCA expression
Among 123 patients with available tumor, 65 (53%) had tumors that were positive for PSCA cell surface staining defined by an H-score of ≥100. Although patient numbers were limited for these exploratory analyses, the survival time appeared longer among patients receiving gemcitabine who had PSCA-positive tumors versus those who had PSCA-negative tumors (Table 2; Figure 2A), indicating a possible prognostic effect of this marker. However, the HRs for OS comparing the two treatment arms were similar in PSCA-positive (HR, 0.83; 95% CI 0.46–1.48) and PSCA-negative (HR, 0.83; 95% CI, 0.47–1.50) groups, suggesting that tumor staining for PSCA by IHC, as defined by an H-score of ≥100, may not be predictive of treatment benefit from AGS-1C4D4 (Figure 2B).
Figure 2.
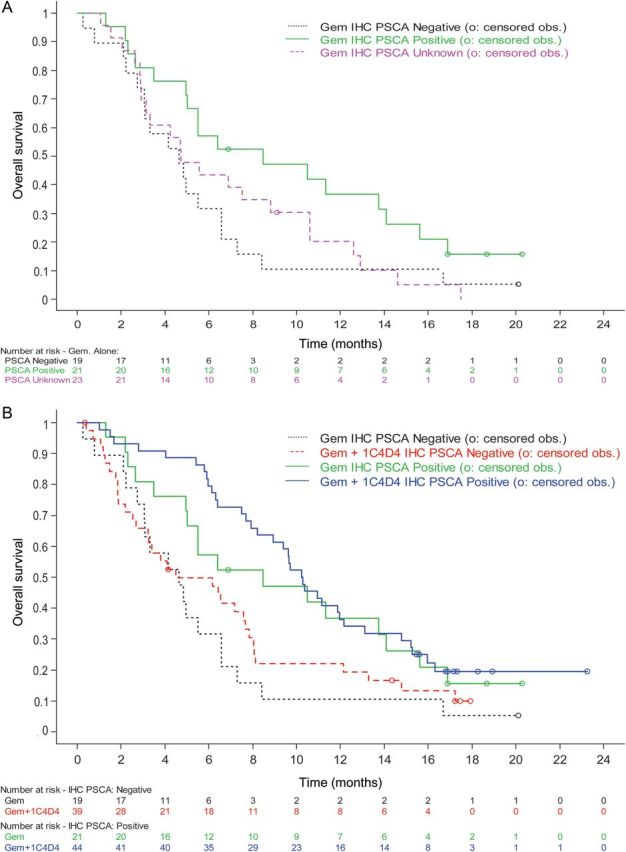
Overall survival (OS) of patients receiving (A) gemcitabine and (B) gemcitabine versus gemcitabine plus AGS-1C4D4 by tumor PSCA staining. (A) Gemcitabine arm only. (B) Gemcitabine arm and gemcitabine plus AGS-1C4D4 arm.
tolerability
Grade 3/4 treatment-emergent adverse events (TEAEs) are summarized in Table 4. Grade 3/4 TEAEs were seen in 40 (63.5%) patients on the gemcitabine arm and 105 (78.9%) patients on the gemcitabine plus AGS-14D4 arm, although no specific toxicity signal was evident in the gemcitabine plus AGS-1C4D4 arm. Three (2.3%) patients had a grade 1 and five (3.8%) patients had a grade 2 infusion reaction attributed to AGS-1C4D4. One patient with a grade 2 infusion reaction opted to discontinue AGS-1C4D4 and remained on gemcitabine. No patients receiving AGS-1C4D4 developed antibodies to the drug.
Table 4.
Grade 3/4 treatment-emergent adverse events by treatment arm
| Treatment-emergent adverse events (frequency ≥5%) | Gemcitabine (n = 63) |
Gemcitabine + AGS-1C4D4 (n = 133) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Hematologic | ||||
| Anemia | 2 | 3.2 | 9 | 6.8 |
| Neutropenia | 4 | 6.3 | 11 | 8.3 |
| Thrombocytopenia | 1 | 1.6 | 7 | 5.3 |
| Non-hematologic | ||||
| Abdominal pain | 1 | 1.6 | 13 | 9.8 |
| Abdominal pain upper | 5 | 7.9 | 4 | 3.0 |
| Ascites | 1 | 1.6 | 11 | 8.3 |
| Asthenia | 4 | 6.3 | 16 | 12.0 |
| Bile duct obstruction | 3 | 4.8 | 8 | 6.0 |
| Fatigue | 7 | 11.1 | 12 | 9.0 |
| Hyperbilirubinemia | 5 | 7.9 | 7 | 5.3 |
| Hyperglycemia | 1 | 1.6 | 8 | 6.0 |
| Pulmonary embolism | 2 | 3.2 | 8 | 6.0 |
| Vomiting | 4 | 6.3 | 4 | 3.0 |
| Any grade 3/4 adverse event | 40 | 63.5 | 105 | 78.9 |
All-cause 60-day mortality was 7.9% in the gemcitabine arm and 13.5% in the gemcitabine plus AGS-1C4D4 arm. The number of deaths that occurred during treatment or within 30 days of the last treatment was 11 (17.5%) on the gemcitabine arm and was 23 (17.3%) on the gemcitabine plus AGS-1C4D4 arm.
discussion
This large, global, randomized, phase II trial evaluating the addition of AGS-1C4D4 to gemcitabine met its primary end point of improved 6-month SR in patients with previously untreated, metastatic pancreatic adenocarcinoma. Specifically, the 6-month SR was 44.4% in the gemcitabine alone arm and 60.9% in the gemcitabine plus AGS-1C4D4 arm (P = 0.03). Data from the study's secondary end points, including OS, PFS and RR, were also suggestive of benefit from the addition of AGS-1C4D4 to gemcitabine; however, definitive conclusions are limited in the context of a phase II study.
Multiple clinical trials have attempted to improve upon the efficacy of single-agent gemcitabine in patients with metastatic pancreatic cancer by the addition of a second cytotoxic chemotherapy or targeted therapy [4–12]. In many instances, preclinical data and a single-arm phase II study appeared promising, but the subsequent randomized, phase III trial did not confirm a significant improvement in survival. The current study enrolled a larger number of subjects to include a randomized, control group, which received gemcitabine alone. This study design has the advantages of reducing selection bias and allowing for the formal comparison of an intermediate end point (such as 6-month SR), with the goal of more reliably determining the appropriateness of a subsequent randomized, phase III study [36]. Furthermore, in the current study, survival of patients on the gemcitabine alone arm was comparable with that in several recent randomized, phase III trials [6, 10, 11], lowering the likelihood that patient selection was primarily responsible for the improved survival in the gemcitabine plus AGS-1C4D4 arm. Specifically, the median OS in the gemcitabine plus placebo arm was 5.9 months in NCIC CTG PA.3 [6], SWOG-S0205 [11], and CALGB 80303 [10], with 24%, 22%, and 16% of patients, respectively, on these trials having locally advanced disease. In the current study, the median OS in the gemcitabine arm was 5.5 months, with all patients required to have distant metastases.
As suggested by data from prior phase I studies in prostate cancer [30, 31], toxicity due to AGS-1C4D4 appeared modest, with similar adverse event rates in the gemcitabine and gemcitabine plus AGS-1C4D4 arms. It is believed that PSCA has limited normal tissue expression [18], which may reduce the incidence of on-target, non-tumor effects and is currently being exploited for novel imaging approaches and immune-based therapies [23, 37]. Additionally, safety data were followed during the study by an independent DSMC and no concerning toxicity signal emerged. This manageable toxicity profile is of particular importance in patients with metastatic pancreatic cancer, as they are commonly symptomatic from their disease.
The mechanisms by which PSCA may promote malignant transformation and progression remain to be defined. It is a member of the Thy-1/Ly-6 family of GPI-linked surface proteins, which have no transmembrane domain, and PSCA has no known ligand [16, 17]. Nevertheless, GPI-linked proteins appear to participate in a diverse array of cellular functions, including signal transduction, cell–cell adhesion and immune modulation [38]. Furthermore, a growing number of preclinical studies have demonstrated the ability of PSCA-targeted therapy to inhibit tumor growth and spread, including in models of pancreatic cancer [21–25].
We obtained adequate tumor specimens from 123 patients enrolled on study. Among the collected specimens, 53% were positive for PSCA cell surface staining, as defined by an H-score of ≥100. This H-score was assigned to each tumor specimen by a pathologist blinded to treatment assignment and the cut-off for a ‘positive’ score was determined before analysis of the data. This rate of PSCA ‘positive’ tumors is slightly lower than that seen in a limited number of other studies, in which rates ranged from 60% to 80% [19, 21, 22]. However, the methods used to define PSCA positivity varied across these studies and further work is necessary to better define what constitutes a PSCA-positive tumor.
In a pre-planned exploratory analysis, we investigated tumor PSCA staining as a possible predictive marker for efficacy of AGS-1C4D4. Although patient numbers were limited for this analysis, tumor PSCA staining did not appear to select for those patients who would benefit from AGS-1C4D4, using our predefined definition of PSCA positivity. The HR of death was ∼0.80 comparing the two treatment arms in both the PSCA-positive and PSCA-negative subgroups. Further studies of AGS-1C4D4 should address the utility of PSCA staining as a predictive marker by collecting diagnostic tumor specimens from a larger group of patients receiving AGS-1C4D4.
Interestingly, among patients who received gemcitabine, the 6-month SR was higher among those with PSCA-positive tumors (57.1%) versus those with PSCA-negative tumors (31.6%). These data would suggest that tumor PSCA staining may act as a prognostic marker, independent of treatment with AGS-1C4D4. However, this analysis was not pre-planned and any prognostic value of PSCA staining must be confirmed in additional populations.
A role for the PSCA in tumorigenesis has also been suggested in large studies evaluating germline genetic variants and risk of bladder and gastric cancers [26–28]. A missense SNP (rs2294008) in the first exon of PSCA was associated with cancer risk and functional evaluation suggested that the rs2294008 SNP modulated transcriptional activity of the PSCA promoter [26, 27, 29]. Whether this SNP is associated only with the risk of cancer or whether it may also impact survival of patients with cancer remains to be determined [39]. We did not collect germline DNA in the current trial, so exploratory studies of germline PSCA variants and survival were not possible. Nevertheless, this is an interesting area for further study. Of note, several GWASs of pancreatic cancer have not identified germline PSCA variants as related to risk, although the sample sizes of these studies have been somewhat modest [40–42].
In sum, this large, global, randomized, phase II trial evaluating the addition of AGS-1C4D4 to gemcitabine met its primary end point of improved 6-month SR in patients with previously untreated, metastatic pancreatic adenocarcinoma. These data support further research involving AGS-1C4D4 in pancreatic cancer, with the hope of improving outcomes for patients with this highly lethal malignancy.
funding
This work was supported by Agensys, Inc., an affiliate of Astellas Pharmaceuticals Inc.
disclosure
BMW is an advisor for Agensys Inc., Genentech, Merimack Pharma, and Momenta Pharma. EMO received research funding from Agensys Inc. YJK is an advisor for Agensys Inc. EC is an advisor for Amgen and Genentech and received research funding from Agensys, ImClone, Bristol-Myers Squibb, Sanofi-Aventis, Pfizer, Genentech. AH, KM, LJ, MV, and LR are employees of Agensys Inc. MH is an advisor for Agensys Inc. All the remaining authors have declared no conflicts of interest. The institutions of all investigators received research funding to conduct the clinical trial.
acknowledgements
We are gratefully indebted to the patients who participated in this trial and to their families. We acknowledge the study site staffs, PRA International, and the following individuals from Agensys Inc.: Ebonie McDaniel for IHC sample management; Huafeng Zhou for biostatisical support; Yao Huang for programming support; Patrick Stern for data management; Anna Butturini, Jennifer Poulakos, and Kathy Jelaca-Maxwell, for extensive and critical data review.
references
- 1.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 5.Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 10.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Ko AH. FOLFIRINOX: a small step or a great leap forward? J Clin Oncol. 2011;29:3727–3729. doi: 10.1200/JCO.2011.37.3464. [DOI] [PubMed] [Google Scholar]
- 15.Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncol. 2011;12:8–9. doi: 10.1016/S1470-2045(10)70237-0. [DOI] [PubMed] [Google Scholar]
- 16.Reiter RE, Gu Z, Watabe T, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeki N, Gu J, Yoshida T, et al. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer Res. 2010;16:3533–3538. doi: 10.1158/1078-0432.CCR-09-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raff AB, Gray A, Kast WM. Prostate stem cell antigen: a prospective therapeutic and diagnostic target. Cancer Lett. 2009;277:126–132. doi: 10.1016/j.canlet.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argani P, Rosty C, Reiter RE, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- 20.Amara N, Palapattu GS, Schrage M, et al. Prostate stem cell antigen is overexpressed in human transitional cell carcinoma. Cancer Res. 2001;61:4660–4665. [PubMed] [Google Scholar]
- 21.Wente MN, Jain A, Kono E, et al. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas. 2005;31:119–125. doi: 10.1097/01.mpa.0000173459.81193.4d. [DOI] [PubMed] [Google Scholar]
- 22.Katari UL, Keirnan JM, Worth AC, et al. Engineered T cells for pancreatic cancer treatment. HPB (Oxford) 2011;13:643–650. doi: 10.1111/j.1477-2574.2011.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saffran DC, Raitano AB, Hubert RS, et al. Anti-PSCA mAbs inhibit tumor growth and metastasis formation and prolong the survival of mice bearing human prostate cancer xenografts. Proc Natl Acad Sci USA. 2001;98:2658–2663. doi: 10.1073/pnas.051624698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Z, Yamashiro J, Kono E, et al. Anti-prostate stem cell antigen monoclonal antibody 1G8 induces cell death in vitro and inhibits tumor growth in vivo via a Fc-independent mechanism. Cancer Res. 2005;65:9495–9500. doi: 10.1158/0008-5472.CAN-05-2086. [DOI] [PubMed] [Google Scholar]
- 25.Bossow S, Grossardt C, Temme A, et al. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Ther. 2011;18:598–608. doi: 10.1038/cgt.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamoto H, Yoshimura K, Saeki N, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730–740. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Ye Y, Kiemeney LA, et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat Genet. 2009;41:991–995. doi: 10.1038/ng.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lochhead P, Frank B, Hold GL, et al. Genetic variation in the prostate stem cell antigen gene and upper gastrointestinal cancer in white individuals. Gastroenterology. 2011;140:435–441. doi: 10.1053/j.gastro.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu YP, Kohaar I, Rothman N, et al. Common genetic variants in the PSCA gene influence gene expression and bladder cancer risk. Proc Natl Acad Sci USA. 2012;109:4974–4979. doi: 10.1073/pnas.1202189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris MJ, Eisenberger MA, Pili R, et al. A phase I/IIA study of AGS-PSCA for castration-resistant prostate cancer. Ann Oncol. 2012;23:2714–2719. doi: 10.1093/annonc/mds078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonarakis ES, Carducci MA, Eisenberger MA, et al. Phase I rapid dose-escalation study of AGS-1C4D4, a human anti-PSCA (prostate stem cell antigen) monoclonal antibody, in patients with castration-resistant prostate cancer: a PCCTC trial. Cancer Chemother Pharmacol. 2012;69:763–771. doi: 10.1007/s00280-011-1759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarty KS, Jr., Szabo E, Flowers JL, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244s–4248s. [PubMed] [Google Scholar]
- 33.Agresti A. An introduction to categorical data analysis. New York: John Wiley & Sons; 2007. [Google Scholar]
- 34.Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 35.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 36.Wieand HS. Randomized phase II trials: what does randomization gain? J Clin Oncol. 2005;23:1794–1795. doi: 10.1200/JCO.2005.10.956. [DOI] [PubMed] [Google Scholar]
- 37.Lepin EJ, Leyton JV, Zhou Y, et al. An affinity matured minibody for PET imaging of prostate stem cell antigen (PSCA)-expressing tumors. Eur J Nucl Med Mol Imaging. 2010;37:1529–1538. doi: 10.1007/s00259-010-1433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulick MG, Bertozzi CR. The glycosylphosphatidylinositol anchor: a complex membrane-anchoring structure for proteins. Biochemistry. 2008;47:6991–7000. doi: 10.1021/bi8006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Bai J, Tan Y, et al. Genetic variant in PSCA predicts survival of diffuse-type gastric cancer in a Chinese population. Int J Cancer. 2011;129:1207–1213. doi: 10.1002/ijc.25740. [DOI] [PubMed] [Google Scholar]
- 40.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, Miao X, Huang L, et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet. 2012;44:62–66. doi: 10.1038/ng.1020. [DOI] [PubMed] [Google Scholar]


