Abstract
Introduction:
The hydatid disease most often involves the liver and the lungs. The disease can involve any part of the body except the hair, teeth and nails. Primary extrahepatico-pulmonary hydatid cysts are rare and only a few sporadic cases have been reported.
Materials and Methods:
Two hundred and forty-four patients with hydatid cysts managed surgically from January 2005 to December 2009 were evaluated retrospectively. Fourteen (5.7%) patients had isolated involvement of the atypical sites, while six (2.4%) also had a primary involvement of liver.
Results:
The cysts were present in gall bladder (0.4%), peritoneum (1.6%), spleen (1.6%), ovary (0.4%), subcutaneous (0.8%), seminal vesicle (0.4%), spinal (0.4%), pancreas (0.4%), kidney (0.4%), mediastinal (0.4%), muscle (0.4%), and brain (0.8%).
Discussion and Conclusions:
Involvement of sites other than liver and lungs by hydatid disease is rare. Symptoms are related to size, location or possible complication of the cyst. It should be strongly suspected in differential diagnosis of all abdominal cysts especially in an endemic area. Proper surgical and medical management to avoid any recurrences, and a regular follow-up, are of utmost importance to detect any late complications such as local recurrence of the disease and development of hydatidosis at the primary sites.
Keywords: Atypical locations, hydatid, echinococcosis
INTRODUCTION
Echinococcal disease in humans is a parasitic tapeworm infection caused by a larval stage (the metacestode) of Echinococcus species (Echinococcus granulosus, Echinococcus multilocularis, Echinococcus oligarthus, or Echinococcus vogelis). The infection can be asymptomatic or severe, causing extensive organ damage and even the death of the patient.[1]
Hydatid cyst has a worldwide distribution and has been recognized since ancient times. Human hydatidosis is a parasitic infection of the liver and other organs caused by the flatworm Echinococcus, most commonly Echinococcus granulosus which is a 5 mm long hermaphroditic tapeworm that has dog, foxes or coyotes as the definitive host and sheep, swine, cattle, and zebra as the intermediate host. Man is an accidental, intermediate host, and infection of humans represents a terminal event (dead end) for the parasite. Once within the man or other intermediate host the ingested eggs hatch in the duodenum to release the true larvae (oncospheres) that penetrate the mucosa of small intestine and enter the portal circulation. Liver acts as the first effective filter for most of the larvae and therefore being the most common site of involvement (65-75%). If the larvae pass through the first filter, they reach the lungs which are the second most frequently involved site (10-25%). If the larvae are not trapped in either liver or lungs, or if they by-pass the liver by traveling via lymphatics, it may lodge itself in any part of the body including the peritoneal cavity (8-18%), spleen (2-3%), kidneys (1-4%), uterus and adnexia (0.5-1%), retroperitoneum (0.5-1%), pancreas (0.5-0.8%),[2] subcutaneous (1-2%),[3] mediastinum (0.1-0.5%),[4] gall bladder (≤1%),[5] brain (2%),[6] seminal vesicle,[7] spinal,[8] and others (0.1-3%).[2] We present our experience with atypical sites of hydatidosis, including the diagnostic evaluation and surgical treatment.
MATERIALS AND METHODS
Two hundred and forty-four patients with hydatid cysts managed surgically from January 2005 to December 2009 at S.K.I.M.S., Soura, Srinagar, Jammu and Kashmir, India, were evaluated retrospectively. Of these patients 142 (58.1%) were males and 102 (41.8%) females. Patient age ranged from 16 to 76 years. Liver was involved in 187 patients, of which 161 (65.9%) had had isolated liver disease, 20 (8.1%) had associated lung cysts, and another 6 (2.4%) patients had concomitant echinococcosis at atypical locations. Isolated lung involvement was seen in 43 (17.6%) patients. Fourteen patients (5.7%) had primary isolated hydatid cysts involving atypical locations. Only those patients with hydatid disease at atypical locations (n=20) were included in the study, including the six patients with concomitant disease of the liver. The atypical locations of the disease included the gall bladder, peritoneum, spleen, ovary, subcutaneous, seminal vesicle, spinal, pancreas, right kidney, mediastinal, muscle, and the brain [Table 1].
Table 1.
Atypical localization of hydatid cysts
Preoperative diagnosis was established by the history, clinical examination, complete blood counts, liver and kidney function tests, serological tests (enzyme linked immunosorbent assay and indirect hemagglutination test), X-ray chest, ultrasound and magnetic resonance imaging (MRI) or contrast enhanced computerized tomography (CECT). The treatment for all the patients was surgical. All the patients received preoperative and postoperative albendazole chemotherapy.
Nonspecific abdominal pain and a nontender palpable abdominal lump were the most predominant symptoms; other symptoms varied according to the localization of the cyst. Palpable lump was also a presenting symptom in patients with subcutaneous and muscle cysts. Four patients with atypical locations were asymptomatic, which included two cases with peritoneal hydatid, one case each of isolated splenic and renal hydatid. Patients with brain and spinal hydatid presented with neurological symptoms, while the one with seminal vesicle hydatid presented with lower urinary tract symptoms (LUTS). Acute popliteal artery embolism due to a laminated membrane was the presentation in the patient with a primary mediastinal hydatid cyst, which had ruptured into descending thoracic aorta [Table 1].
Eosinophilia was observed in 14 of 20 patients. Diagnosis was confirmed by hydatid serology, ultrasonography, MRI/CECT Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8, and histopathological examination of the specimen. These investigations were applied to all the patients.
Figure 1.
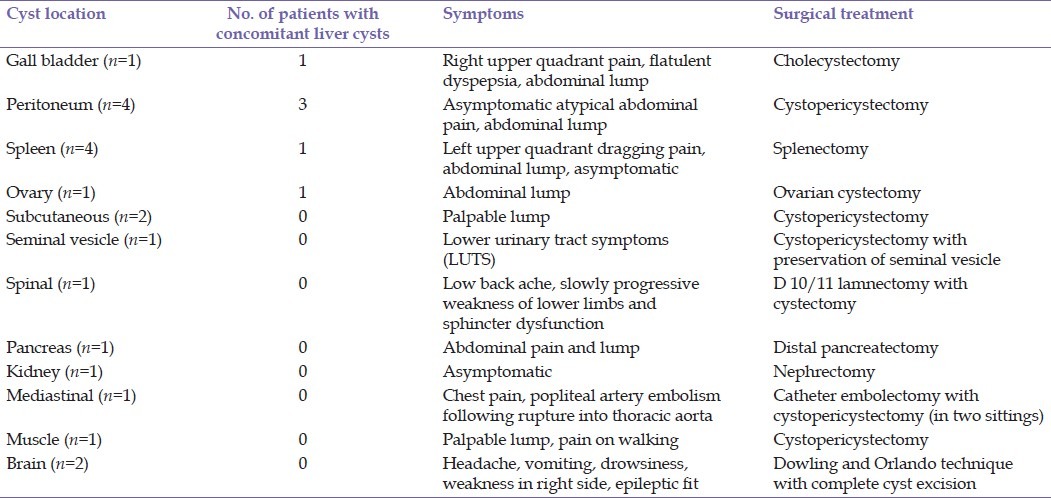
CECT abdomen: Documented hydatid cyst in right lobe of the liver. Another cystic lesion with thick calcified wall was seen in segment-VI of liver measuring 5×5 cm. A calcified area 2 cm in size was also seen medial to the above-calcified cystic lesion. The gall bladder was not seen separately from the calcified cystic lesion. IHBR and CBD were normal
Figure 2.
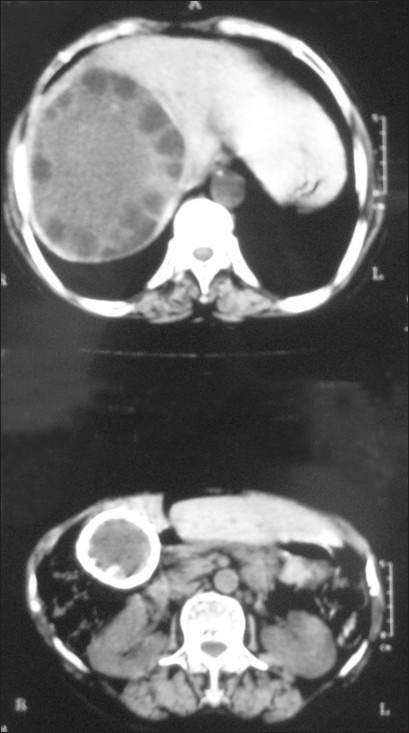
CECT abdomen showing cystic lesion of the spleen involving upper half of the organ
Figure 3.
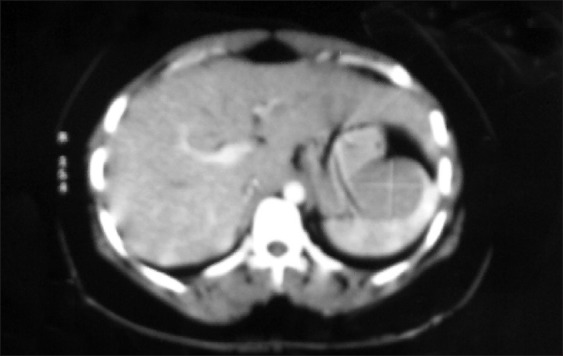
MRI showing multicystic swelling overlying gluteus maximus which is hyperintence on T2WI/STIR and hypointence on T1WI images. Thick septa seen within and surrounding tissue showing edema
Figure 4.
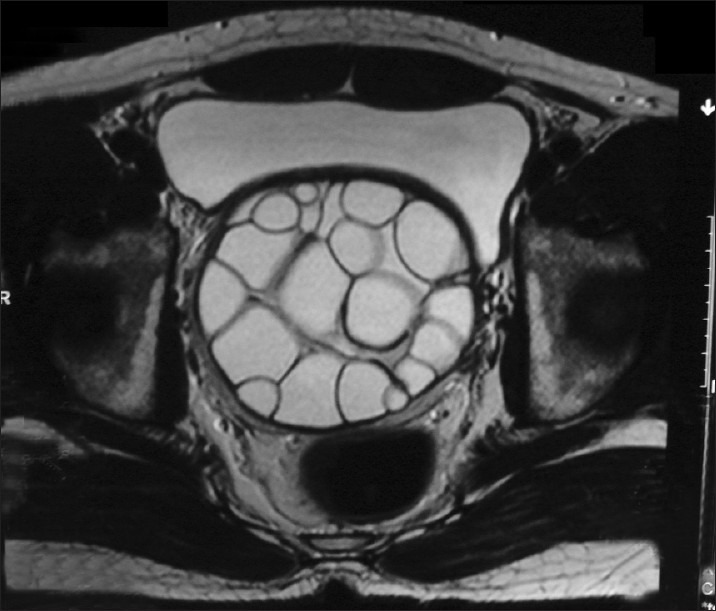
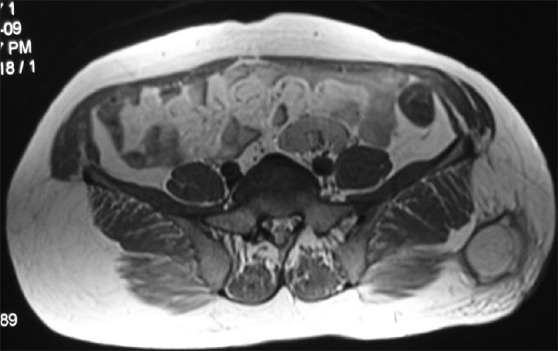
Axial T2WI (MRI) showing hyper intense, multicystic lesion with multiple daughter cysts in relation to the right seminal vesicle
Figure 5.
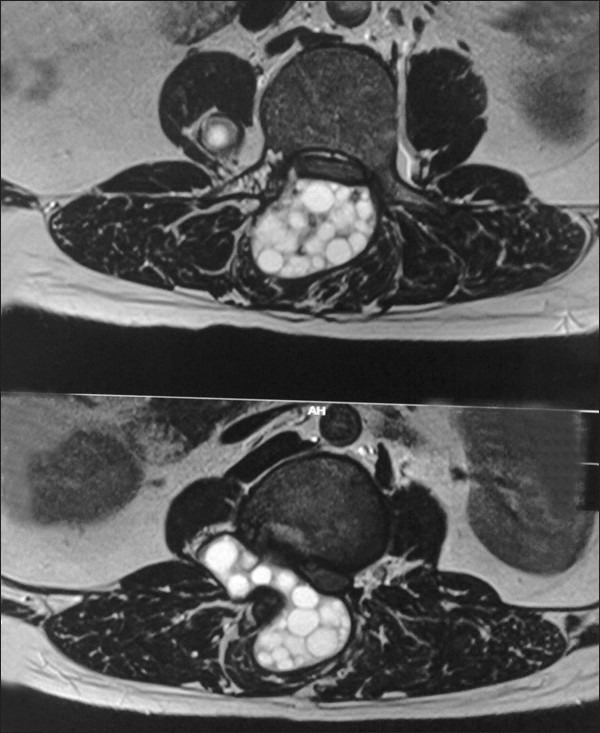
Axial T2WI (MRI) showing hyper intense, multicystic lesion with multiple daughter cysts in the para spinal region with extension into epidural space
Figure 6.
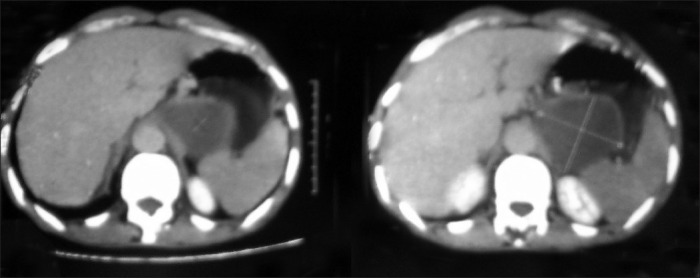
CECT abdomen showing a cystic lesion (unilocular) involving pancreas
Figure 7.
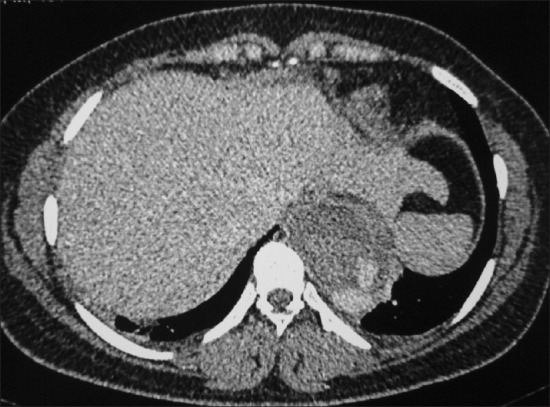
CECT chest documented a primary mediastinal hydatid cyst (cystic laminated structure) eroding into the descending thoracic aorta with contrast leak from aorta into the mediastinal cyst
Figure 8.
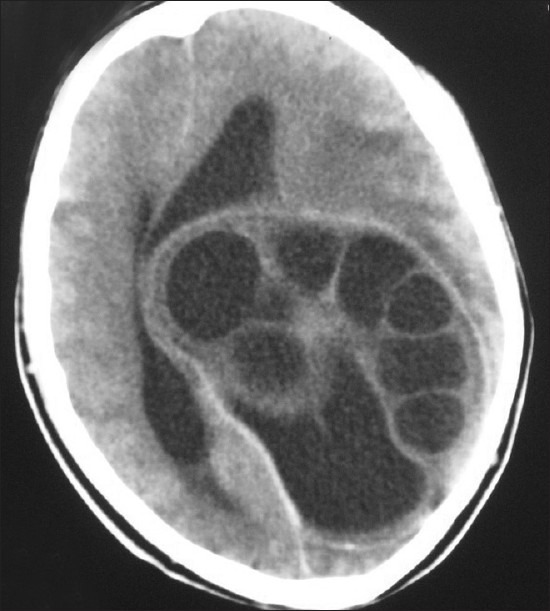
NCCT head showing a multicystic lesion, with multiple daughter cysts in left cerebral hemisphere and compressing the ventricles and opposite lobe
RESULTS
Hydatid disease involving atypical sites was seen in a total of 20 (8.1%) patients. Among this study group, primary isolated hydatid cysts involving atypical locations were present in 14 (5.7%) patients. These included solitary cases of seminal vesicle (0.4%), spinal (0.4%), pancreatic (0.4%), renal (0.4%), mediastinal (0.4%), and muscular (0.4%) hydatid cysts, while involvement of subcutaneous tissue and brain was seen in two each (0.8% each) of the patients. Three patients (1.2%) had isolated involvement of the spleen. Six (2.4%) patients also had a primary disease in the liver. Atypical locations involved in these included peritoneum in 3 (1.2%) and gall bladder (0.4%), spleen (0.4%), and ovary (0.4%) in one each [Table 1].
Surgical treatments include complete cyst excision (cystopericystectomy) in most of the patients. Nephrectomy, cholecystectomy, ovarian cystectomy, distal pancreatectomy and splenectomy, were performed whenever cysts invaded these organs. Classic manoeuvre by Dowling and Orlando was used in management of cerebral hydatid cyst. Epidural hydatid with paravertebral extension was dealt by D 10/11 lamnectomy and complete cyst excision. All the patients were given postoperative chemotherapy (Albendazole 10 mg/kg/day) for three cycles of 21 days each with a gap of 1 week between each cycle. The postoperative hospital stay ranged between 2 and 14 days. Morbidity was minimal, with a wound infection recorded postoperatively in one case treated with splenectomy. The diagnosis of hydatid cyst was confirmed on histopathological examination of the specimen in all cases. Follow-up was performed twice a year with CT scan and antibody titers, with no documented recurrence.
DISCUSSION
Hydatid disease is seen endemically among sheep raising communities. The disease still continues to be a serious problem in countries like Australia, New Zealand, Middle East, Africa, India, South America, Turkey, and Southern Europe.[9] The disease is attributed to occupational exposure during farming practices (sheep rearing), particularly in rural areas; ingestion of contaminated vegetables, drinking of egg-contaminated water (containing hexacanth larvae), and traditional intake of mutton and beef. Because the practice of close contact with domestic animals such as dogs is rare in Kashmir, which is Muslim dominated, the sheep-dog association apparently seems to be most commonly implicated in the life cycle of the parasite. Buffalo-dog, goat-dog, cow-dog, and horse-dog associations are also possible.
Although liver and lungs are the most common sites involved, various atypical sites involved by hydatid cysts and reported in literature include those of peritoneal cavity, spleen, kidneys, uterus and adnexia, retroperitoneum, pancreas, subcutaneous, mediastinum, gall bladder, brain, seminal vesicle, para-spinal, and others.[2,3,4,5,6,7,8,10,11,12,13,14,15,16,17,18,19] In our study, we presented atypical locations of hydatid cysts from a single tertiary care center. Diagnostic and therapeutic strategies are also summarized from the surgical and radiological perspective. Although portal blood stream remains the main pathway of parasite spread, normally existing porto-caval shunts, lymphatic invasion by the parasite, and retrograde migration from vena cava to subclavian vein have been documented and explain some of these rare sites.[1,12]
Atypical localization of hydatid disease usually poses a diagnostic dilemma. It is well known that specific local or general symptoms and signs of hydatid disease do not exist. The majority of cases are diagnosed following incidental findings at imaging examinations for sometimes unrelated complaints. The uncommon localization frequently causes diagnostic problems and specific diagnostic tests do not have 100% reliability in these cases.[7] Hydatid disease in extrahepatic locations usually follows a silent clinical course unless it grows and produces pressure symptoms or develops complications which may include local pressure, rupture, secondary infection, and an allergic reaction.[20] Most of these cases are associated with cysts at the primary location, but in our series all except six patients had isolated primary location of hydatid disease at an atypical site.
In our series, a lump was the presenting feature in cases with hydatid disease involving subcutaneous tissue and thigh muscle. Patient with para spinal disease presented with low back pain, weakness, and paraplegia. Pain abdomen and organomegaly/lump was seen in gall bladder, pancreatic, and splenic hydatid. Seminal vesicle hydatid presented with lower urinary tract symptoms, while the patient with brain hydatid presented with severe headache, vomiting, drowsiness, and hemiparesis. Acute popliteal artery embolism due to a laminated membrane was the presentation in the patient with a primary mediastinal hydatid cyst, which had ruptured into descending thoracic aorta. Four patients with atypical location of hydatid were asymptomatic. It is important to think of hydatid disease as a possibility in patients with nonspecific symptoms with a cystic lesion at any location, especially in endemic areas like India.
Routine laboratory tests can only reveal eosinophilia. Diagnosis is established by imaging and serological tests. Highly sensitive tests include an indirect hemagglutination test (IHA), and a latex agglutination test (LA). Specific tests include double diffusion test (DD), immunoelectrophoresis (IEP), enzyme linked immunosorbent assay (ELISA), and radioallergosorbant test (RAST).[2,21] We performed IHA and ELISA in all our patients, both suggesting hydatid disease. Abdominal USG, CECT, and MRI are highly sensitive and specific and can reveal the morphological characteristics of a cyst, its exact site, size, number, its relation with surrounding structures, and can distinguish it from other lesions.[1,7,9,12,13,14,15,18]
Medical treatment alone may be effective in 30-40% of cases. It is most effective in alveolar hydatid, less so for liver infection, and essentially ineffective for the diseases of bone, brain, eye, gall bladder and other sites.[2,12,22] Many authors recommend preoperative use of antihelmenthics to sterilize the cyst, and reduce the chances of spillage, anaphylaxis, and dissemination at surgery.[23] Postoperative medical treatment reduces the chances of recurrence.[24,25] Our patients received albendazole (10 mg/kg/day) for 4 weeks preoperatively.
Surgery still remains the mainstay of treatment for hydatid disease with special attention to avoid any spread of hydatid with subsequent secondary echinococcosis. There are many surgical procedures for the management of hydatid cysts. Indications for surgery include large cysts with multiple daughter cysts, superficial location amenable to rupture, cysts exerting pressure on adjacent organs, and cysts in ectopic locations such as seminal vesicle, brain, bones, spleen, kidneys, etc.[26,27,28] The type of procedure is selected by taking into consideration the cyst location. In our series of atypical and rare hydatid cyst locations, we performed total cystectomy (cystopericystectomy) in patients with disease involving the muscle, subcutaneous tissue, mediastinum, seminal vesicle and peritoneum. Cholecystectomy, nephrectomy, ovarian cystectomy, distal pancreatectomy, and splenectomy was performed in patients with gall bladder, renal, ovarian, pancreas, and spleen hydatidosis respectively to eradicate the disease, avoiding secondary hydatid spread. Classic manoeuvre by Dowling and Orlando was used in management of cerebral hydatid cyst. Epidural hydatid with paravertebral extension was dealt by D 10/11 lamnectomy and complete cyst excision. During exploration of hydatid cysts, the operative area was carefully packed with pads immersed in Scolicidal agents to avoid local spillage and contamination from the surgical manipulations. Late complications which should be kept in mind are the local recurrence of the disease and development of hydatidosis at the primary sites. The patient has to be kept on regular follow-up paying attention to these possibilities.[7,12,18]
CONCLUSION
Echinococcosis can appear at any site of the human body, and so should always be considered in the differential diagnosis of cystic space-occupying lesions or unidentified tumor formations in patients from endemic areas. Proper surgical and medical management to avoid any recurrences, and a regular follow-up, are of utmost importance to detect any late complications such as local recurrence of the disease and development of hydatidosis at the primary sites.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Delis SG, Bakoyiannis A, Exintabelones T, Triantopoulou C, Papailiou J, Dervenis C. Rare localizations of the hydatid disease.Experience from a single center. J Gastrointest Surg. 2007;11:195–8. doi: 10.1007/s11605-006-0036-4. [DOI] [PubMed] [Google Scholar]
- 2.Palaivelu C. Jaypee Publishers; 2007. Laparoscopic management of hydatid cysts of liver Art of laparoscopic surgery- Textbook and Atlas; pp. 757–83. [Google Scholar]
- 3.Zulfikaroglu B, Koc M, Ozalp N, Ozmen MM. A rare primary location of echinococcal disease: Report of a case. Ups J Med Sci. 2005;110:167–71. doi: 10.3109/2000-1967-078. [DOI] [PubMed] [Google Scholar]
- 4.Kabiri el H, Zidane A, Atoini F, Arsalane A, Bellamari H. Primary hydatid cyst of the posterior mediastinum. Asian Cardiovasc Thorac Ann. 2007;15:e60–2. doi: 10.1177/021849230701500526. [DOI] [PubMed] [Google Scholar]
- 5.Raza MH, Harris SH, Khan R. Hydatid cyst of gall bladder. Indian J Gastroenterol. 2003;22:67–8. [PubMed] [Google Scholar]
- 6.Greenberg SM. 1st ed. New York: Thieme Medical Publisher; 2001. Handbook of Neurosurgery; pp. 238–9. [Google Scholar]
- 7.Safioleas M, Stamatakos M, Zervas A, Agapitos E. Hydatid disease of the seminal vesicle: A rare presentation of hydatid cyst. Int Urol Nephrol. 2006;38:287–9. doi: 10.1007/s11255-006-6652-9. [DOI] [PubMed] [Google Scholar]
- 8.Drimousis PG, Stamou KM, Koutras A, Tsekouras DK, Zografos G. Unusual site of recurrent musculoskeletal hydatid cyst: Case report and brief review of the literature. World J Gastroenterol. 2006;12:5577–8. doi: 10.3748/wjg.v12.i34.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora V, Nijjar IS, Gill KS, Singh G. Case report: Primary hydatid cyst of muscle -a rare site. Indian J Radiological Imaging. 2006;16:239–41. [Google Scholar]
- 10.Moosavi SR, Kermany HK. Epigastric mass due to a hydatid cyst of the pancreas.A case report and review of the literature. JOP. 2007;8:232–4. [PubMed] [Google Scholar]
- 11.Dirican A, Unal B, Kayaalp C, Kirimlioglu V. Subcutaneous hydatid cysts occurring in the palm and the thigh: Two case reports. J Med Case Reports. 2008;2:273. doi: 10.1186/1752-1947-2-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mushtaque M, Malik AA, Malik RA. Hydatid cyst of the gall bladder: A rare location. Eastern Journal of Medicine. 2011;16:83–6. [Google Scholar]
- 13.Kiresi DA, Karabacakoglu A, Odev K, Karakose S. Uncommon locations of hydatid cysts. Acta Radiol. 2003;44:622–36. doi: 10.1080/02841850312331287749. [DOI] [PubMed] [Google Scholar]
- 14.Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid cyst from head to toe. Radiographics. 2003;23:475–94. doi: 10.1148/rg.232025704. [DOI] [PubMed] [Google Scholar]
- 15.Dahniya MH, Hanna RM, Ashebu S, Muhtaseb SA, el-Beltagi A, Badr S, et al. The imaging appearance of hydatid disease at some unusual sites. Br J Radiol. 2001;74:283–9. doi: 10.1259/bjr.74.879.740283. [DOI] [PubMed] [Google Scholar]
- 16.Karavias DD, Vagianos CE, Kakkos SK, Panagopoulos CM, Androulakis JA. Peritoneal echinococcosis. World J Surg. 1996;20:337–40. doi: 10.1007/s002689900054. [DOI] [PubMed] [Google Scholar]
- 17.Arora M, Gupta CR, Jindal S, Kapoor N. An unusual case of hydatid cyst of broad ligament. JIACM. 2005;6:86–7. [Google Scholar]
- 18.Wani RA, Malik AA, Chowdri NA, Wani KA, Naqash SH. Primary extrahepatic abdominal hydatidosis. Int J Surg. 2005;3:125–7. doi: 10.1016/j.ijsu.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Vasileios R, Athanasios P, Stavros T. Echinococcal cyst of the seminal vesicle; a case-report and literature review. Int Urol Nephrol. 2002;34:527–30. doi: 10.1023/a:1025662818745. [DOI] [PubMed] [Google Scholar]
- 20.Tsaroucha AK, Polychronidis AC, Lyrantzopoulos N, Pitiakoudis MS, J Karayiannakis A, Manolas KJ, et al. Hydatid disease of the abdomen and other locations. World J Surg. 2005;29:1161–5. doi: 10.1007/s00268-005-7775-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saimot AG. Medical treatment of liver hydatidosis. World J Surg. 2001;25:15–20. doi: 10.1007/s002680020003. [DOI] [PubMed] [Google Scholar]
- 23.Saenz de San Pedro B, Cazaña JL, Cobo J, Serrano CL, Quiralte J, Contreras J, et al. Anaphylactic shock by rupure of hepatic hydatid cyst.Follow-up by specific IgE serum antibodies. Allergy. 1992;47:568–70. doi: 10.1111/j.1398-9995.1992.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 24.Senyuz OF, Yesildag E, Celayir S. Albendazole therapy in the treatment of hydatid liver disease. Surg Today. 2001;31:487–91. doi: 10.1007/s005950170106. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Eshy SA. Some rare presentations of hydatid cyst (Echinococcus granulosus) J R Coll Surg Edinb. 1998;43:347–52. [PubMed] [Google Scholar]
- 26.Safioleas M, Misiakos EP, Kakisis J, Manti C, Papachristodoulou A, Lambrou P, et al. Surgical treatment of human echinococcosis. Int Surg. 2000;85:358–65. [PubMed] [Google Scholar]
- 27.Gollackner B, Längle F, Auer H, Maier A, Mittlböck M, Agstner I, et al. Radical surgical therapy of abdominal cystic hydatid disease: Factors of recurrence. World J Surg. 2000;24:717–21. doi: 10.1007/s002689910115. [DOI] [PubMed] [Google Scholar]
- 28.Dervenis C, Delis S, Avgerinos C, Madariaga J, Milicevic M. Changing concepts in the management of liver hydatid disease. J Gastrointest Surg. 2005;9:869–77. doi: 10.1016/j.gassur.2004.10.016. [DOI] [PubMed] [Google Scholar]


