Abstract
Purpose
Extracts of the saw palmetto berry are used by many men in the U.S. as self-treatment for lower urinary tract symptoms due to benign prostatic hyperplasia. While the most recent data from double-blind clinical trials do not support efficacy superior to that of placebo, there are few data on the toxicity of saw palmetto.
Materials and Methods
369 patients were randomized in the Complementary and Alternative Medicine for Urological Symptoms (CAMUS) trial; 357 participants are included in this modified intention-to-treat analysis. Participants were randomized to 320mg, 640mg, and 960mg daily of an ethanolic saw palmetto extract or an identical-appearing placebo, in an escalating manner at 6-month intervals, for a total of 18 months follow-up. Adverse-event assessments, vital signs, and blood and urine laboratory tests were obtained at regular intervals.
Results
There were no statistically significant differences between groups in rates of serious or non-serious adverse events, changes in vital signs, digital prostate exam findings, or study withdrawal rates. Overall, there were no significant inter-group differences in the occurrence of laboratory-test abnormalities; differences in individual laboratory tests were uncommon and small in magnitude. No evidence of significant dose-response phenomena were identified.
Conclusions
The saw palmetto extract used in the CAMUS trial showed no evidence of toxicity at doses up to three times the usual clinical dose over a period of 18 months.
Keywords: Serenoa, Drug Toxicity, Phytotherapy
INTRODUCTION
Extracts of the saw palmetto (SP) berry have consistently ranked among the most commonly used dietary supplements in the United States 1. They are taken primarily by men to treat lower urinary tract symptoms (LUTS), most frequently due to benign prostatic hyperplasia (BPH) 2,3. Earlier clinical studies suggested that SP had modest efficacy for relieving LUTS 4,5, an assertion challenged by the Saw palmetto Treatment for Enlarged Prostates (STEP) trial, a single-center study that found no evidence of benefit of SP, at a dose of 160mg twice daily, for any subjective or objective outcome 6. Importantly, the STEP study also found no evidence of toxicity associated with this typical dose of the extract over the one-year study period 7.
In order to help resolve the inconsistency of the reported findings and address the issue of dose-response, the National Institutes of Health initiated a multicenter, dose-ranging double-blind clinical trial of SP for men with LUTS 8. Doses up to three times that employed in the STEP trial were used in a graduated manner over an 18-month period, providing a unique opportunity to assess potential SP toxicity under more challenging clinical conditions. This study, the Complementary and Alternative Medicine for Urinary Symptoms (CAMUS) trial (clinicaltrails.gov #NCT00603304) again found no evidence of benefit of SP over placebo for any outcome 9.
Though the efficacy results from these two studies suggest that SP is not superior to placebo, many men who perceive benefit from the supplement will likely continue to take it, despite these negative studies 10. Therefore, it remains important to better define any potential toxicities associated with its use. We report here the adverse-event data from the CAMUS trial with an emphasis on dose-related observations.
MATERIALS AND METHODS
The design of the CAMUS trial has been described previously 8,9. Briefly, eligible men were ≥45 years old, had an American Urological Association Symptom Index (AUASI 11) score between 8 and 24, with a peak urinary flow rate ≥4 mL/s with a voided volume ≥125 mL. Between June 2008 and May 2009, 369 men were randomized at 11 centers in the U.S. and Canada, of whom 357 are included in this modified intention-to-treat analytic sample (12 participants did not take any doses of study medication and/or did not have at least one follow-up visit).
Men randomized to active therapy took one 320mg gelcap of SP daily for the first 24 weeks (weeks 1–24), two gelcaps for the second 24 weeks (weeks 25–48), then three gelcaps for the third 24-week period (weeks 49–72). Placebo participants received the same escalating number of identical-appearing placebo gelcaps. The primary outcome for the study was change in the AUASI; several secondary outcomes (both symptomatic and objective measures of urine flow and post-void residual volume) were also defined 8,9.
The active preparation used was an ethanolic extract of SP berries (Serenoa repens (W. Bartram) Small (Arecaceae)) manufactured by and supplied to the trial by Rottapharm/Madaus (Cologne, Germany). This extract is sold commercially as PROSTA-URGENIN UNO and was standardized to a reference chromatogram with 85%–95% fatty acids. The placebo consisted primarily of polyethylene glycol.
Adverse-event assessments were conducted at 12-week intervals and at 4 weeks after the initiation of each dose increase. In addition to open-ended questions about adverse experiences, participants also completed questionnaires assessing erectile and ejaculatory function, continence, prostatitis, and sleep; vital signs were obtained at each clinic visit. In this report, laboratory tests are generally reported dichotomously as normal or abnormal since these measurements were performed locally at each clinical site (with differing normal ranges) so these data could not be combined across sites on a continuous scale. For each laboratory test, Fisher’s exact test was used to compare treatment groups with respect to the proportions of patients who had abnormal results at each dose level, and the Cochran-Mantel-Haenszel test was used to compare the treatment arms across dose levels with respect to the proportions with abnormal results. Prostate-specific antigen (PSA) levels were measured at baseline, 24, 48, and 72 weeks and a urinalysis was obtained at baseline and closeout. Serious and non-serious adverse events (as defined by the Food and Drug Administration (17)) were categorized by organ system.
All participants gave written informed consent; the study was approved by the institutional review boards of all clinical sites and the Data Coordinating Center at the University of Alabama, Birmingham.
For dichotomous outcomes measured only at baseline and closeout, inter-group differences were assessed with Fisher’s Exact Test. For other outcomes with multiple measurements over the follow-up period, overall comparisons were conducted with generalized estimating equations (GEE) to assess the relationship between treatment groups over time, adjusting for intra-patient variation 12. For adverse-event categories where at least 5% of study participants experienced an event, the proportions of study participants who experienced an adverse event were calculated by treatment arm and dose level and compared with GEE to assess the relationship between the frequency of adverse events with treatment arm and dose level, adjusting for intra-patient variation. A test for overall dose-response was also conducted for the total of all adverse events by treatment arm. No missing data were imputed. Analyses were conducted with SAS software, v9.2 13
RESULTS
The two treatment groups were well-matched at baseline (Table 1). Overall, 176 men were randomized to the SP arm and 181 men to placebo.
Table 1.
Demographics of CAMUS study participants
| Characteristic (units, [summary statistics]) | Saw palmetto N = 176 | Placebo N= 181 |
|---|---|---|
| Categorical characteristics [N (%)] | ||
| Age (years [N (%)]) | ||
| 50–59 years | 45 (40%) | 42 (37%) |
| 60–69 years | 46 (41%) | 48 (42%) |
| 70–79 years | 21 (18%) | 23 (20%) |
| Race [N (%)] | ||
| African-American | 4 (4%) | 8 (7%) |
| Asian or Pacific Islander | 7 (6%) | 8 (7%) |
| White | 94 (84%) | 89 (79%) |
| Hispanic | 6 (5%) | 5 (4%) |
| Other | 1 (1%) | 2 (2%) |
| Continuous characteristics [mean (SD)] | ||
| American Urological Association Symptom Index (score) | 15.7 (5.7) | 15.0 (5.3) |
| Maximum urinary flow rate (ml/sec) | 11.4 (3.5) | 11.6 (4.3) |
| Post-void residual bladder volume (ml) | 80.0 (51.9) | 84.5 (63.8) |
| BPH Impact Index (score) | 3.4 (2.2) | 3.7 (2.8) |
| PSA (ng/ml) | 2.2 (1.9) | 2.3 (1.1) |
Serious Adverse Events
As previously reported, a total of 36 serious adverse events (SAEs) were identified among CAMUS participants, with 18 SAEs occurring in 17 participants in the SP group and 17 SAEs occurring in 17 participants in the placebo group (p = 1.00, two-sided Fisher’s exact test) (the table from the main publication can be accessed at http://jama.jamanetwork.com/article.aspx?articleid=1104439 9). The most common category of serious adverse events in both groups was hospitalization for surgery or trauma (6 events in the active-treatment arm and 8 events in the placebo group).
No deaths occurred in either treatment group.
Non-Serious Adverse Events
As previously reported, 1006 non-serious adverse events (NSAEs) occurred among all CAMUS participants 9. The most common NSAEs were minor gastrointestinal symptoms, genitourinary problems, musculoskeletal complaints, and upper respiratory tract infections. Overall, there was a slightly, but non-significantly, greater frequency of NSAEs among those participants randomized to the SP group, with an observed NSAE rate among the active-treatment group of 3.01 per participant vs. a rate of 2.63 among placebo-allocated participants (p = 0.16). Overall, 136 participants in the active-treatment group and 137 participants in the placebo group experienced at least 1 NSAE (p = 0.80).
Only 12 NSAEs (1.2%) were considered by the site investigator to be at least probably related to the blinded study intervention while 841 (83.6%) were considered unlikely to be related or definitely unrelated to the intervention. The remaining 153 events (15.2%) were considered possibly related to the study medication.
Laboratory Data
Extensive laboratory testing was conducted throughout the trial and few significant differences between treatment arms were detected (Table 2). Significant between-group differences were found for the hemoglobin level at the highest dose level, and for sodium at the lowest dose level. Across dose levels, the proportions of participants with abnormal hemoglobin levels was higher in the placebo arm than in the SP arm, and sodium abnormalities were reported more frequently in the SP arm, though the magnitude of the differences in both measurements were small (0.6 g/dL for the hemoglobin level and 0.5 mEq/dL for sodium). It should be noted that these differences may not have achieved levels of statistical significance had adjustments been made for multiple comparisons. No differences were detected with respect to urine dipstick levels at week 72 (Table 3).
Table 2.
Proportion of study participants who had an abnormal lab test value by treatment arm and dose level
| Lab Test | Dose Level | Saw palmetto (%) | Placebo (%) | p-value for comparing treatment arms at each dose level* | p-value for comparing treatments across dose levels** |
|---|---|---|---|---|---|
| Bicarbonate | 1 | 14.9 | 14.5 | 1.00 | 0.19 |
| 2 | 14.8 | 13.7 | 0.88 | ||
| 3 | 18.3 | 11.0 | 0.07 | ||
| Chloride | 1 | 13.2 | 7.8 | 0.12 | 0.08 |
| 2 | 11.2 | 7.4 | 0.27 | ||
| 3 | 10.4 | 9.9 | 1.00 | ||
| Creatinine | 1 | 7.5 | 6.7 | 0.84 | 0.69 |
| 2 | 8.3 | 7.4 | 0.84 | ||
| 3 | 7.9 | 7.6 | 1.00 | ||
| GGT | 1 | 11.5 | 8.5 | 0.46 | 0.10 |
| 2 | 10.2 | 8.6 | 0.71 | ||
| 3 | 12.2 | 7.6 | 0.20 | ||
| Glucose | 1 | 35.1 | 38.0 | 0.58 | 0.14 |
| 2 | 33.1 | 38.3 | 0.37 | ||
| 3 | 37.8 | 43.0 | 0.37 | ||
| Hemoglobin | 1 | 12.7 | 19.4 | 0.11 | 0.001 |
| 2 | 12.4 | 19.4 | 0.08 | ||
| 3 | 11.0 | 19.2 | 0.048 | ||
| Potassium | 1 | 9.2 | 8.4 | 0.85 | 0.39 |
| 2 | 8.3 | 8.0 | 1.00 | ||
| 3 | 7.3 | 4.1 | 0.24 | ||
| RBC | 1 | 16.8 | 20.6 | 0.41 | 0.31 |
| 2 | 18.9 | 20.6 | 0.79 | ||
| 3 | 17.8 | 19.8 | 0.68 | ||
| SGOT | 1 | 11.3 | 7.9 | 0.35 | 0.84 |
| 2 | 10.1 | 12.0 | 0.61 | ||
| 3 | 9.8 | 9.9 | 1.00 | ||
| SGPT | 1 | 13.2 | 11.0 | 0.61 | 0.46 |
| 2 | 14.2 | 17.0 | 0.55 | ||
| 3 | 12.2 | 16.3 | 0.35 | ||
| Sodium | 1 | 14.9 | 7.8 | 0.04 | 0.01 |
| 2 | 13.6 | 8.0 | 0.12 | ||
| 3 | 9.8 | 8.1 | 0.70 | ||
| WBC | 1 | 7.5 | 6.7 | 0.84 | 0.57 |
| 2 | 4.1 | 5.7 | 0.62 | ||
| 3 | 6.8 | 8.7 | 0.55 |
Fisher’s exact test
Cochran-Mantel-Haenszel test
Table 3.
Proportion of study participants who had abnormal* urine dipstick values at week 72
| Urine Test | Saw palmetto (%) | Placebo (%) | p-value |
|---|---|---|---|
| Glucose | 3.1 | 1.8 | 0.50 |
| Blood | 4.3 | 3.0 | 0.57 |
| Ketones | 1.8 | 2.4 | 1.00 |
| Protein | 3.7 | 5.3 | 0.60 |
| Leukocyte esterase | 1.23 | 0 | 0.24 |
any abnormality (trace, 1+, 2+, 3+, or 4+)
Vital Signs
Assessments of systolic blood pressure, diastolic blood pressure, and heart rate showed small declines over the course of the study in both study groups (Figure 1). None of the inter-group tests of changes over time were significant.
Figure 1.
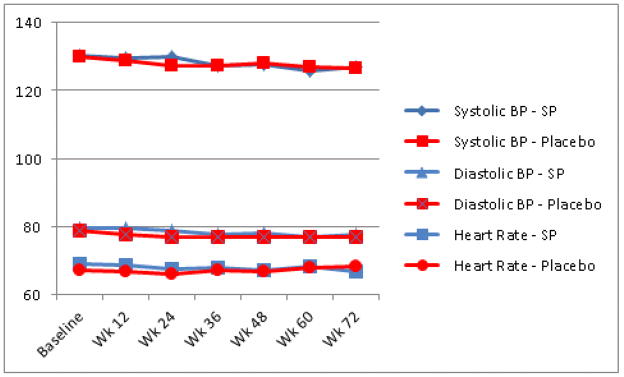
Changes in vital signs among CAMUS participants, stratifed by treatment group
Digital Prostate Exam
No evidence of an increased rate of palpable prostate abnormalities was observed and there were no significant inter-group differences (Table 4).
Table 4.
Prostate digital rectal exam findings*
| Baseline | Closeout | Change | p-value* | |
|---|---|---|---|---|
| Nodules (%) [N(%)] | 1.00 | |||
| Saw Palmetto | 0 (0%) | 0 (0%) | 0 (0%) | |
| Placebo | 0 (0%) | 0 (0%) | 0 (0%) | |
| Asymmetry [N(%)] | 1.00 | |||
| Saw Palmetto | 8 (4.6%) | 5 (3.1%) | −3 (−1.5%) | |
| Placebo | 12 (6.6%) | 8 (4.7%) | −4 (−1.9%) | |
| Tenderness (%) | 1.00 | |||
| Saw Palmetto | 2 (1.1%) | 0 (0%) | −2 (−1.1%) | |
| Placebo | 3 (1.7%) | 1 (0.6%) | −2 (−1.1%) |
p-values for comparison of saw palmetto vs. placebo groups by Fisher’s exact test
Discontinuation Rates
The CAMUS study experienced a low rate of study withdrawal. Overall, 335 of the originally randomized 369 participants completed the study (93.8% of the modified intention-to-treat subset and 90.8% of all randomized participants). While the crude discontinuation rate was slightly higher among participants in the active-treatment group (7.5% vs. 5.0%), this difference was not significant (p = 0.39, Table 5). Only 2 participants withdrew from the study for perceived adverse events and both of these were in the SP group. However, this inter-group difference was also non-significant (p = 0.25).
Table 5.
Discontinuation rates by treatment group and dose
| Treatment Group | Single Dose | Double Dose | Triple Dose | Total | p-value* |
|---|---|---|---|---|---|
| Discontinuation for Any Reason [N (%)] | |||||
| Saw palmetto | 4 (2.3%) | 8 (4.6%) | 1 (0.6%) | 13 (7.5%) | 0.39 |
| Placebo | 2 (1.1%) | 6 (3.3%) | 1 (0.6%) | 9 (5.0%) | |
| Discontinuation for Adverse Event [N (%)] | |||||
| Saw palmetto | 1 (0.6%) | 1 (0.6%) | 0 (0%) | 2 (1.2%) | 0.25 |
| Placebo | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
p-value for comparison of Saw palmetto vs. placebo groups by Fisher’s exact test
Dose-Response Data
Potentially important toxicities associated with higher doses of SP may be obscured by grouping response data for all three doses together. Therefore, we analyzed all NSAEs that occurred with a frequency ≥5% for evidence of dose-response phenomena in their frequency (Table 6). Only upper respiratory tract infections and oral/dental problems showed evidence of a significant dose-response relationship but for both of these NSAEs, the trend was toward a reduced frequency of events with increasing doses of study medication. An overall test of dose-related differences in the frequency of NSAEs was non-significant (p > 0.2).
Table 6.
Proportions of participants who experienced each category of adverse event by dose level *
| Adverse Event | Saw palmetto (%) | Placebo (%) | p-value for drug | p-value (Double vs Single)** | p-value (Triple vs single)** |
|---|---|---|---|---|---|
| Arrhythmia | 0.67 | 0.41 | 0.41 | ||
| Single dose | 2.3 | 2.2 | |||
| Double dose | 1.1 | 1.7 | |||
| Triple dose | 1.1 | 1.7 | |||
| Elevated blood pressure | 0.10 | 0.20 | 0.12 | ||
| Single dose | 4.0 | 1.7 | |||
| Double dose | 2.8 | 0 | |||
| Triple dose | 0.6 | 1.7 | |||
| Upper respiratory infection | 0.64 | 0.17 | <0.001 | ||
| Single dose | 11.8 | 15.9 | |||
| Double dose | 10.6 | 10.2 | |||
| Triple dose | 6.3 | 6.0 | |||
| Flu-like symptoms | 0.60 | 0.28 | 0.45 | ||
| Single dose | 4.5 | 3.3 | |||
| Double dose | 3.4 | 1.7 | |||
| Triple dose | 2.3 | 3.3 | |||
| Oral/dental | 0.10 | 0.72 | <0.001 | ||
| Single dose | 5.7 | 5.0 | |||
| Double dose | 6.8 | 2.8 | |||
| Triple dose | 1.7 | 0 | |||
| Musculoskeletal | 0.44 | 0.28 | 0.41 | ||
| Single dose | 15.6 | 15.5 | |||
| Double dose | 15.6 | 9.9 | |||
| Triple dose | 13.4 | 13.0 | |||
| Genitourinary | 0.90 | 0.98 | 0.78 | ||
| Single dose | 10.6 | 8.7 | |||
| Double dose | 9.5 | 9.7 | |||
| Triple dose | 8.4 | 9.3 | |||
| Elevated PSA | 0.81 | 0.65 | 0.35 | ||
| Single dose | 1.1 | 2.8 | |||
| Double dose | 2.8 | 2.2 | |||
| Triple dose | 4.0 | 2.2 | |||
| Gastrointestinal | 0.69 | 0.72 | 0.09 | ||
| Single dose | 12.4 | 10.9 | |||
| Double dose | 10.1 | 11.3 | |||
| Triple dose | 6.2 | 9.3 | |||
| Dermatologic | 0.30 | 0.73 | 0.56 | ||
| Single dose | 2.8 | 4.9 | |||
| Double dose | 3.9 | 2.7 | |||
| Triple dose | 2.8 | 6.6 | |||
| Physical injury/trauma | 0.02 | 0.37 | 0.18 | ||
| Single dose | 4.0 | 1.1 | |||
| Double dose | 5.1 | 2.2 | |||
| Triple dose | 6.2 | 2.8 | |||
| Abnormal serum chemistry | 0.66 | 0.69 | 0.07 | ||
| Single dose | 0 | 1.7 | |||
| Double dose | 0.6 | 0.6 | |||
| Triple dose | 4.5 | 1.7 |
Includes only those adverse events that occurred in at least 5% of study participants
p-value for comparing dose levels across treatments and time adjusting for intra-patient variation using generalized estimating equations
Other Outcomes
As previously reported, there were no significant differences between treatment groups in changes over time for the International Index of Erectile Function, the Male Sexual Health Questionnaire - Ejaculatory Dysfunction Scale, the National Institutes of Health Chronic Prostatitis Symptom Index, the International Continence Society Male Incontinence Scale, and the Jenkins Sleep Dysfunction Scale 9.
DISCUSSION
Consistent with the results of the STEP study 7, the multicenter CAMUS trial found no evidence of important toxicity associated with higher-than-usual SP doses in a larger patient cohort with dose escalation and an 18-month period of product exposure.
While there was a higher rate of NSAEs among SP-allocated participants than in placebo-treated participants, the difference was not significant, and the absolute difference between the rates in the two study groups was small. The great majority of these NSAEs were not thought to be related to the study medication by the local site investigators. The rates of SAEs were nearly identical between the study groups. There were also few clinically important between-group differences in rates of laboratory abnormalities, as well as no differences in vital signs or palpable prostate abnormalities. Finally, overall and cause-specific withdrawal rates were low and not significantly different between the two study arms.
Taken together, the evidence from the two large NIH-funded clinical trials of SP are highly consistent in suggesting that, for periods up to 18 months, there are no serious safety concerns associated with use of this dietary supplement, even at a dose of nearly 1 gm/day, three times the typical dose. In addition, no other common or serious toxicity has been noted in any of the prior trials of SP, though most of these studies did not routinely report extensive data on safety and toxicity in a standardized manner 14. Recent reviews of reported toxicities associated with the use of herbal supplements have generally concluded that SP appears to be relatively safe 3,14–17. Importantly, these data, as well as additional published data, strongly suggest that SP does not alter PSA values 7,18.
While reassuring, these data do not preclude the occurrence of rare and potentially serious toxicities of SP, since such events would be too uncommon to be recognized in trials of this size. Indeed, there are a handful of case reports implicating SP in a disparate set of adverse events including hepatotoxicity 19, coagulopathy 20, pancreatitis 21,22, and intraoperative floppy-iris syndrome 23. However, the causal relationship between the use of SP and each of these adverse events is not firmly established for most of these, as these reports are complicated by concomitant use of other drugs or supplements, absence of re-challenge, and missing information about temporal relationships. Nonetheless, as with most herbal therapies, SP contains many organic compounds and individuals may be at risk for idiosyncratic toxicities. The potential for significant herb-drug interactions has been described for other widely used products, such as St. John’s Wort, but has not been explored for most marketed supplements 24.
Reliable data on the safety of dietary supplements are critical since current U.S. regulatory policy permits distributing and marketing of supplements without governmental pre-market review of clinical data on safety and efficacy 25. Despite lack of safety and efficacy data on dietary supplements, use of these products by the public is widespread, with regular use by approximately 20% of the public 1. In view of extensive public use, large trials such as CAMUS provide much-needed safety data. Reports indicate that negative results of large trials of dietary supplements do impact product sales and patterns of use by the public 1,26,27. It is also recognized that even when efficacy data demonstrate no clear superiority of a supplement over placebo, many people will continue to take these over-the-counter supplements because they perceive a benefit, despite the average null effect observed in the studies 10. Hence, even if published data do not support the efficacy of a dietary supplement, many individuals will continue to self-medicate with these substances. Toxicity data remain vital for patients’ informed decision making regarding how they manage their health care, as well as for healthcare providers in counseling patients.
While the CAMUS trial was the largest clinical study of SP that included detailed and repeated structured assessments of symptomatic adverse effects and asymptomatic physical and laboratory abnormalities, its limitations should be acknowledged. As noted above, the size of the trial was too small to rule out uncommon but potentially serious SP-related toxicity. While we examined a large set of laboratory tests, there are many more measurements which were not assessed that might have revealed unrecognized toxicity. Though our safety data (and those of the STEP study) are reassuring for those supplements used in these trials, they may not generalize to all other manufacturers or extraction techniques 28. We did not have analyses relevant to the issue of potential interactions between SP and prescription medications or other dietary supplements. We conducted a large number of statistical tests without adjustment for multiple comparisons and the potential for false positives must be considered. Finally, the CAMUS trial lasted only 18 months and these results may not generalize well to longer time frames (a particular concern since men often use SP supplements for many years).
CONCLUSIONS
Our data suggest that SP is unlikely to be associated with important and common toxicity for a period up to 18 months. While the most recent clinical evidence does not support the superiority of SP over placebo, it appears that those men who elect to try the supplement are unlikely to suffer substantial adverse medical consequences from its short-term use. This information should help the many men who suffer from LUTS and their care providers make more informed and personally appropriate decisions for managing this common and bothersome condition.
Acknowledgments
This study was funded by cooperative agreements from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: U01 DK63795, U01 DK63797, U01 DK63825, U01 DK63835, U01 DK63866, U01 DK63833, U01 DK63862, U01 DK63840, U01 DK63883, U01 DK63831, U01 DK63778 and U01 DK63788. Support was also provided by the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, NIH. Saw palmetto extract and matching placebo were donated by Rottapharm/Madaus, Cologne, Germany.
Glossary
- AUASI
American Urological Association Symptom Index
- BPH
Benign Prostatic Hyperplasia (BPH)
- CAMUS
Complementary and Alternative Medicine for Urinary Symptoms trial
- LUTS
Lower Urinary Tract Symptoms
- NSAE
Non-Serious Adverse Event
- PSA
Prostate Specific Antigen
- SAE
Serious Adverse Event
- SP
Saw palmetto
- STEP
Saw palmetto Treatment for Enlarged Prostates (STEP) trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. [PubMed] [Google Scholar]
- 2.Tacklind J, MacDonald R, Rutks I, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;(2):CD001423. doi: 10.1002/14651858.CD001423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avins AL, Bent S. Saw palmetto and lower urinary tract symptoms: what is the latest evidence? Curr Urol Reports. 2006;7:260–5. doi: 10.1007/s11934-996-0004-2. [DOI] [PubMed] [Google Scholar]
- 4.Wilt TJ, Ishani A, Stark G, et al. Saw palmetto extracts for treatment of benign prostatic hyperplasia. JAMA. 1998;280:1604–9. doi: 10.1001/jama.280.18.1604. [DOI] [PubMed] [Google Scholar]
- 5.Wilt T, Ishani A, Mac Donald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002:CD001423. doi: 10.1002/14651858.CD001423. [DOI] [PubMed] [Google Scholar]
- 6.Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557–66. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 7.Avins AL, Bent S, Staccone S, et al. A detailed safety assessment of a saw palmetto extract. Complement Ther Med. 2008:147–154. doi: 10.1016/j.ctim.2007.10.005. 2008/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Andriole G, Avins A, et al. Redesigning a large-scale clinical trial in response to negative external trial results: The CAMUS study of Phytotherapy for Benign Prostatic Hyperplasia. Clinical Trials. 2009 doi: 10.1177/1740774509352199. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry MJ, Meleth S, Lee JY, et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: a randomized trial. JAMA. 2011;306:1344–1351. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilburt JC, Emanuel EJ, Miller FG. Does the evidence make a difference in consumer behavior? Sales of supplements before and after publication of negative research results. J Gen Intern Med. 2008;23:1495–1498. doi: 10.1007/s11606-008-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry MJ, JFF, MPOL, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 12.Diggle PJ, Heagerty P, Liang KY, et al. Analysis of longitudinal data. Oxford: Oxford University Press; 2002. [Google Scholar]
- 13.SAS Institute I. SAS statistical software. 2008. [Google Scholar]
- 14.Agbabiaka TB, Pittler MH, Wider B, et al. Serenoa repens (saw palmetto): a systematic review of adverse events. Drug Saf. 2009;32:637–647. doi: 10.2165/00002018-200932080-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ernst E. The risk-benefit profile of commonly used herbal therapies: gingko, St. John’s wort, ginseng, echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136:42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- 16.Kane CJ, Raheem OA, Bent S, et al. What do I tell patients about saw palmetto for benign prostatic hyperplasia? Urol Clin North Am. 2011;38:261–277. doi: 10.1016/j.ucl.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald R, Tacklind JW, Rutks I, et al. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): an updated Cochrane systematic review. [accessed May 12, 2012];BJU International. 2012 doi: 10.1111/j.1464-410X.2012.11172.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22551330. [DOI] [PMC free article] [PubMed]
- 18.Carraro JC, Raynaud JP, Koch G, et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. Prostate. 1996;29:231–40. doi: 10.1002/(SICI)1097-0045(199610)29:4<231::AID-PROS4>3.0.CO;2-E. discussion 241–2. [DOI] [PubMed] [Google Scholar]
- 19.Lapi F, Gallo E, Giocaliere E, et al. Acute liver damage due to Serenoa repens: a case report. Br J Clin Pharmacol. 2010;69:558–560. doi: 10.1111/j.1365-2125.2010.03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheema P, El-Mefty O, Jazieh AR. Intraoperative haemorrhage associated with the use of extract of Saw Palmetto herb: a case report and review of literature. J Intern Med. 2001;250:167–9. doi: 10.1046/j.1365-2796.2001.00851.x. [DOI] [PubMed] [Google Scholar]
- 21.Wargo KA, Allman E, Ibrahim F. A possible case of saw palmetto-induced pancreatitis. South Med J. 2010;103:683–685. doi: 10.1097/SMJ.0b013e3181e1e3ee. [DOI] [PubMed] [Google Scholar]
- 22.Jibrin I, Erinle A, Saidi A, et al. Saw palmetto-induced pancreatitis. South Med J. 2006;99:611–2. doi: 10.1097/01.smj.0000215642.76198.44. [DOI] [PubMed] [Google Scholar]
- 23.Yeu E, Grostern R. Saw palmetto and intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2007;33:927–928. doi: 10.1016/j.jcrs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Hu Z, Yang X, Ho PCL, et al. Herb-drug interactions: a literature review. Drugs. 2005;65:1239–1282. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 25.Glisson JK, Walker LA. How physicians should evaluate dietary supplements. Am J Med. 2010;123:577–582. doi: 10.1016/j.amjmed.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PM, Powell-Griner E, McFann K, et al. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 27.Nutrition Business Journal: Supplement Business Report 2011. Boulder, CO: New Hope Natural Media, Penton Media, Inc; 2011. [Google Scholar]
- 28.Habib FK, Wyllie MG. Not all brands are created equal: a comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer Prostatic Dis. 2004;7:195–200. doi: 10.1038/sj.pcan.4500746. [DOI] [PubMed] [Google Scholar]


