Abstract
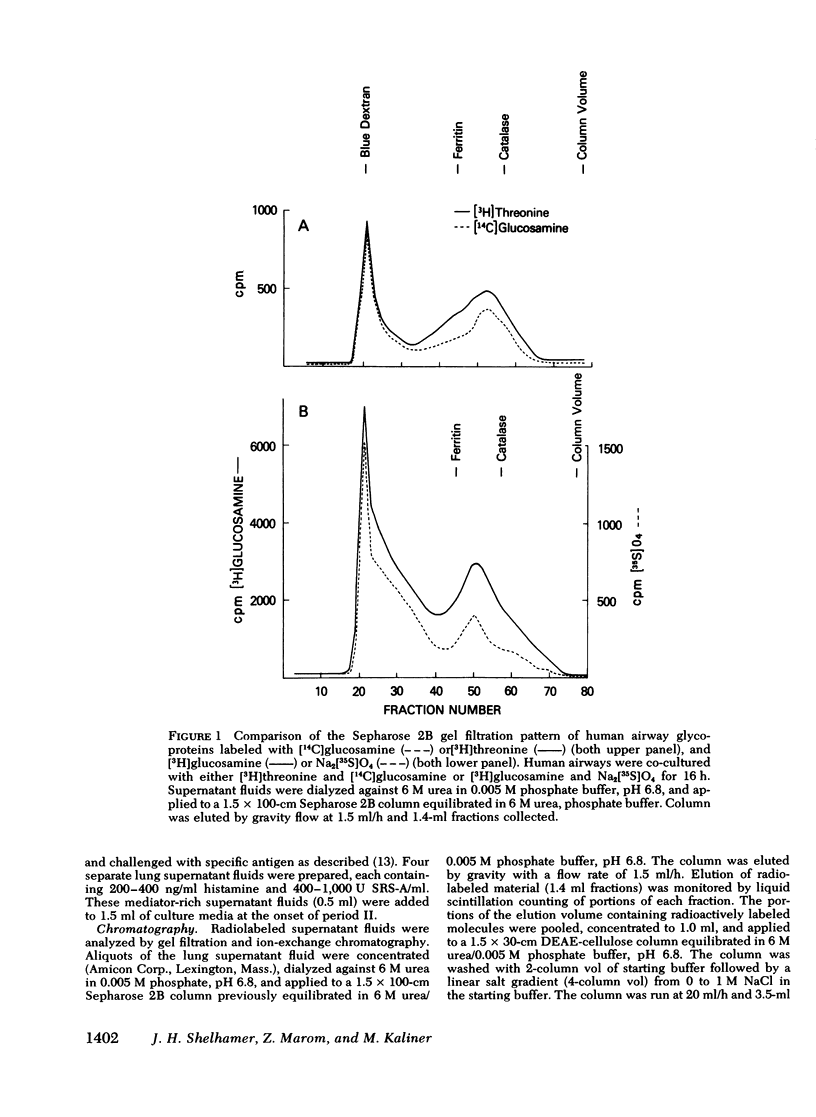
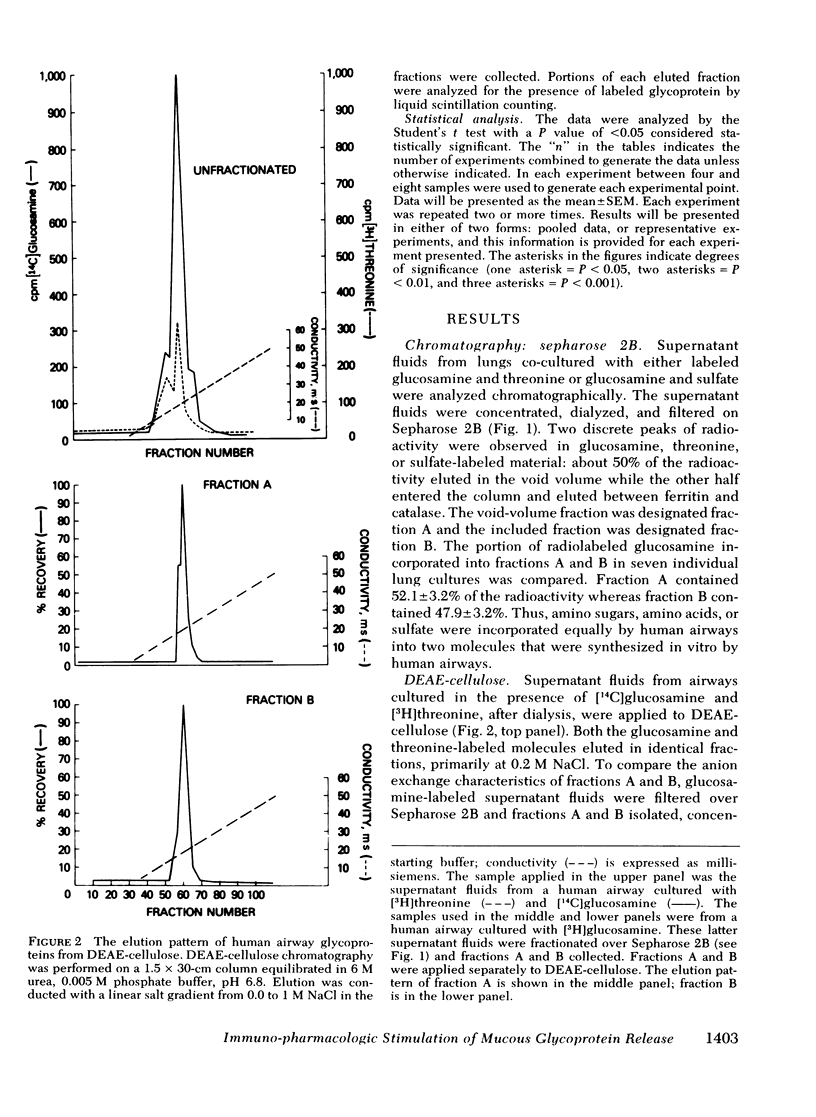
Human bronchial airways obtained after surgical resection were maintained in tissue culture for 24-48 h. Incorporation of [3H]- or [14C]-glucosamine, [14C]threonine, or Na2[35S]O4 to the culture media resulted in biosynthesis of two radiolabeled glycoproteins—one filtering in the exclusion volume of Sepharose 2B, and the other filtering with an approximate molecular weight of 400,000. Both fractions had similar elution patterns from DEAE-cellulose anion exchange chromatography. [3H]Glucosamine was incorporated equally into the two fractions.
The effects of anaphylaxis, histamine, and several neurohormones upon the release of [3H]glucosamine-labeled glycoproteins were analyzed, making no attempt to separate the two glycoprotein fractions. Three lines of evidence were found suggesting that mast-cell degranulation increases mucous release from cultured airways. (a) Supernatant fluids from anaphylaxed peripheral human lung that contained 200-400 ng/ml histamine and 400-1,000 U/ml slow-reacting substance of anaphylaxis (SRS-A) increased release by 40±18%. (b) The addition of antigen to IgE-sensitized airways led to the release of 26±7% of the total histamine and a 36±14% increase in mucous release. (c) Reversed anaphylaxis with anti-IgE antibodies induced a 36±6% release of histamine from the airways and an increase in the release of mucous glycoproteins of 25±9%.
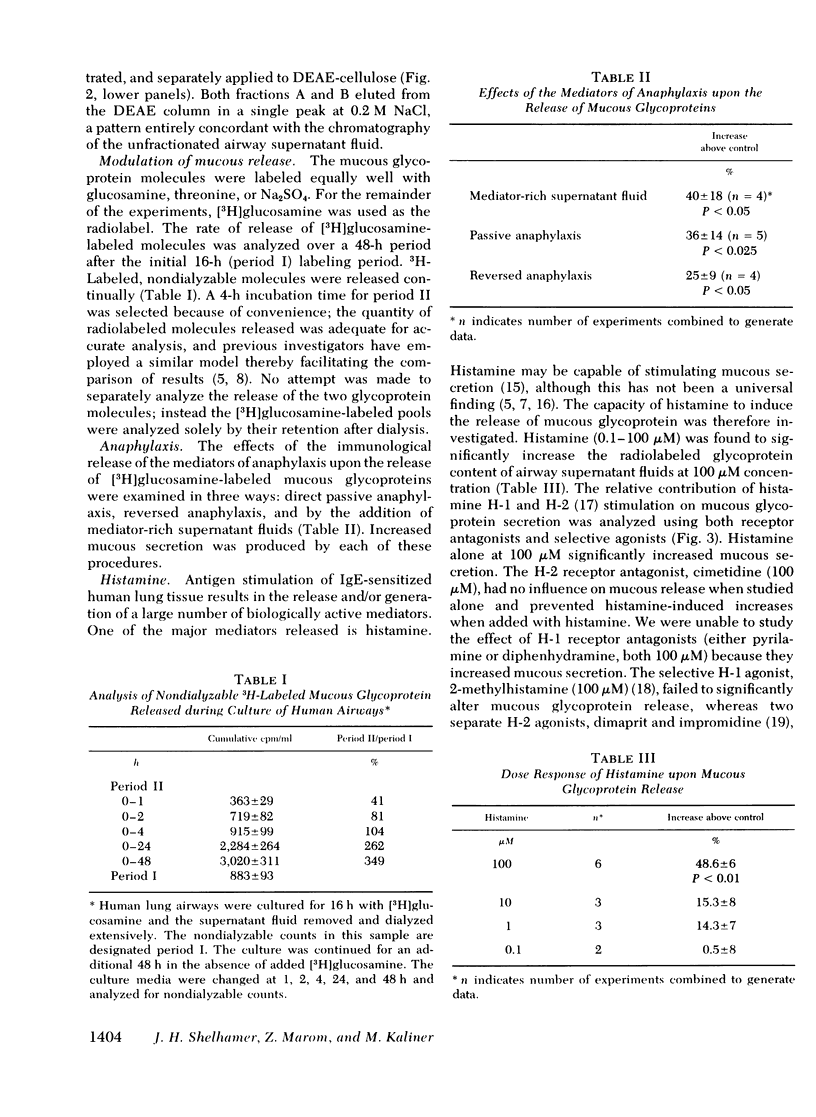
Exogenous histamine added to airways increased mucous glycoprotein release, an effect prevented by cimetidine, an H-2 antagonist. Selective histamine H-2, but not H-1 agonists increased mucous glycoprotein release, suggesting the possibility that anaphylaxis of airways results in increased mucous glycoprotein release partly through histamine H-2 stimulation.
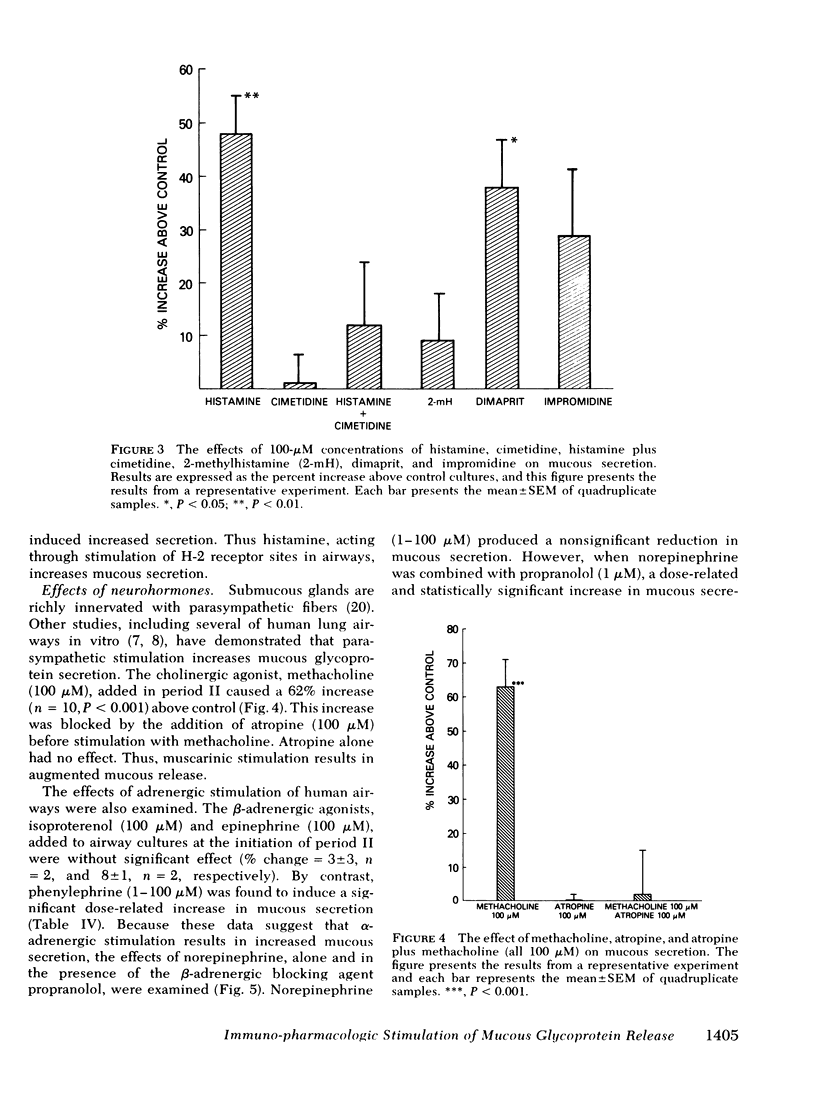
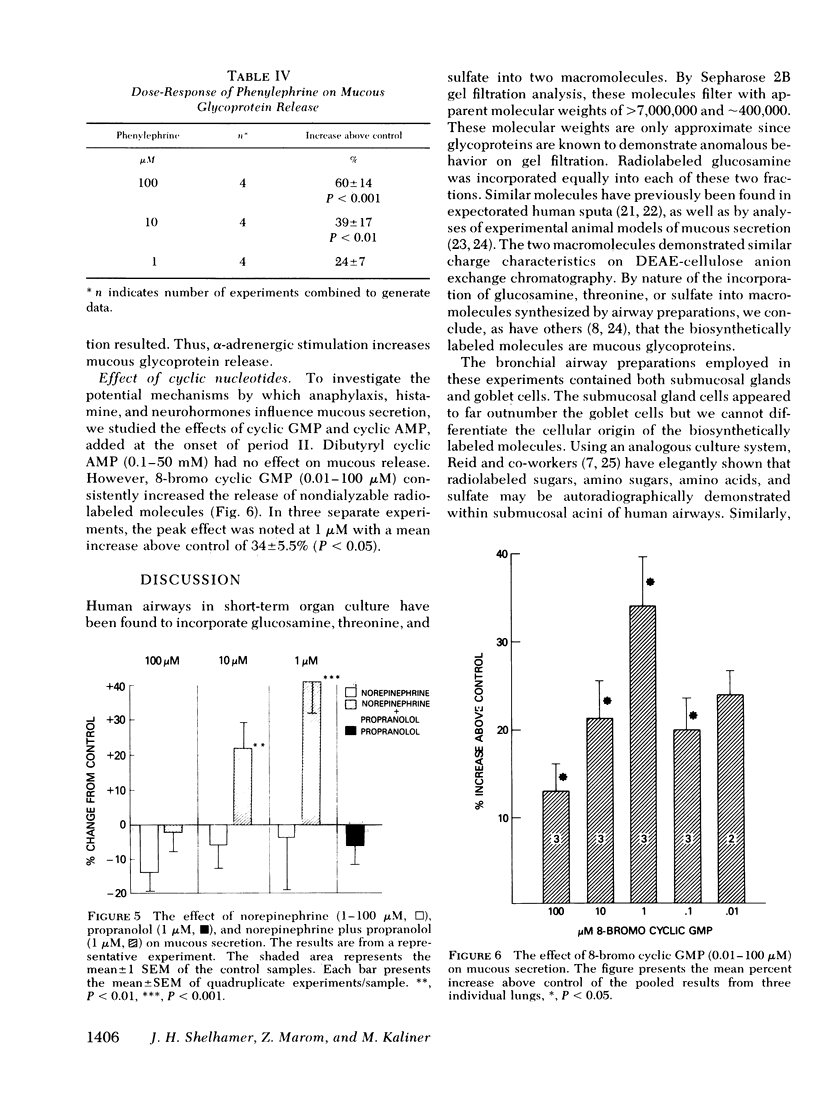
A cholinomimetic agonist, methacholine, increased mucous release; this response was prevented by atropine which alone had no effect. No response to β-adrenergic stimulation with either isoproterenol or epinephrine was noted. However, α-adrenergic stimulation with either norepinephrine combined with propranolol or phenylephrine alone resulted in dose-related increases in glycoprotein release. Both α-adrenergic and cholinergic stimulation of human tissues induce the formation of guanosine 3′,5′-phosphoric acid (cyclic GMP), and 8-bromo cyclic GMP added to the airways led to increased mucous secretion. Thus, it seems likely that neurohormones capable of stimulating cyclic GMP formation in human airways may lead to increased mucous glycoprotein release.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball J. H., Kaminsky N. I., Hardman J. G., Broadus A. E., Sutherland E. W., Liddle G. W. Effects of catecholamines and adrenergic-blocking agents on plasma and urinary cyclic nucleotides in man. J Clin Invest. 1972 Aug;51(8):2124–2129. doi: 10.1172/JCI107019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L. A., McDowell E. M., Frank A. L., Harris C. C., Trump B. F. Long-term organ culture of human bronchial epithelium. Cancer Res. 1976 Mar;36(3):1003–1010. [PubMed] [Google Scholar]
- Beaven M. A. Histamine (second of two parts). N Engl J Med. 1976 Feb 5;294(6):320–325. doi: 10.1056/NEJM197602052940608. [DOI] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W., Iyer R. N., Carlson D. M., Polony I. Human respiratory tract secretion. Mucous glycoproteins of nonpurulent tracheobronchial secretions, and sputum of patients with bronchitis and cystic fibrosis. Arch Biochem Biophys. 1976 Nov;177(1):95–104. doi: 10.1016/0003-9861(76)90419-7. [DOI] [PubMed] [Google Scholar]
- Chakrin L. W., Baker A. P., Christian P., Wardell J. R., Jr Effect of cholinergic stimulation on the release of macromolecules by canine trachea in vitro. Am Rev Respir Dis. 1973 Jul;108(1):69–76. doi: 10.1164/arrd.1973.108.1.69. [DOI] [PubMed] [Google Scholar]
- Chakrin L. W., Baker A. P., Spicer S. S., Wardell J. R., Jr, De Sanctis N., Dries C. Synthesis and secretion of macromolecules by canine trachea. Am Rev Respir Dis. 1972 Mar;105(3):368–381. doi: 10.1164/arrd.1972.105.3.368. [DOI] [PubMed] [Google Scholar]
- Davis B., Phipps R. J., Nadel J. A. A new method for studying tracheal secretion in vitro: effect of adrenergic agonists in cats. Chest. 1979 Feb;75(2 Suppl):224–225. [PubMed] [Google Scholar]
- Durant G. J., Duncan W. A., Ganellin C. R., Parsons M. E., Blakemore R. C., Rasmussen A. C. Impromidine (SK&F 92676) is a very potent and specific agonist for histamine H2 receptors. Nature. 1978 Nov 23;276(5686):403–405. doi: 10.1038/276403a0. [DOI] [PubMed] [Google Scholar]
- Ellis D. B., Stahl G. H. Biosynthesis of respiratory-tract mucins. Incorporation of radioactive precursors into glycoproteins by canine tracheal explants in vitro. Biochem J. 1973 Dec;136(4):837–844. doi: 10.1042/bj1360837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Kent P. W., Passatore M., Phipps R. J., Richardson P. S. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):49–76. doi: 10.1098/rspb.1975.0151. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Shelhamer J. H., Reingold D. B., Smith L. J., Evans R., 3rd, Kaliner M. Alpha-adrenergic hyper-responsiveness in asthma. N Engl J Med. 1979 Mar 22;300(12):642–647. doi: 10.1056/NEJM197903223001203. [DOI] [PubMed] [Google Scholar]
- Kaliner M. Human lung tissue and anaphylaxis. I. The role of cyclic GMP as a modulator of the immunologically induced secretory process. J Allergy Clin Immunol. 1977 Sep;60(3):204–211. doi: 10.1016/0091-6749(77)90125-7. [DOI] [PubMed] [Google Scholar]
- Kaliner M. The cholinergic nervous system and immediate hypersensitivity. 1. Eccrine sweat responses in allergic patients. J Allergy Clin Immunol. 1976 Aug;58(2):308–315. doi: 10.1016/0091-6749(76)90136-6. [DOI] [PubMed] [Google Scholar]
- Lake A. M., Bloch K. J., Neutra M. R., Walker W. A. Intestinal goblet cell mucus release. II. In vivo stimulation by antigen in the immunized rat. J Immunol. 1979 Mar;122(3):834–837. [PubMed] [Google Scholar]
- Lake A. M., Bloch K. J., Sinclair K. J., Walker W. A. Anaphylactic release of intestinal goblet cell mucus. Immunology. 1980 Feb;39(2):173–178. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vidriero M. T., Das I., Smith A. P., Picot R., Reid L. Bronchial secretion from normal human airways after inhalation of prostaglandin F2alpha, acetylcholine, histamine, and citric acid. Thorax. 1977 Dec;32(6):734–739. doi: 10.1136/thx.32.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESSER J. W., PETERS G. A., BENNETT W. A. Causes of death and pathologic findings in 304 cases of bronchial asthma. Dis Chest. 1960 Dec;38:616–624. doi: 10.1378/chest.38.6.616. [DOI] [PubMed] [Google Scholar]
- Mann S. P. The innervation of mammalian bronchial smooth muscle: the localization of catecholamines and cholinesterases. Histochem J. 1971 Sep;3(5):319–331. doi: 10.1007/BF01005014. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. In vitro incorporation of (3H)threonine and (3H)glucose by the mucous and serous cells of the human bronchial submucosal gland. A quantitative electron microscope study. J Cell Biol. 1975 Nov;67(2PT1):320–344. doi: 10.1083/jcb.67.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D. V. Pharmacology of mucus. Br Med Bull. 1978 Jan;34(1):89–94. doi: 10.1093/oxfordjournals.bmb.a071465. [DOI] [PubMed] [Google Scholar]
- Paul W., Weir D. M. Histamine release from human lung by specific antisera. Clin Exp Immunol. 1969 Sep;5(3):311–316. [PMC free article] [PubMed] [Google Scholar]
- Platshon L. F., Kaliner M. The effects of the immunologic release of histamine upon human lung cyclic nucleotide levels and prostaglandin generation. J Clin Invest. 1978 Dec;62(6):1113–1121. doi: 10.1172/JCI109230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reasor M. J., Adams G. K., 3rd, Proctor D. F., Rubin R. J. Tracheobronchial secretions collected from intact dogs. I. Protein and mucous glycoprotein composition. J Appl Physiol Respir Environ Exerc Physiol. 1978 Aug;45(2):182–189. doi: 10.1152/jappl.1978.45.2.182. [DOI] [PubMed] [Google Scholar]
- Reasor M. J., Cohen D., Proctor D. F., Rubin R. J. Tracheobronchial secretions collected from intact dogs. II. Effects of cholinomimetic stimulation. J Appl Physiol Respir Environ Exerc Physiol. 1978 Aug;45(2):190–194. doi: 10.1152/jappl.1978.45.2.190. [DOI] [PubMed] [Google Scholar]
- Reed C. E. Abnormal autonomic merchanisms in asthma. J Allergy Clin Immunol. 1974 Jan;53(1):34–41. doi: 10.1016/0091-6749(74)90097-9. [DOI] [PubMed] [Google Scholar]
- Roberts G. P. The role of disulfide bonds in maintaining the gel structure of bronchial mucus. Arch Biochem Biophys. 1976 Apr;173(2):528–537. doi: 10.1016/0003-9861(76)90289-7. [DOI] [PubMed] [Google Scholar]
- Sturgess J., Reid L. An organ culture study of the effect of drugs on the secretory activity of the human bronchial submucosal gland. Clin Sci. 1972 Oct;43(4):533–543. doi: 10.1042/cs0430533. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Wu M., Bloch K. J. Stimulation by immune complexes of mucus release from goblet cells of the rat small intestine. Science. 1977 Jul 22;197(4301):370–372. doi: 10.1126/science.877559. [DOI] [PubMed] [Google Scholar]
- Wardell J. R., Jr, Chakrin L. W., Payne B. J. The canine tracheal pouch. A model for use in respiratory mucus research. Am Rev Respir Dis. 1970 May;101(5):741–754. doi: 10.1164/arrd.1970.101.5.741. [DOI] [PubMed] [Google Scholar]


