Abstract
Medial patellofemoral ligament reconstruction is a commonly performed procedure for patellofemoral instability. In recent years the surgery has evolved considerably as the anatomy and goals of reconstruction have become more defined. This has resulted in numerous surgical options involving various fixation devices. Furthermore, as biomechanical data accumulate regarding the importance of graft position and tension, surgical techniques for applying this knowledge with precision are needed. This technical note with an accompanying video details one such technique that may be applied to various methods of fixation, allowing for improved precision and possibly resulting in a stronger fixation construct.
Surgical treatment of patellar instability can be complex and requires a thorough understanding of the anatomy and biomechanics of the various soft-tissue and osseous structures involved. Of these, an injury to the medial patellofemoral ligament (MPFL) is considered the essential lesion of a lateral patellar dislocation, providing 60% of the restraining force to lateral patellar translation.1 In the absence of an osteochondral fracture, nonoperative treatment remains the gold standard for a first-time traumatic patellar dislocation. However, if recurrent instability develops and the frequency and symptoms associated with these episodes are incompatible with the patient's functional demands, surgery is often pursued. It is important to note, however, that the recurrent instability, not retropatellar pain or chondromalacia, is the primary indication for surgery.
In the consideration of surgical options, addressing MPFL pathology may be performed in isolation or in conjunction with additional procedures (e.g., distal realignment procedures or lateral release), based on the presence of contributing factors, such as patellar alta, patellar tilt, an increased tibial tubercle-trochlear groove (TT-TG) distance, and significant coronal-plane malalignment. Decisions to repair or reconstruct the MPFL are also based on various factors, including the acuity and site of ligament injury, the presence of recurrent instability and/or generalized ligamentous laxity, a history of surgery (e.g., failed medial reefing or distal realignment procedure2), and the presence of trochlear dysplasia. The clinical and diagnostic evaluation of the unstable patella must be performed with these issues in mind, before proceeding with surgical treatment.
The MPFL originates on the femur in the “saddle” region between the medial epicondyle and the adductor tubercle and inserts on the upper two-thirds of the medial patella, just deep to the vastus medialis obliquus insertion.3,4 Acting as a checkrein, the MPFL serves as the primary static soft-tissue restraint to lateral patellar translation in the first 20° to 30° of flexion and becomes lax in higher degrees of flexion.1,3,5-7 In the absence of trochlear dysplasia, the trochlea produces osseous restraint beyond 30° of flexion, maintaining patellofemoral stability.8
The goal of MPFL reconstruction is to restore normal functional anatomy. Given this, it is crucial to place the graft anatomically and under normal physiologic tension. During graft placement, identifying the central point of the femoral insertion by dissection alone can be challenging because of alteration or absence of the normal tissue after injury.9 To address this, Schöttle et al.10 performed an anatomic and radiographic cadaveric study, describing the femoral insertion to be 1.3 mm anterior to the posterior cortical extension line, 2.5 mm distal to the posterior origin of the medial femoral condyle, and 3 mm proximal to a perpendicular line intersecting the posterior point of the Blumensaat line on a lateral radiograph. Although several other anatomic and radiographic studies have suggested that the femoral insertion of the MPFL is either slightly anterior11 or posterior9 to the radiographic point described by Schöttle et al., it has also been shown that altering this insertion by 5 mm or less does not change the isometry.12 In our experience, Schöttle's point serves as a reliable landmark that localizes fairly consistently to the central aspect of the MPFL femoral insertion, posterior to the femoral epicondyle and anteroinferior to the adductor tubercle.
In terms of graft tensioning, Burks and colleagues13 showed that normal physiologic lateral patellar translation and patellofemoral contact forces were restored by tensioning the MPFL graft with a force of only 2 N whereas tensioning the ligament at 10 N and 40 N produced excessive medial patellar facet contact pressures. With the concern of over-tensioning the graft leading to accelerated patellofemoral arthrosis, a technique that allows for fine adjustments in graft tension is beneficial.
Farr and Schepsis14 first described the “reverse-loop” technique in which a double-loaded anchor is unloaded and then reloaded with the looped end of one suture back through the anchor islet. After the femoral side of the graft is secured, the patellar side of the graft limb(s) is passed through the looped end of the anchor suture and provisionally secured while the tension is assessed clinically. Fine adjustments may be applied before definitive fixation. Schepsis and Rogers2 have recently described a slight modification in which a second suture is threaded through the looped end of the first suture, “capturing” the suture. With both of these techniques, however, only one doubled-over suture is actually threaded through the anchor islet, yielding 2 suture limbs on each side of the islet. In this technical note, we describe a modification of this technique, allowing for 2 sutures to be threaded through the anchor with 3 suture limbs exiting each side of the eyelet. Video 1 illustrates the modification of the reverse-loop technique.
Surgical Technique
The key points of the surgical technique are shown in Table 1. We begin with an examination of the patient under anesthesia, evaluating both the operative and nonoperative extremities and observing the lateral patellar glide at varying degrees of knee flexion (Fig 1). The operative extremity is then prepared and draped from the mid thigh distally with a sterile stockinet secluding the distal leg and foot. A thorough diagnostic arthroscopy of the knee is performed. The patellofemoral joint is evaluated from this intra-articular perspective with an assessment of patellar congruence and tilt during gentle knee range of motion. Visible injury to the capsular or retinacular structures, as well as chondral surfaces, is noted, and any necessary intra-articular procedures are performed at that time.
Table 1.
Key Points to Modified Reverse-Loop MPFL Reconstruction Technique
| Perform a preoperative examination under anesthesia of lateral patellar glide, allowing for a direct comparison after provisional fixation. |
| Use a Kirschner wire and fluoroscopy to confirm placement of the femoral tunnel before drilling. |
| Remain extracapsular, carefully dissecting between retinacular layers 2 and 3. |
| Ensure that each limb of the allograft tendon is at least 6 cm long, and mark the midpoint to identify the femoral fixation site. |
| Place the femoral anchor (Super QuickAnchor Plus DS), and secure the graft in this location before addressing the patella. |
| During patellar anchor (Super QuickAnchor Plus DS) preparation, cut and remove one of the 2 No. 2 sutures (Fig 4A), pass a suture threader (ConMed Linvatec) through the eyelet of the anchor (Fig 4B), place the looped end of the suture within the loop of the suture threader (Fig 4C), and then pull the threader back through the anchor eyelet (Figs 4D and 4E). |
| Center the patellar anchors within the proximal two-thirds of the patella. |
| While provisionally tensioning the graft, maintain the knee at 30° of flexion by placing a towel bump under the knee. |
| Use appropriate tension to remove any graft redundancy at 30°, maintain the patella centered within the trochlea from 0° to 30°, and allow for mild graft laxity in hyperflexion. |
| Evaluate patellofemoral tracking arthroscopically before definitive graft fixation. |
| During closure, imbricate retinacular layers 1 and 2 to match the resting tone of the reconstructed ligament and then assess lateral patellar glide once more to ensure that over-tensioning has not occurred. |
Fig 1.
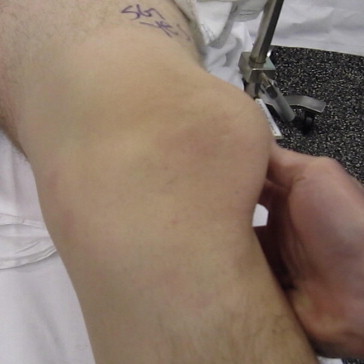
Examination under anesthesia of left knee with patient in supine position showing a lateral patellar dislocation during assessment of lateral patellar glide.
In preparation for the femoral incision, the posterior aspect of the medial epicondyle is identified by manual palpation. This approximation of the MPFL femoral insertion is then confirmed and adjusted under fluoroscopy by identifying Schöttle's point on a lateral knee view. With the knee in 30° to 45° of flexion, a 2-cm longitudinal incision is made, dissecting sharply through subcutaneous tissue down to the first of 3 layers of the medial retinaculum. A limited blunt dissection is performed, freeing the areolar and subcutaneous tissues from the underlying fascia. This deep fascia is then incised sharply, and manual palpation of the anatomic landmarks is again performed. The adductor tubercle is palpated slightly proximal and posterior to the medial epicondyle. Between these 2 landmarks, the dissection is carried down to bone with use of electrocautery and a periosteal elevator. A 0.062-mm Kirschner wire is then placed in the center of the “saddle” region between the adductor tubercle and the medial epicondyle, and Schöttle's point is again verified on fluoroscopy. Fine adjustments of the Kirschner wire are made as needed.
The patellar incision is centered over the medial proximal two thirds. A 2-cm longitudinal incision is made with a knife through skin and subcutaneous tissue. By use of tenotomy scissors, dissection proceeds down to the plane between layers 2 and 3, remaining extracapsular. Care must be taken during this step, because the layers are variably adherent more anteriorly,4 especially in the setting of a patellar-sided avulsion. Once these layers are defined, dissection continues subperiosteally to patellar bone, exposing the anterior and proximal two-thirds of the medial patella deep, the distal extent of the vastus medialis obliquus insertion. A small curette is then used to abrade the cortical surface as a means of generating a healing stimulus for the allograft tendon. A blunt clamp is used to fully develop the retinacular tunnel connecting the 2 incisions between layers 2 and 3. The 2 free ends of a passing suture are then retrieved by the clamp from the femoral incision, shuttled out the patellar incision, and clamped together with the looped end.
The femoral fixation consists of a single 2.9-mm metal anchor (Super QuickAnchor Plus DS; DePuy Mitek, Raynham, MA) loaded with 2 No. 2 high-strength polyethylene sutures (Orthocord; DePuy Mitek) (Fig 2). In preparation for placement of our femoral anchor, the 0.062-mm Kirschner wire is removed and replaced with a 2.9-mm drill for the anchor pilot hole. Before drilling, the placement of the drill is again confirmed on fluoroscopy. The pilot hole is then drilled, and the anchor is tapped into the bone with a mallet to the appropriate depth, as determined by the laser-etched line on the anchor driver. The anchor sutures are freed from the handle, which is subsequently removed, and the anchor is firmly seated against the bone by pulling gently several times on the sutures.
Fig 2.

Metal anchor: 2.9-mm Super QuickAnchor Plus DS.
A thawed semitendinosus allograft tendon is folded in half, and the length is assessed. With the knowledge that the native MPFL is approximately 59 mm in length,15 each limb of the graft should exceed this by at least several centimeters, both for fixation and for ease of handling. The apex of the looped end of the graft is then marked with a surgical marker, and one of the No. 2 sutures from the femoral anchor is passed around the tendon at this location and tied securely (Figs 3A and 3B). Each suture limb from the remaining anchor suture is passed in the same direction through both limbs of the graft immediately above the previously tied knot (Fig 3C). These suture limbs are then tied together, and all suture tails are cut, completing the femoral fixation of the graft. The limbs of the graft are shuttled through the retinacular tunnel with the passing suture such that both limbs are protruding from the patellar incision.
Fig 3.
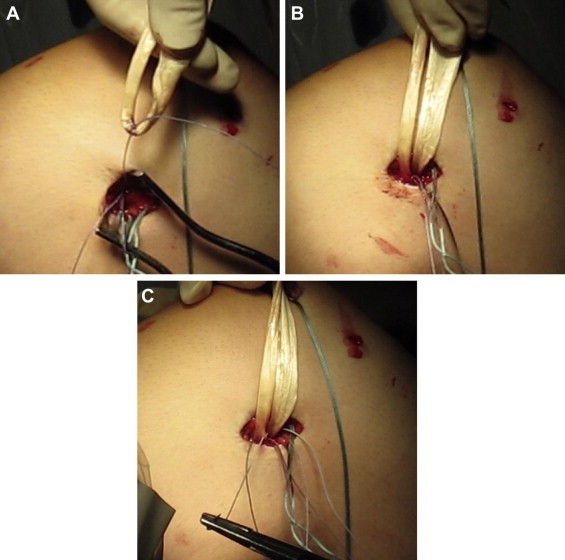
Graft fixation to femoral Super QuickAnchor Plus DS. (A) One suture limb from the first femoral anchor suture is passed around the graft. (B) Secured graft after tying of first suture from femoral anchor. (C) Both suture limbs from the second suture are passed in the same direction through both graft limbs and tied together.
The location of proximal and anterior two-thirds of the medial patella is verified on fluoroscopy, and preparation of the patellar fixation commences. On the back table, two 2.9-mm metal anchors (Super QuickAnchor Plus DS) double loaded with No. 2 high-strength polyethylene sutures (Orthocord) are altered from their packaged state in the same fashion. First, one of the 2 No. 2 sutures is cut, unloaded from the anchor, and discarded (Fig 4A). A suture threader (ConMed Linvatec, Largo, FL) is then passed through the eyelet of the anchor in preparation for shuttling a new No. 2 high-strength suture (Fig 4B). The new suture is held with both free suture limbs together, and the looped end is placed within the loop of the suture threader (Fig 4C). The threader is then pulled back through the anchor eyelet, loading the anchor with the new suture (Figs 4D and 4E). The suture limbs are secured to the anchor handle with tape to maintain tension on the anchor and prevent it from falling off (Fig 4F). This results in an anchor construct with 2 No. 2 high-strength sutures, one of which is doubled over such that the looped end on one side of the anchor eyelet may be tensioned by pulling on that suture's free limbs from the other side of the anchor eyelet. It is also of note that 3 suture limbs are exiting on either side of the anchor eyelet, rendering this construct functionally equivalent to a triple-loaded anchor.
Fig 4.
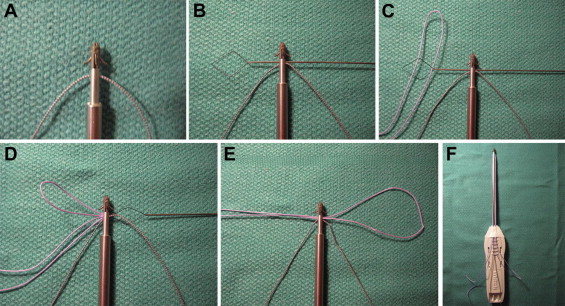
Preparation of patellar anchors (Super QuickAnchor Plus DS) with high-strength No. 2 Orthocord suture. (A) Anchor with single suture after removal of 1 suture. (B) Placement of suture threader (ConMed Linvatec) through eyelet of anchor. (C) Passing of looped end of suture through suture threader. (D) Pulling suture threader with new suture through eyelet of anchor. (E) Appearance of anchor and suture after shuttling looped end of new suture through eyelet of anchor. (F) Finished anchor construct with sutures taped to driver handle.
With the anchors prepared, attention is turned back to the patella in preparation for anchor placement. The central aspect of the native MPFL inserts at the proximal one-third–two-thirds junction of the patella, and with its width measuring approximately 12 mm, the ligament in its entirety inserts over the proximal two-thirds.15 Given this, the anchors are evenly centered within the proximal two-thirds of the patella. Each anchor is placed in the same fashion as before, beginning by drilling a 2.9-mm pilot hole and followed by tapping in the anchor to the appropriate depth with a mallet. Anchor placement is confirmed under fluoroscopy.
With the anchors firmly seated in bone, the graft limbs are provisionally secured to the patella to assess for the appropriate degree of lateral patellar restraint. This is performed by first placing a towel bump below the knee, maintaining a flexion angle of 30°. The distal graft limb is then passed through the looped suture in the distal anchor and the proximal graft limb through the looped suture in the proximal anchor (Fig 5A). Pulling on the 2 free suture tails from each looped suture firmly reduces each graft limb to the patella. This provisional fixation is maintained both by the friction of the looped suture within the eyelet of the anchor and by continued tension on the free suture limbs. The graft can be loosened by gently pulling on the looped end with a blunt instrument. This allows for the precise amount of desired tension to be applied to each limb of the graft independently.
Fig 5.
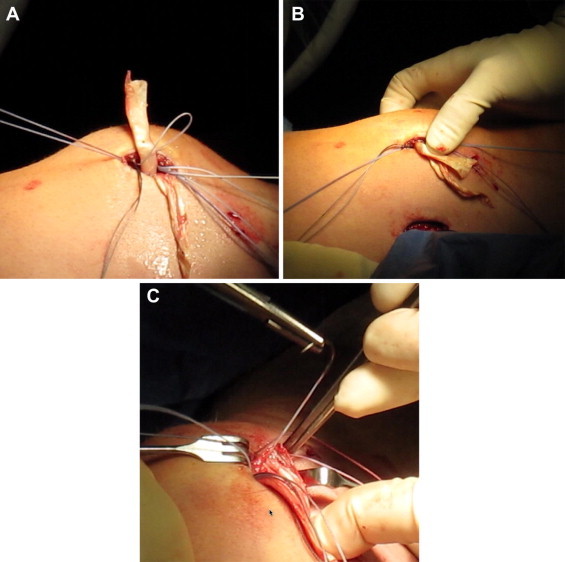
Graft fixation to patellar anchors (Super QuickAnchor Plus DS). (A) Proximal limb of graft as it is placed through looped suture of proximal anchor. (B) Assessment of lateral patellar glide after provisional fixation. (C) Definitive fixation of graft with second suture from each patellar anchor.
Because the function of the MPFL is primarily that of a checkrein to lateral patellar translation, minimal tension is required. The goal is to restore normal anatomy. So, by simply removing all redundancy from the graft, restoration of a normal one quadrant of lateral patellar glide and maintenance of the patella in a central position throughout the first 30° of flexion are achieved (Fig 5B). The graft should also loosen slightly in flexion and then return to a state of minimal tension near full extension. With each limb of the graft provisionally held at the desired position, the reconstruction is evaluated arthroscopically. The patella should be observed tracking centrally in the trochlear groove, and extra-articular placement of the graft should be confirmed.
Once the graft is appropriately tensioned, definitive fixation to the patella is performed. The free limbs of both looped sutures are pulled tightly and tied. The tails of these tied sutures are then each passed in opposite directions through both limbs of the graft immediately above the previously tied knots and tied again. This links the fixation of each graft limb not only to its own respective anchor in the patella but also to the opposite graft limb and anchor. An unaltered No. 2 high-strength suture still remains in each of the patellar anchors. For each of these, one of the suture limbs is passed immediately above the previously tied knots through both graft limbs away from the respective anchor. This same suture limb is then passed back through both limbs again and tied to its partner suture limb (Fig 5C).
All suture limbs from both incisions are cut, and the wounds are thoroughly irrigated. During closure of the patellar incision, layers 1 and 2 are gently imbricated with nonabsorbable suture only to match the resting tone of the reconstructed ligament. After closure of these layers, lateral patellar glide is again assessed to ensure that normal mobility has been restored without over-tensioning of the retinacular layers. The remaining closure is then performed in standard fashion, and a sterile dressing is applied.
Postoperatively, the extremity is placed in a hinged knee brace locked in extension. On postoperative day 2, the brace is unlocked and passive knee motion is instituted with use of a continuous passive motion machine. The continuous passive motion parameters are set at 0° to 40° of flexion and then increased by 10° twice daily as tolerated by the patient. Formal supervised physical therapy is begun at 1 week after surgery, emphasizing quadriceps isometrics and range of motion. Non–weight-bearing precautions are maintained for 3 weeks, and return to sporting activities typically occurs 3 to 6 months after surgery.
Discussion
Reconstruction of the MPFL restores the deficient medial restraints to lateral patellar translation. However, a deficient MPFL must be considered in the context of all pathomechanics about the knee and the patient as a whole. With much of the available literature being fraught with methodologic flaws and consisting of small numbers of patients, mixed results are found in comparing nonoperative and operative treatments.
In a randomized controlled Level II study of 33 patients undergoing primary medial retinacular repair versus nonoperative management, the repair group was found to have a lower rate of recurrent instability and higher functional scores.16 In contrast, a prospective nonrandomized Level II study of 76 patients comparing nonoperative treatment with acute primary repair did not show differences in patellar instability after a median 7-year follow-up.17 The following year, however, the same group published a prospective randomized Level I study of 40 young adults comparing nonoperative treatment with surgical stabilization, including both primary repairs and some reconstructions, showing lower rates of recurrent instability after surgery.18 Lastly, a recent Level I randomized controlled trial of 39 young patients (41 knees) comparing nonoperative treatment versus MPFL reconstruction using a patellar tendon autograft after an acute traumatic patellar dislocation showed improved subjective outcomes and lower recurrent instability rates with reconstruction.19 Again, however, many of these studies reflect the results of varying numbers of patients, surgeons, and regions, as well as varying patient demographics. As such, the generalizability of these results should be questioned until multicenter randomized controlled trials can corroborate or refute these findings.
Within the context of MPFL reconstruction, there are numerous options in terms of fixation constructs and graft selection. Given that the MPFL functions primarily as a checkrein to lateral patellar translation, in the absence of abnormal mechanical alignment and rotation or aberrant soft-tissue forces, an MPFL graft should not require resistance to tensile loads beyond that which is provided by normal anatomy. With the native MPFL tensile strength measuring just over 200 N,20 a fixation construct should approximate or exceed this value.
The technique described in this article uses a semitendinosus allograft tendon and suture anchors for fixation. With the doubled-over allograft semitendinosus yielding a strength of approximately 2,300 N and a stiffness of approximately 470 N/mm,21 the limiting factor in the construct is clearly the anchor fixation. When combined with a single No. 5 polyester suture, the 2.9-mm metal anchor used in this technique was found to have a pullout strength of 232.7 N in a cadaveric cancellous bone model.22 However, failure of the construct in 100% of specimens (10 of 10) was due to suture failure either at the knot or at the suture–anchor eyelet interface, not due to anchor pullout. Of the 3 anchors described in this technique, the femoral anchor would be expected to be the weakest link, because it does not share the load with an adjacent anchor. With polyethylene suture yielding a strength 2- to 2.5-fold greater than that of polyester suture,23 the 2 No. 2 high-strength polyethylene sutures loaded in the femoral anchor would be expected to yield an even greater pullout strength, as compared with the anchor loaded with a single No. 5 polyester suture in the study. Biomechanical data evaluating the pullout strength of interference screw fixation in the setting of MPFL reconstruction have yielded similar results, with through-tunnel fixation failing at 195 N and aperture fixation failing at between 126 and 241 N.20,24 It is also of note, however, that despite the previous biomechanical argument in support of this construct, the principle of the reverse-loop technique can be applied to a variety of fixation options. For anchor fixation, the primary limitation is simply the ability of the anchor eyelet to accept the looped suture of choice and the desired number of sutures. A looped suture could also be passed through a small 2-mm bone tunnel in either the patella or femur for assessment of tension and provisional fixation. One potential advantage of using anchors over interference screws or through-tunnel fixation, however, is the smaller diameter of the drill hole required, minimizing the risk of patellar fracture. Conversely, it may be advantageous, in terms of graft-to-bone healing, to position the graft intraosseously.25
In summary, the modification to the reverse-loop MPFL technique described by Farr and Schepsis14 allows for fine adjustments in graft tension such that an appropriate degree of physiologic tension can be easily obtained. With the use of additional high-strength sutures in each anchor, it may be possible to achieve greater resistance before construct failure.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
Supplementary Data
Modification of reverse-loop technique: examination under anesthesia (15 seconds); diagnostic arthroscopy (22 seconds); femoral dissection (32 seconds); patellar dissection (1 minute 12 seconds); description of fixation construct (1 minute 46 seconds); insertion of femoral anchor (2 minutes); definitive femoral fixation (2 minutes 18 seconds); preparation of patellar anchors (2 minutes 48 seconds); insertion of patellar anchors (3 minutes 11 seconds); provisional graft tensioning (3 minutes 28 seconds); arthroscopic evaluation of reconstruction (4 minutes 10 seconds); definitive patellar fixation (4 minutes 23 seconds); wound closure (4 minutes 56 seconds); and postoperative protocol (5 minutes 15 seconds).
References
- 1.Desio S.M., Burks R.T., Bachus K.N. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26:59–65. doi: 10.1177/03635465980260012701. [DOI] [PubMed] [Google Scholar]
- 2.Schepsis A.A., Rogers A.J. Medial patellofemoral ligament reconstruction: Indications and technique. Sports Med Arthrosc Rev. 2012;20:162–170. doi: 10.1097/JSA.0b013e318264188b. [DOI] [PubMed] [Google Scholar]
- 3.Nomura E., Fijikawa T., Takeda T. Anatomic study of the medial patellofemoral ligament. Orthop Surg Suppl. 1992;22:2–5. [Google Scholar]
- 4.Balwin J.L. The anatomy of the patellofemoral ligament. Am J Sports Med. 2009;37:2355–2361. doi: 10.1177/0363546509339909. [DOI] [PubMed] [Google Scholar]
- 5.Conlan T., Garth W.P., Jr., Lemons J.E. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75:682–693. doi: 10.2106/00004623-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Huatamaa P., Fithian D.C., Kaufman K.R. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998:174–182. doi: 10.1097/00003086-199804000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Burks R.T., Desio S.M., Bachus K.N. Biomechanical evaluation of lateral patellar dislocations. Am J Knee Surg. 1998;11:24–31. [PubMed] [Google Scholar]
- 8.Lee T.Q., Yang B.Y., Sandusky M.D., McMahon P.J. The effects of tibial rotation on the patellofemoral joint: Assessment of the changes in in situ strain in the peripatellar retinaculum and the patellofemoral contact pressures and areas. J Rehabil Res Dev. 2001;38:463–469. [PubMed] [Google Scholar]
- 9.Redfern J., Kamath G., Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38:293–297. doi: 10.1177/0363546509347602. [DOI] [PubMed] [Google Scholar]
- 10.Schöttle P.B., Schmeling A., Rosenstiel N., Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:801–804. doi: 10.1177/0363546506296415. [DOI] [PubMed] [Google Scholar]
- 11.Wijdicks C.A., Griffith C.J., LaPrade R.F. Radiographic identification of the primary medial knee structures. J Bone Joint Surg Am. 2009;91:521–529. doi: 10.2106/JBJS.H.00909. [DOI] [PubMed] [Google Scholar]
- 12.Smirk C., Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10:221–227. doi: 10.1016/s0968-0160(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 13.Beck P., Brown N.A., Greis P.E., Burks R.T. Patellofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:1557–1563. doi: 10.1177/0363546507300872. [DOI] [PubMed] [Google Scholar]
- 14.Farr A., Schepsis A.A. Reconstruction of the medial patellofemoral ligament for recurrent patellar instability. J Knee Surg. 2006;19:307–316. doi: 10.1055/s-0030-1248123. [DOI] [PubMed] [Google Scholar]
- 15.Nomura E., Inoue M., Osada N. Anatomical analysis of the medial patellofemoral ligament of the knee, especially the femoral attachment. Knee Surg Sports Traumatol Arthrosc. 2005;13:510–515. doi: 10.1007/s00167-004-0607-4. [DOI] [PubMed] [Google Scholar]
- 16.Camanho G.L., Viegas A.C., Bitar A.C. Conservative versus surgical treatment for repair of the medial patellofemoral ligament in acute dislocations of the patella. Arthroscopy. 2009;25:620–625. doi: 10.1016/j.arthro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Sillapaa P.J., Maenpaa H.M., Mattila V.M. Arthroscopic surgery for primary traumatic patellar dislocation: A prospective, nonrandomized study comparing patients treated with and without acute arthroscopic stabilization with a median 7-year follow-up. Am J Sports Med. 2008;36:2301–2309. doi: 10.1177/0363546508322894. [DOI] [PubMed] [Google Scholar]
- 18.Sillapaa P.J., Mattila V.M., Maenpaa H. Treatment with and without initial stabilizing surgery for primary traumatic patellar dislocation. A prospective randomized study. J Bone Joint Surg Am. 2009;91:263–273. doi: 10.2106/JBJS.G.01449. [DOI] [PubMed] [Google Scholar]
- 19.Bitar A.C., Demange M.K., D'Elia C.O., Camanho G.L. Traumatic patellar dislocation: Nonoperative treatment compared with MPFL reconstruction using patellar tendon. Am J Sports Med. 2012;40:114–122. doi: 10.1177/0363546511423742. [DOI] [PubMed] [Google Scholar]
- 20.Mountney J., Senavongse W., Amiss A.A. Tensile strength of the medial patellofemoral ligament before and after repair or reconstruction. J Bone Joint Surg Br. 2005;87:36–40. [PubMed] [Google Scholar]
- 21.Hamner D.L., Brown C.H., Jr., Steiner M.E. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: Biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am. 1999;81:549–557. doi: 10.2106/00004623-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Rupp S., Georg T., Gauss C. Fatigue testing of suture anchors. Am J Sports Med. 2002;30:239–247. doi: 10.1177/03635465020300021601. [DOI] [PubMed] [Google Scholar]
- 23.Wust D.M., Meyer D.C., Favre P. Mechanical and handling properties of braided polyblend polyethylene sutures in comparison to braided polyester and monofilament polydioxanone sutures. Arthroscopy. 2006;22:1146–1153. doi: 10.1016/j.arthro.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Hapa O., Aksahin E., Ozden R. Aperture fixation instead of transverse tunnels at the patella for medial patellofemoral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20:322–326. doi: 10.1007/s00167-011-1582-1. [DOI] [PubMed] [Google Scholar]
- 25.Greis P.E., Burks R.T., Bachus K., Luker M.G. The influence of tendon length and fit on the strength of a tendon-bone tunnel complex. A biomechanical and histologic study in the dog. Am J Sports Med. 2001;29:493–497. doi: 10.1177/03635465010290041901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Modification of reverse-loop technique: examination under anesthesia (15 seconds); diagnostic arthroscopy (22 seconds); femoral dissection (32 seconds); patellar dissection (1 minute 12 seconds); description of fixation construct (1 minute 46 seconds); insertion of femoral anchor (2 minutes); definitive femoral fixation (2 minutes 18 seconds); preparation of patellar anchors (2 minutes 48 seconds); insertion of patellar anchors (3 minutes 11 seconds); provisional graft tensioning (3 minutes 28 seconds); arthroscopic evaluation of reconstruction (4 minutes 10 seconds); definitive patellar fixation (4 minutes 23 seconds); wound closure (4 minutes 56 seconds); and postoperative protocol (5 minutes 15 seconds).


