Abstract
Aged mice exhibit ~ 5-10 fold increases in an ordinarily minor CD21/35− CD23− mature B cell subset termed age-associated B cells (ABC). ABC from old, but not young, mice induce apoptosis in pro-B cells directly through secretion of TNFα. In addition, aged ABC, via TNFα, stimulate bone marrow cells to suppress pro-B cell growth. ABC effects can be prevented by the anti-inflammatory cytokine IL-10. Notably, CD21/35+ CD23+ follicular (FO) splenic and FO-like recirculating bone marrow B cells in both young and aged mice contain a subpopulation which produces IL-10. Unlike young adult FO B cells, old FO B cells also produce TNFα; however, secretion of IL-10 within this B cell population ameliorates the TNFα-mediated effects on B cell precursors. Loss of B cell precursors in the bone marrow of old mice in vivo was significantly associated with increased ABC relative to recirculating FO-like B cells. Adoptive transfer of aged ABC into RAG-2 KO recipients resulted in significant losses of pro-B cells within the bone marrow. These results suggest that alterations in B cell composition during old age, in particular the increase in ABC within the B cell compartments contribute to a pro-inflammatory environment within the bone marrow. This provides a mechanism of inappropriate B cell “feedback” which promotes down-regulation of B lymphopoiesis in old age.
INTRODUCTION
The decline in B lymphopoiesis within the bone marrow of aged mice has been well characterized over the last two decades (reviewed in Allman and Miller, 2005; Cancro et al., 2009; Linton and Dorshkind, 2004; Riley et al., 2005) . Multiple mechanisms have been shown to contribute to this phenomenon including increased apoptosis among B cell precursors (Kirman et al., 1998; Sherwood et al., 2003; Van der Put et al., 2003); diminished growth factor expression within the bone marrow and reduced capacity of B cell precursors to respond to these cytokines (Stephan et al., 1997; Stephan et al., 1998); and reduced expression of key transcription factors (E2A; EBF1) (Frasca et al., 2003; King et al., 2007; Lescale et al., 2010; Sherwood et al., 2000; Van der Put et al., 2004) and expression of their targeted gene products (RAG-1,RAG-2; surrogate light chain) (Alter-Wolf et al., 2009; Labrie et al., 2004; Sherwood et al., 1998; Sherwood et al., 2000). These processes can affect a variety of stages of B lymphopoiesis, including hematopoietic stem cell commitment to the B lineage (Guerrettaz et al., 2008; Muller-Sieburg et al., 2012); generation of common lymphoid progenitors (CLPs) (Miller and Allman, 2003); as well as development of more differentiated B cell precursors, e.g., pro-B cells and their progression through the pro-B to pre-B cell checkpoint (Riley et al., 1991; Stephan et al., 1996; Van der Put et al., 2003). The decline in B lymphopoiesis coincides with alterations, not only in new B cell development, but also in the readout of the antibody repertoire within the bone marrow and periphery (reviewed in Klinman and Kline, 1997; Song et al., 1997). Although clearly important to our understanding of B cell functional deficits in old age, the cellular and molecular “triggers” leading to altered B lymphopoiesis in old age remain poorly defined.
Recently, Keren, et. al. (2011b), demonstrated that serial rounds of depletion of mature B cells in aged mice, followed by autoreconstitution, resulted in progressive recovery of B lymphopoiesis to levels seen in young adults. This suggested that there is “negative feed-back” from mature B cells in aged mice that impairs new B cell development within the bone marrow (Keren et al., 2011a; Keren et al., 2011b). We hypothesize that a newly defined population of B cells, termed age-associated B cells (ABC) (Hao et al., 2011), characterized as CD21/35− CD23−, increases in the bone marrow and spleen in old age and inhibits the development and maintenance of B cell precursors. Our studies reveal that ABC, through TNFα expression, regulate inhibition of B lymphopoiesis in aged mice.
RESULTS
ABC increase in the spleen and bone marrow of aged mice
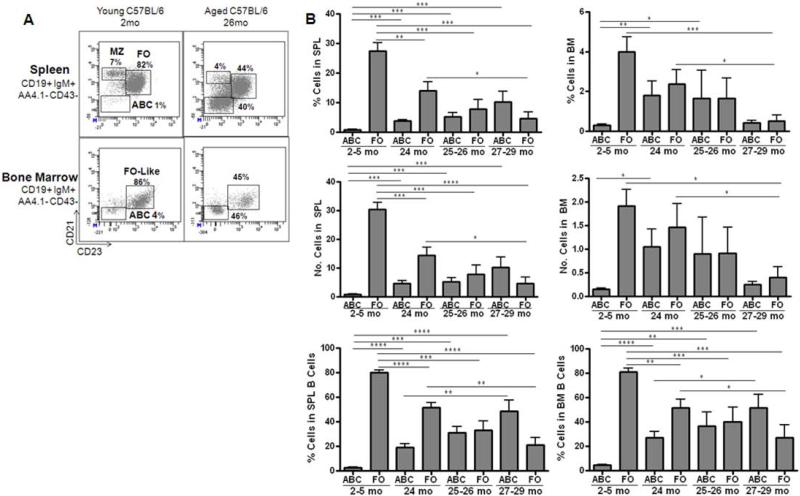
With old age, the representations of B cell subsets within the spleen and bone marrow are considerably altered. Recently, Hao, et. al. (2011), have shown that B cells in the spleens of aged mice are increasingly comprised of a novel B cell subset bearing little CD21/35 or CD23. Among mature (AA4.1−) B cells, CD21/35− CD23− “age-associated B cells” (ABC) were increased an average of 5-fold in proportion and number in the spleens of 24 months old C57BL/6 (B6) mice and were 12-fold increased by 27-29 months of age (Fig. 1). Our ABC were comparable in surface phenotype to those described by Hao, et. al. (2011); e.g., CD21/35− CD23− CD5low/negative CD43/S7− AA4.1− IgM+ CD19+ CD45R (B220)+, but differed from another CD21/35− B cell subset that increases in aged mice described by Rubtsov, et. al (2011), and also called “ABC”, in that the ABC in our studies were negative for CD11b and CD11c (data not shown).
Figure 1. Age-associated B cells (ABC) accumulate in spleen and bone marrow of aged C57BL/6 mice.
Panel A: Spleen and bone marrow were obtained from young (2 mo.) and old (26 mo.) C57BL/6 mice, processed, and stained for follicular B cells (FO; IgM+ CD21/35+ CD23+ CD19+), age-associated B cells (ABC; IgM+ CD21/35− CD23− CD19+), and marginal zone B cells (MZ; IgM+ CD21/35high CD23− CD19+). In the analyses shown, immature B cells (AA4.1+) and B1 B cells (CD43/S7+) were gated out. Data are shown as the percentage of these B cell populations within the total mature, non-B1 pool. Panel B: Cumulative results showing the percentages and numbers of ABC and FO B cells in spleens and of ABC and FO-like, recirculating mature B cells in bone marrow of aged and young mice. Results shown are for 22 young mice (2-5 mo), 12 aged mice at 24 mo, 5 aged mice at 25-26 mo, and 8 aged mice at 27-29 mo of age. Total spleen cells averaged 124 × 106 in young mice and 112 × 106 in old mice; total bone marrow cells averaged 48 × 106 for young and 55 × 106 for aged mice for combined femur/tibia pairs. Statistical significance as shown: *, p<0.045, **, p<0.0083; ***, p<0.0009; ****, p<0.0001.
Concomitantly, as ABC increased in aged spleens, follicular (FO) B cells were reduced (Fig. 1). Marginal zone (MZ) (CD21/CD35hi CD23−) and B1 (CD43/S7+) B cells were also examined and each represented minor populations of <10% of B cells in our aged B6 mice (Fig. 1; data not shown). ABC constituted, on average, ~20-50% of total mature B cells in the spleens of 24-29 months old B6 mice. ABC were also increased in the blood (data not shown) and in the bone marrow of old mice compared to young controls (Fig. 1). In the bone marrow, ABC levels were variable, but on average, represented ~20-60% of mature B cells in old mice depending upon the age (Fig. 1).
ABC from old mice inhibited IL-7 mediated pro-B cell, but not pre-B cell, growth via TNFα mediated apoptosis
As an initial test of whether B cells with the ABC phenotype could alter B cell precursor functions, we asked whether the expanded ABC seen in aged spleen could affect B cell precursor growth in vitro. In these experiments, splenic ABC from aged mice were cultured in the upper chambers of transwell cultures separated by a semi-permeable membrane from unfractionated bone marrow cells as a source of young adult B cell precursors in the lower chamber. After 3 days of co-culture in the presence of IL-7, the bone marrow cells were harvested and IL-7 mediated B cell precursor growth capacity was measured in IL-7 colony forming assays (IL-7 CFU). IL-7 CFU are generated by both pro-B cells and early pre-B cells; therefore, any effects on IL-7 CFU are targeted at relatively early stages in the development of new B cells. As shown previously, the decline in B cell precursors in aged mice coincides with losses in IL-7 responding B cell precursors (IL-7 CFU) within the bone marrow (Riley et. al., 1991). As shown in Figure 2A, co-culture of bone marrow cells with splenic ABC from old mice resulted in diminished growth of B cell precursors with the extent of inhibition dependent upon the numbers of ABC employed.
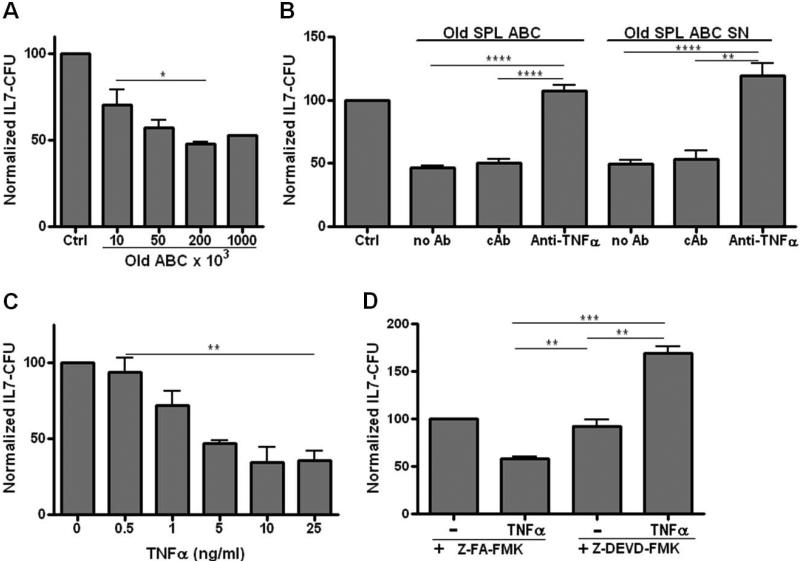
Figure 2. ABC from old mice produce TNFα which induces apoptosis in IL-7 responsive B cell precursors.
Panel A: Isolated ABC from aged spleens were co-cultured for 3 days in transwells at the numbers shown, separated from unfractionated bone marrow cells (1 × 106) as a source of B cell precursors. Cultures included IL-7 (5ng/ml) to support B cell precursor growth. At end of co-culture, bone marrow cells were removed and placed in triplicate IL-7 CFU assays as per the Materials and Methods. B cell precursor colonies were counted on days 7-9. Cumulative data is from 3-10 experiments with averages and SE shown. To allow for comparison among experiments, data were normalized to control bone marrow IL-7 CFU values which were set at “100”. Panel B: ABC (2 × 105) isolated from old spleens were co-cultured in transwells separated from bone marrow cells (1 × 106) from young mice, plus 5ng/ml rmIL-7, for 3 days as in Panel A. Some cultures had added anti-TNFα neutralizing antibody (1 μg/ml) or an isotype control antibody (cAb). Control cultures (Ctrl) had no added ABC. After co-culture, bone marrow cells were placed in triplicate IL-7 CFU assays. Data are cumulative for 4 experiments with data normalized as in Panel A. Supernatants were derived from old splenic ABC after 3 days of culture at 1 × 106/ml in complete media. Unfractionated bone marrow cells (1 × 106) from young mice were cultured for 3 days with 20% supernatant (SN) plus 5ng/ml rmIL-7; bone marrow cells were then harvested and placed into triplicate IL-7 CFU cultures. Some cultures contained 1μg/ml anti-TNFα neutralizing antibody or an isotype control antibody (cAb) in addition to SN. The control culture had no added SN. Data are cumulative for 3 experiments with data normalized to control cultures. Panel C: Bone marrow cells (1 × 106) from young mice were co-cultured with rmTNFα at the concentrations shown in IL-7 CFU assays. Bone marrow cells were then harvested and seeded into triplicate IL-7 CFU assays. Data are cumulative for 3 experiments, normalized to control cultures. Panel D: Bone marrow cells (1 × 106) from young mice were cultured with the control compound Z-FA-FMK (50μM) or the caspase 3 inhibitor Z-DEVD-FMK (50μM) for 30 minutes prior to seeding bone marrow cells into triplicate IL-7 CFU assays with or without rmTNFα (10 ng/ml). The control compound or the caspase 3 inhibitor were also added to maintain their initial concentrations in the IL-7 CFU cultures. Data are cumulative for 3 experiments, normalized to their respective control cultures. Statistical significance as shown: *, p<0.03, **, p<0.0063; ***, p<0.0002; ****, p<0.0001.
The capacity of aged ABC to inhibit B cell precursor growth in vitro was prevented by anti-TNFα neutralizing antibodies (Fig. 2B). IL-7, which was present in the transwell cultures, was not required to induce ABC-mediated inhibition of B cell precursor growth. Culture of aged ABC, in the absence of added cytokines, for 3 days resulted in supernatants which reduced B cell precursor growth in IL-7 CFU assays (Fig. 2B). Inclusion of neutralizing anti-TNFα antibodies prevented the inhibition of IL-7 CFU by aged ABC supernatants (Fig. 2B).
ABC from aged mice have been shown previously to express TNFα mRNA (Frasca et al., 2012); recombinant mouse (rm)TNFα also was a potent inhibitor of IL-7 CFU (Fig. 2C). Inhibition of B cell precursor growth by TNFα was primarily via apoptosis since addition of the caspase 3 inhibitor Z-DEVD-FMK reversed TNFα inhibition of IL-7 CFU (Fig. 2D). Indeed, inhibition of caspase activation revealed a positive effect of TNFα on IL-7 CFU. Notably, TNFα acts via multiple pathways leading to activation of NFκB, which may promote survival, as well as caspases (Leong and Karsan, 2000).
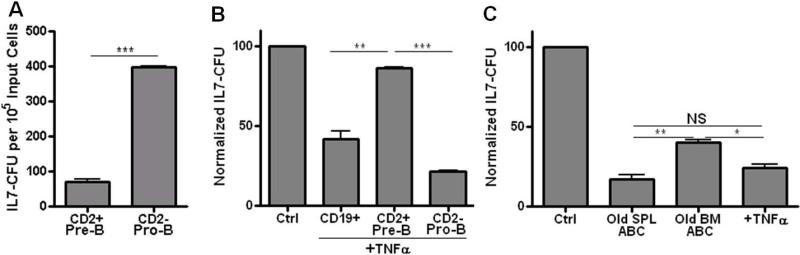
The maximal inhibition of IL-7 CFU from unfractionated bone marrow that was induced by old ABC as well as TNFα was ~50-60% of control values (Figs. 2A, C). Since ~40-50% of IL-7 CFU were relatively resistant to ABC (as well as TNFα) mediated growth inhibition, a subset of B cell precursors was apparently not affected by ABC and TNFα. This likely reflected the generation of IL-7 CFU from both bone marrow pre-B and pro-B cells. Pre-B cells (CD2+; 7% ± 0.1% SE) in bone marrow of young adult B6 mice are 3-4 fold higher in incidence than are pro-B cells (CD2−; 2% ± 0.1% SE). However, as shown in Figure 3A, the cloning efficiency of pre-B cells, most of which fail to respond to IL-7 (Hardy et al., 1991), is about 5-fold lower than for pro-B cells. Therefore, on balance, roughly equal numbers of IL-7 CFU in unfractionated bone marrow derive from pre-B versus pro-B cells.
Figure 3. Pro-B cell, but not pre-B cell, growth is inhibited by ABC and TNFα.
Panel A: Isolated pre-B cells (IgM− CD2+ CD19+) and pro-B cells (IgM− CD2− CD19+) from young adult B6 mice were cultured with 5ng/ml rmIL-7 in triplicate IL-7 CFU assays. The IL-7 CFU incidence is provided normalized to 105 input B cell precursors. Panel B: CD2+ pre-B cells (6 × 105) and CD2− pro-B cells (2 × 105) from young adult B6 mice, isolated as in Panel A, were treated with rmTNFα (10ng/ml) in IL-7 CFU assay. Triplicate IL-7 CFU assays were performed. Data are normalized to IL-7 CFU for each cell population, in the absence of TNFα, set to “100”. Panel C: Either old splenic ABC (2 × 105), old bone marrow ABC (2 × 105), or rmTNFα (10ng/ml) were cultured in transwells separated from CD19+ pro-B cells (3 × 105) isolated from RAG-2 gene knockout B6 mice. Culture conditions were as described in Figure 2. IL-7 CFU cultures in each individual experiment were performed in triplicate; each bar represents cumulative results of 2-3 individual experiments with averages and SE shown. In order to compare across experiments, results from each individual experiment were normalized by setting the control (Ctrl) culture IL-7 CFU colony counts (no added ABC or FO B cells or TNFα) to 100. Statistical significance is shown: *, p<0.023 ; **, p< 0.009, ***, p<0.0009.
We asked if pre-B cell and pro-B cell IL-7 CFU differed in susceptibility to TNFα. Most (~80%) of pre-B cell derived IL-7 CFU are resistant to TNFα induced apoptosis while, conversely, most (~80%) of pro-B cell derived IL-7 CFU are susceptible to TNFα growth inhibition (Fig. 3B). Therefore, as B cell precursors transit the pro-B to pre-B cell transition, their susceptibility to TNFα-mediated apoptosis is reduced. This results from signaling via the pre-B cell receptor in IL-7 responsive early pre-B cells (Ratliff, M., et al., manuscript in preparation).
IL-7 CFU from isolated pro-B cells from RAG-2 knockout mice, which lack development beyond the pro-B cell stage, were susceptible (~75-80%) to inhibition by splenic ABC as well as TNFα (Fig. 3C), consistent with wild-type CD2− pro-B cell susceptibility to TNFα Fig. 3B). Importantly, ABC present in the bone marrow of aged mice, like ABC from aged spleen, mediated inhibition of IL-7 CFU from pro-B cells, albeit old bone marrow ABC were slightly, but significantly, less effective than splenic ABC (Fig. 3C). Therefore, ABC from aged mice, via TNFα, specifically inhibited the growth of pro-B cells, but not pre-B cells, in vitro.
Inhibition of B cell precursor growth is acquired by ABC with age and is not seen with follicular B cells in old mice
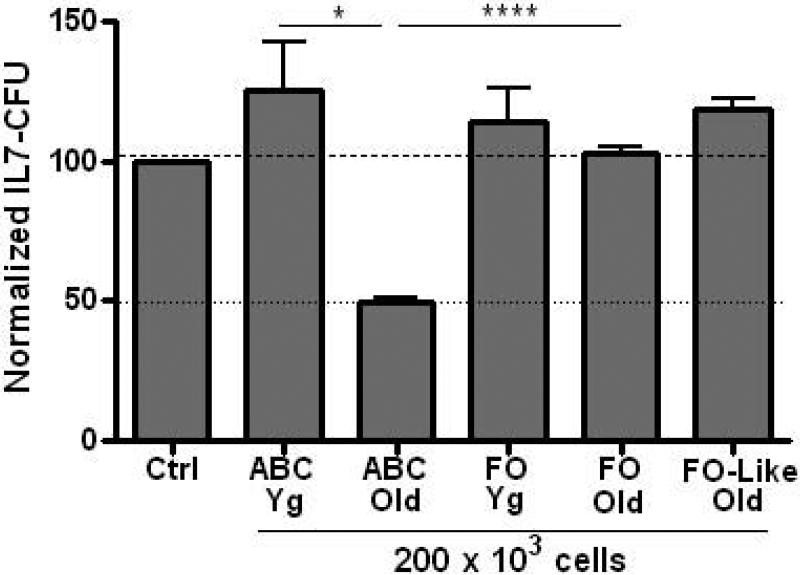
While ABC from aged mice inhibited IL-7 CFU, B cells with the same ABC phenotype from young adult mice did not affect B cell precursor growth in vitro (Fig. 4). Therefore, capacity to inhibit IL-7 CFU was gained in ABC with advanced age. Moreover, FO B cells from either young or aged spleen failed to inhibit B cell precursor growth (Fig. 4). Similar results were also obtained with isolated mature B cells from the bone marrow whose surface phenotype was analogous to that of FO splenic B cells (IgM+ CD21+ CD23+). With these “FO-like” recirculating B cells from aged bone marrow, again no inhibition of B cell precursor growth was observed in co-cultures (Fig. 4).
Figure 4. Inhibition of B cell precursor growth is acquired with old age by ABC and is not seen with follicular (FO) B cells.
ABC, FO splenic B cells, or FO-like bone marrow B cells (2 × 105) from young or aged B6 mice were co-cultured in transwells with young adult bone marrow cells (1 × 106) as in Figures 1-3. IL-7 CFU data are cumulative from 4-12 experiments, with the exception of FO-like old cells used in 2 experiments. Statistical significance as shown: *, p<0.04; ****, p<0.0001.
A subset within splenic and bone marrow B cells produces IL-10 and rescues TNFα induced growth suppression of B cell precursors
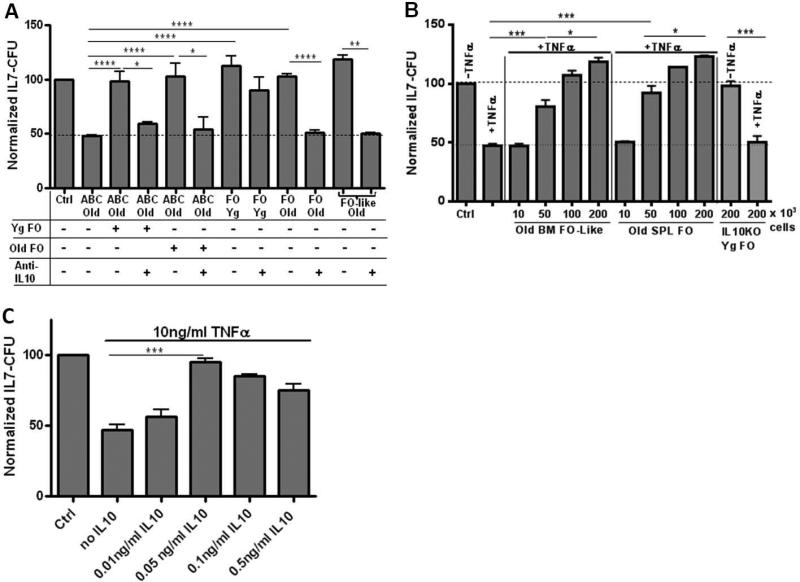
We found it surprising that FO B cells from old mice failed to inhibit IL-7 CFU. We have previously reported that mature splenic B cells with an FO phenotype (IgM+ CD21/35+ CD23+) in old mice produced TNFα in even greater amounts than did ABC (Frasca et al., 2012). In mixing experiments, FO B cells, from either young or aged mice, prevented old ABC from inhibiting B cell precursor growth in vitro (Fig. 5A). Neutralization of IL-10 resulted in abrogation of the protective effects of young adult FO B cells (Fig. 5A). Moreover, when IL-10 was neutralized, FO B cells, as well as FO-like recirculating bone marrow B cells, from aged mice now became inhibitory (Fig. 5A). Similarly, FO splenic B cells, as well as FO-like recirculating B cells from the bone marrow, of old mice were capable of protecting B cell precursors from growth inhibition by recombinant (rm)TNFα (Fig. 5B).
Figure 5. Splenic FO and recirculating FO-like bone marrow B cells produce IL-10 which prevents ABC and TNFα-mediated inhibition of B cell precursor growth.
Panel A: Bone marrow cells (1 × 106) from young mice as a source of B cell precursors were co-cultured, separated in transwells, with old splenic ABC (2 × 105) or old splenic FO B cells or old bone marrow FO-like B cells as shown. In addition, as indicated, certain cultures of old splenic ABC also were mixed with either young splenic FO B cells (2 × 105), old splenic FO B cells (2 × 105). Particular cultures also contained anti-IL-10 neutralizing antibody (1μg/ml) or isotype control (cAb) antibody (data not shown; cAb had no effects on IL-7 CFU values). Cultures also contained 5ng/ml rmIL-7. After 3 days of co-culture, the bone marrow cells were harvested from the transwell compartment and seeded into triplicate IL-7 CFU assays. Data is cumulative for 3-11 experiments with data normalized to their respective control cultures. Panel B: FO splenic B cells and FO-like recirculating bone marrow B cells from old mice or splenic FO B cells from young adult IL-10 gene knockout mice, were co-cultured with bone marrow cells (1 × 106) with or without rmTNFα (10ng/ml) for 3 days and bone marrow cell IL-7 CFU were determined in triplicate cultures. Data are cumulative for 2-3 experiments, with the exception of TNFα alone for 5 experiments, with data normalized to their respective control cultures. Panel C: Isolated CD19+ B lineage cells from young bone marrow were cultured with 10ng/ml rmTNFα and with graded concentrations of rmIL-10 as shown for 3 days together with 5ng/ml rmIL-7. B lineage cells were then harvested and seeded into triplicate IL-7 CFU assays. Data are cumulative for 3 experiments with data normalized to their respective controls. Control (Ctrl) cultures had B lineage cells with no added cytokines other than rmIL-7. Statistical significance as shown: *, p<0.04; **, p<0.003; ***, p<0.0006; ****, p<0.0001.
FO B cells from young IL-10 KO mice were not capable of preventing rmTNFα-mediated inhibition of B cell precursor growth unlike wild-type FO B cells (Fig. 5B). These results indicated that some FO B cells in both young and aged mice secreted IL-10 which prevented TNFα mediated apoptosis in B cell precursors. Notably, TNFα inhibition of IL-7 CFU was highly sensitive to the presence of IL-10. As little as a ~1:200 molar ratio of IL-10 to TNFα was sufficient to abrogate the inhibition of CD19+ B lineage IL-7 CFU seen with TNFα (Fig. 5C). Since isolated CD19+ bone marrow B cell precursors served as “targets” for both TNFα and IL-10, both the apoptotic effects of TNFα and the protective effects of IL-10 act directly on IL-7 responsive B cell precursors.
ABC mediate decline of pro-B cells in vivo
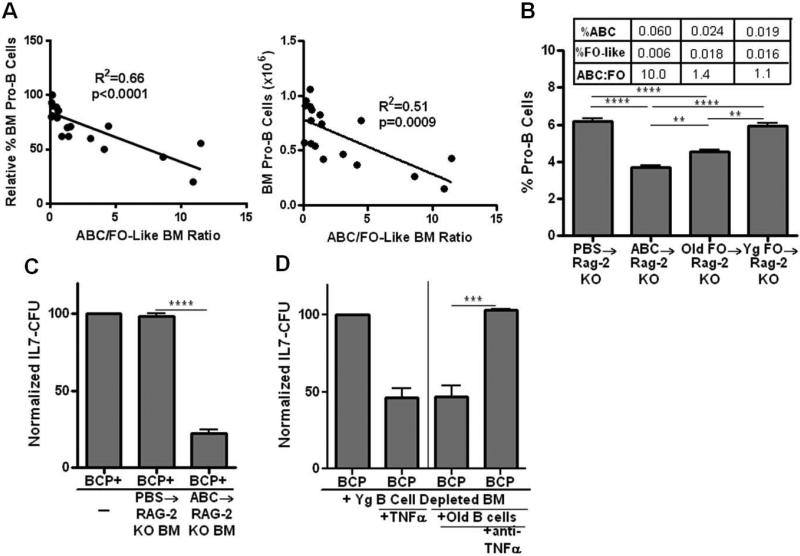
Based upon the capacity of ABC in old mice to inhibit pro-B cell survival and IL-7 CFU responses in vitro, we reasoned that as inhibitory, pro-inflammatory ABC capable of TNFα production increased in bone marrow, but protective IL-10 producing FO-like B cells decreased, this would be associated with progressive losses of pro-B cells in individual aged mice. This was seen to be the case: there were reductions in bone marrow pro-B cells, both in percentage and in number, which were proportional to the increased ABC to FO-like B cell ratio (Fig. 6A). This suggests the hypothesis that as the balance of mature B cells within the bone marrow favors a higher ABC to FO-like B cell ratio, the targets of ABC and TNFα, e.g., pro-B cells, are proportionally reduced.
Figure 6. ABC are associated with loss of pro-B cells in old mice and promote pro-B cell loss upon adoptive transfer.
Panel A: The proportions of ABC, FO-like B cells, and pro-B cells (AA4.1+ IgMCD43/S7+ CD2− CD19+) within the bone marrow of individual aged B6 mice were determined by fluorescence flow cytometry. The ABC/FO-like B cell ratios, based on their relative percentages, were plotted as a function of the relative percentage of pro-B cells (young adults = 100) and absolute pro-B cell numbers. Panel B: Splenic ABC from old mice (1.7 × 106) and FO B cells from old or young mice (9.4 × 106) were transferred i.v. into unmanipulated RAG-2 KO recipients. Pro-B cells in the bone marrow, and ABC and FO-like B cells, were assessed 4 weeks later. The bone marrow ABC and FO-like B cell composition is shown for each B cell recipient group in the table inset. Panel C: B cell depleted bone marrow (2 × 106) from RAG-2 KO recipients of old ABC or PBS controls (see Panel B) were co-cultured in transwells with young bone marrow CD19+ cells (1 × 106) as a source of B cell precursors (BCP) for 3 days. The precursors were then harvested and seeded into triplicate IL-7 CFU assays. Panel D: Bone marrow cells (2 × 106) from young B6 mice were depleted of B cells by MACS and co-cultured in transwell cultures with either rmTNFα (10ng/ml) alone or instead only with pooled B cells (2 × 105) isolated from the bone marrow of old B6 mice as shown. The old B cells had an ABC:FO-like ratio of 1.6 + 0.5 representing a relatively high ABC content. In some cultures, anti-TNFα (1 μg/ml) was included. Co-cultures went for 3 days. At the end of that co-culture period, the bone marrow cells were washed and co-cultured with young CD19+ B cell precursors (1 × 106) as a source of B cell precursors (BCP) for an additional 3 days in transwell cultures. Finally, B cell precursors were harvested and seeded into triplicate IL-7 CFU assays. Data are cumulative of 3 experiments with data normalized to untreated controls (100). Statistical significance was as shown: **, p<0.002; ***, p=0.0002; ****, p<0.0001.
In order to test whether old ABC directly contributed to loss of pro-B cells in vivo, we adoptively transferred ABC from aged B6 mice into RAG-2 KO young adults. RAG-2 mice have elevated levels of pro-B cells within the bone marrow and, as shown above, RAG-2 KO pro-B cells are sensitive to both old ABC and TNFα mediated suppression. When old ABC were transferred into RAG-2 KO mice, pro-B cells were reduced by ~40% when assessed 4 weeks later (Fig. 6B). This coincided with the presence of ABC, little or no FO-like B cells, and a very high ABC to FO-like B cell ratio in the bone marrow of these recipients (Fig. 6B). It should be noted that within the spleens of these recipients, ABC were readily detected and were equivalent to ~9% of the original ABC inoculums and typical FO B cells were not observed (<1%) (data not shown). These experiments established that upon adoptive transfer, old ABC both populated the spleen and bone marrow and promoted the loss of pro-B cells within the bone marrow in vivo.
When young adult FO B cells were transferred into RAG-2 KO mice, no loss of pro-B cells was observed (Fig. 6B). This was so even though low levels of phenotypic ABC were seen within the bone marrow (Fig. 6B) and ~30% of splenic B cells were now ABC phenotype (data not shown). That ABC were seen upon FO B cell transfer was expected given the derivation of ABC from FO B cells described in vivo by Hao, et. al. (2011). Furthermore, that young FO (or ABC) had no effect on pro-B cell levels was consistent with the failure of either young FO or ABC to inhibit IL-7 CFU (Fig. 3).
In contrast to young FO B cells, when old FO B cells were transferred to RAG-2 KO mice, ~25% loss of pro-B cells was observed (Fig. 6B). This coincided with detection of ABC, derived from old FO B cells, within the bone marrow (Fig. 6B). Unlike young B cells with the ABC phenotype, old ABC can inhibit pro-B cell survival and their presence in these recipients likely explains the reductions seen in pro-B cells.
Our in vitro experiments demonstrated that ABC from old mice, via production of TNFα, promoted apoptosis among pro-B cells and reduced IL-7 CFU responses. However, we also assessed the capacity of old ABC, transferred into young RAG-2 KO mice, to indirectly affect B cell precursors by altering the bone marrow microenvironment. As shown in Figure 6C, bone marrow cells from old ABC→RAG-2 KO recipients, after depletion of any B cells, were capable of suppressing the IL-7 mediated growth of young B cell precursors. ABC could induce this suppressive capacity in bone marrow cells via TNFα. In vitro, mature B cells (ABC:FO-like ratio of 1.6) from the bone marrow of aged mice were cultured with young adult (non-B) bone marrow cells. After co-culture, this bone marrow was now capable of suppressing IL-7 CFU (Fig. 6D). As shown, rmTNFα was capable of inducing IL-7 CFU suppressive capacity in bone marrow cells and TNFα was required for the induction of bone marrow suppression by aged B cells in vitro (Fig. 6D).
DISCUSSION
Recent findings that peripheral B cells exert “negative feed-back” on B lymphopoiesis suggest that a mature B cell population may regulate B cell development in old age (Keren et al., 2011a; Keren et al., 2011b; Van Den Broeck and Cambier, 2011). However, the identification of such B cells and their possible mechanisms of action have yet to be determined. Our results indicate that the subset of mature B cells which are low in both CD21/35 and CD23, and which have recently been named age-associated B cells (ABC) (Hao et al., 2011), have the potential to inhibit B cell precursor growth through effects of TNFα. While capable of initiating apoptosis in pro-B cells, TNFα alters patterns of B lymphopoiesis in mice by reducing stromal cell expression of both CXCL12 and stem cell factor (Ueda et al., 2004). Other cell types within the aged bone marrow may also contribute to a more pro-inflammatory microenvironment. For example, we have shown that bone marrow NK cells from aged mice are increased in number and express TNFα (King et al., 2009).
Adoptive transfer of aged ABC to young adult RAG-2 KO mice resulted in significant reductions in pro-B cell levels, indicating that ABC, from old mice, can suppress bone marrow B cell precursors. In old mice, as ABC become predominant over FO-like (recirculating) B cells in bone marrow, pro-B cells are reduced and this is correlated with the ABC to FO-like B cell ratio. Our studies indicate that aged ABC, via TNFα, can act directly on pro-B cells to induce apoptosis. ABC and pro-B cells may occupy distinct niches within the bone marrow; mature B cells have been found within extravascular perisinusoidal niches in the bone marrow (Pillai and Cariappa, 2008) while pro-B cells are found near the endosteum (Osmond et al., 1992). However, as shown herein, upon transfer of old ABC into RAG-2 KO mice, the bone marrow becomes suppressive to pro-B cell growth. In vitro, old ABC, via TNFα, can cause the bone marrow to now actively suppress pro-B cell growth. TNFα induces production of a variety of biomediators and cytokines, including PGE2 and IL-1, in macrophages and adipose cells which also inhibit B cell precursor proliferation and/or survival (Berg and Scherer, 2005; Feghali and Wright, 1997). Further studies will elucidate the influences of ABC on the bone marrow microenvironment in old age.
While ABC likely contribute to a pro-inflammatory microenvironment in old bone marrow, just as important is the reduction seen in FO-like recirculating B cells which, via secretion of IL-10, can “block” the effects of TNFα on B cell precursors. Notably, some regulatory B cell subsets have surface phenotypes that would be included within the CD21/35+ CD23+ FO B cell pool (Yanaba et al., 2009). These likely are the source of IL-10 producing B cells within the B cell populations in our studies. Further efforts are in progress to define the IL-10 expressing B cells in aged mice. The variation seen in the relative levels of ABC compared to FO-like B cells in individual aged mice may contribute to the heterogeneity of B cell precursor numbers seen in mice of the same chronological old age.
ABC are not limited to old age; B cells with the ABC cell surface phenotype also occur, albeit in low numbers, in young adult mice. While B cells with the ABC surface phenotype are seen throughout adult life, but become expanded in old age, the functions of these B cells change over time. In old mice, ABC produce TNFα and can inhibit IL-7 CFU; this is not the case with ABC from young adult mice. In this regard, ABC resemble follicular B cells where TNFα expression is only seen at substantial levels in old age (Frasca et al., 2012). It is of interest that exogenous TNFα stimulation of follicular B cells from young mice elicits the production of autocrine TNFα (Frasca et al., 2012) and this may also occur with ABC. Our results suggest that B cells with an ABC surface phenotype in young adult mice and old mice differ in their capacity to produce TNFα and promote suppression of B cell precursor growth. Whether the “ABC” seen in young adult mice differ in other functions compared to ABC in aged mice remains to be determined.
In summary, ABC represent a novel B cell subset which, in old age, increases proportionately and numerically in spleen and bone marrow, gains the capacity to produce TNFα, and inhibits pro-B cell growth. Importantly, ABC expansion occurs concomitantly with decreases in typical follicular (or recirculating) B cells. These FO (and FO-like) B cells contain a subpopulation which produces IL-10 and protects pro-B cells from TNFα induced effects. ABC, in old mice, can mediate decline in pro-B cells in vivo. Increased TNFα-producing ABC and diminished IL-10-producing B cells within the bone marrow are associated with loss of B cell precursors in aged mice. This may not only affect the numbers of B cell precursors available for further development along the B lineage, but also may affect such functions as read-out of the antibody repertoire in old mice as we (Alter-Wolf et al., 2009; Wilson et al., 2005) and others (Klinman and Kline, 1997) have reported. As we have previously observed, TNFα may diminish levels of the surrogate light chain λ5 in pro-B cells (King et al., 2009) and this may compromise the pre-B cell receptor checkpoint and influence μ heavy chain selection. Therefore, ABC, as they become more prevalent within the spleen and the bone marrow, may comprise a B cell subset capable of inappropriate “feedback” inhibition of B lymphopoiesis in old age.
Experimental Procedures
Mice
Young adult C57BL/6 mice were originally obtained from The Jackson Laboratory, Bar Harbor, ME and were subsequently bred and maintained at the University of Miami Miller School of Medicine. Young C57BL/6 male or female mice were used at 2-5 months of age. Aged (24-29 months old) B6 female mice were obtained from the National Institute on Aging colony or aged in our breeding colony. Aged mice (<5% of the total) bearing visible tumors in the thoracic or abdominal cavities, as well as mice with splenomegaly, were eliminated from the study. RAG-2 knockout and IL10 knockout mice on the B6 background were purchased from The Jackson Laboratory. Animal studies were carried out under protocols approved by the University of Miami Animal Care and Use Committee.
Cell Staining and Sorting
Spleen, peripheral blood, or bone marrow cells were stained with the following antibodies: anti-IgM-APC (clone II/41), anti-CD43-biotin (clone S7), anti-CD19-APC-Cy7 (clone 1D3), anti-CD21/35-FITC (clone 7G6), anti-CD2-PE (clone RM2-5), and streptavidin-PerCP, all from B-D Biosciences, San Diego, CA, and anti-CD93-PE-Cy7 (AA4.1) and anti-CD23-PE (B3B4) from eBioscience, San Diego, CA. Analysis was performed on LSR II, LSR-Fortessa, or FACS Canto fluorescence flow cytometers (B-D Bioscience). Various cell subsets (ABC, FO, FO-like, pre-B, pro-B) were sorted on a FACSAria IIe (B-D Bioscience) with 97-99% purity in all sorts.
ABC were isolated as surface IgM+ CD19+ CD21/35− CD23− CD43/S7− CD93 (AA4.1)− cells; FO B cells were isolated as IgM+ CD19+ CD23/35+ CD23+ CD43/S7− CD93 (AA4.1)− cells. Pro-B cells were isolated as CD2− surface IgM− CD19+ cells; these B cell precursors also were uniformly CD43/S7+, AA4.1+, <10% cytoplasmic μ+, >80% cytoplasmic λ5+, and >80% c-kit+ as would be expected for pro-B Hardy Fractions B, C (1991) and pre-B I (pro-B) cells as defined by Rolink, et. al. (2000). CD2 has been shown to be absent on pro-B cells (Middendorp et al., 2003; Sen et al., 1990). Pre-B cells were isolated as CD2+ surface IgM− CD19+ cells; these B cell precursors were also shown to be uniformly CD43/S7−, AA4.1+, uniformly cytoplasmic μ+, <10% cytoplasmic λ5+, and <5% c-kit+ as expected (Hardy et al., 1991; Rolink et al., 1993).
In some experiments, CD19+ B lineage cells were isolated by magnetic bead (MACS) sorting using anti-CD19-PE or anti-CD19-APC (B-D Biosciences) and anti-PE or anti-APC microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) following the miniMACS protocols. CD19+ cells were purified over magnetic columns to a purity of >97%. Mature FO B cells from aged spleen (24 mo) were also isolated by magnetic beads. Spleens were first processed using a B cell isolation kit (biotin labeled antibodies against CD43, CD4, and Ter-119; anti-Biotin microbeads) to deplete non-B cells. The B cells were further sorted based on CD23 expression, using anti-CD23-PE and anti-PE microbeads.
Cell Culture
Femur and tibia pairs were flushed to harvest cells from the bone marrow as previously described (Riley et al., 1991) and spleens were mechanically disrupted. Single cell suspensions were washed and counted for use in flow cytometry, cell sorting, or cell culture. In typical experiments, either bone marrow cells or CD19+ bone marrow B lineage cells, isolated as above, from young, aged, or young RAG-2 knockout mice were used as sources of B cell precursors and resuspended at 3 × 105 to 1 × 106 cells/ml in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FCS (low endotoxin; Sigma-Aldrich, St. Louis, MO) plus 1% penicillin-streptomycin, 1% glutamine, and 0.05 mM 2-mercaptoethanol. Recombinant mouse (rm) IL-7 (Invitrogen) was added at 5 ng/ml to promote B cell precursor growth. Cell cultures employed transwells (0.2 micron) where the B cell precursors were cultured in the bottom chamber. In the upper chamber, isolated mature B cells (ABC; FO; FO-like B cells) were cultured at various concentrations. After 3 days co-culture, the B cell precursors were harvested from the lower chamber and seeded equally into triplicate IL-7 CFU assays (see below). In some studies, B cell precursors were cultured with rmTNFα and/or rmIL-10 (Invitrogen) at the concentrations indicated. In other experiments, splenic ABC or FO B cells were cultured in complete media for 3 days at 1 × 106/ml, but without added Cytokines were neutralized by using anti-TNFα (purified NA/LE hamster anti-mouse/rat TNFα monoclonal, clone TN3-19.12); anti-IL-10 (purified NA/LE rat anti-mouse IL-10 monoclonal, clone JES5-2A5); or isotype controls (purified NA/LE hamster IgG1 or rat IgG1) (B-D Biosciences).
In analysis of apoptotic mechanisms, a caspase 3 inhibitor, Z-DEVD-FMK (B-D Biosciences) or negative control, Z-FA-FMK (B-D Biosciences), were used to block caspase 3 activity. Cells were pre-incubated with inhibitor or control for 30 min., then transferred into IL-7 CFU assays (see below). Added inhibitor or control was included to maintain original concentrations (50mM) in culture.
IL-7 CFU Assay
B cell precursors, either from bone marrow or as CD19+ B lineage cells isolated as above, were cultured in semi-solid methylcellulose media to derive pro-B/pre-B cell colonies as previously described (Merchant et al., 1996; Riley et al., 1991). The methylcellulose media (Methocult M3134 base; StemCell Technologies, Vancouver, Canada) was supplemented with 30% FCS, 1% penicillin-streptomycin, 1% glutamine, IMDM (Invitrogen), 0.1mM 2-mercaptoethanol, and 10 ng/ml rmIL-7 for 7-9 days and scored for colony growth (IL7 CFU). Typically, IL-7 CFU in control cultures ranged from 500 to 3000 depending on the experiment. As a consequence of IL-7 CFU control culture variability in colony counts for individual experiments, IL-7 CFU were normalized to that of the control cultures (set at 100%) in each experiment.
Adoptive Transfer
Mature ABC and FO B cell populations were isolated from spleens of aged (24 mo) mice. ABC were isolated by fluorescence activated cell sort as described above. FO B cells were isolated either by fluorescence activated cell sort or MACS sort as described above. B cells were transferred i.v. into unmanipulated RAG-2 KO mice at the following concentrations: 1.7 × 106 cells per mouse for old ABC (3 recipients), 9.4 × 106 cells per mouse for old FO (4 recipients), and 9.4 × 106 cells per mouse for young FO (2 recipients). Four recipients received PBS alone as control. Bone marrow and spleen were collected from recipients 4 weeks following transfer and analyzed by fluorescence flow cytometry.
Statistical Analysis
The various groups were compared by two-tailed Student's t-test or non-parametric Mann-Whitney U-test with p values shown.
Acknowledgements
We wish to thank the Flow Cytometry Core Facility, and in particular Dr. Shannon Opiela, for assistance with isolation of cell populations. We are appreciative of members of the Blomberg and Riley laboratories for helpful insights.
Footnotes
Supported by NIH grants AG 252560 to RLR and AG 023717 to BBB.
AUTHOR CONTRIBUTIONS
Michelle Ratliff carried out experiments and assisted with writing the manuscript. Sarah Alter performed experiments and analysis. Daniela Frasca and Bonnie Blomberg assisted in experimental design. Richard Riley designed experiments and assisted in writing the manuscript.
REFERENCES
- Allman D, Miller JP. B cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:463–467. doi: 10.1016/j.coi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Alter-Wolf S, Blomberg B, Riley R. Deviation of the B cell pathway in senescent mice is associated with reduced surrogate light chain expression and altered immature B cell generation, phenotype, and light chain expression. J Immunol. 2009;182:138–147. doi: 10.4049/jimmunol.182.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Cancro M, Hao Y, Scholz J, Riley R, Frasca D, Dunn-Walters D, Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30:313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- Frasca D, Nguyen D, Riley R, Blomberg BB. Decreased E12 and/or E47 transcription factor activity in the bone marrow as well as in the spleen of aged mice. J Immunol. 2003;170:719–726. doi: 10.4049/jimmunol.170.2.719. [DOI] [PubMed] [Google Scholar]
- Frasca D, Romero M, Diaz A, Alter-Wolf S, Ratliff M, Landin A, Riley R, Blomberg B. A molecular mechanism for TNF-alpha-mediated downregulation of B cell responses. J Immunol. 2012;188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci U S A. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, O'Neill P, Naradikian M, Scholz J, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren Z, Averbuch D, Shahaf G, Zisman-Rozen S, Golan K, Itkin T, Lapidot T, Mehr R, Melamed D. Chronic B cell deficiency from birth prevents age-related alterations in the B lineage. J Immunol. 2011a;187:2140–2147. doi: 10.4049/jimmunol.1100999. [DOI] [PubMed] [Google Scholar]
- Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, Sasaki Y, Schmidt-Supprian M, Lapidot T, Melamed D. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood. 2011b;117:3104–3112. doi: 10.1182/blood-2010-09-307983. [DOI] [PubMed] [Google Scholar]
- King A, Keating P, Prabhu A, Blomberg B, Riley R. NK cells in the CD19− B220+ bone marrow fraction are increased in senescence and reduce E2A and surrogate light chain proteins in B cell precursors. Mech Ageing Dev. 2009;130:384–392. doi: 10.1016/j.mad.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, Van der Put E, Blomberg BB, Riley RL. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. J Immunol. 2007;178:3521–3529. doi: 10.4049/jimmunol.178.6.3521. [DOI] [PubMed] [Google Scholar]
- Kirman I, Zhao K, Wang Y, Szabo P, Telford W, Weksler ME. Increased apoptosis of bone marrow pre-B cells in old mice associated with their low number. Int Immunol. 1998;10:1385–1392. doi: 10.1093/intimm/10.9.1385. [DOI] [PubMed] [Google Scholar]
- Klinman NR, Kline GH. The B-cell biology of aging. Immunol Rev. 1997;160:103–114. doi: 10.1111/j.1600-065x.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Labrie J.r., Sah A, Allman D, Cancro M, Gerstein R. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K, Karsan A. Signaling pathways mediated by tumor necrosis factor alpha. Histol Histopathol. 2000;15:1303–1325. doi: 10.14670/HH-15.1303. [DOI] [PubMed] [Google Scholar]
- Lescale C, Dias S, Maes J, Cumano A, Szabo P, Charron D, Weksler ME, Dosquet C, Vieira P, Goodhardt M. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell. 2010;9:410–419. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Merchant M, Garvy B, Riley R. Autoantibodies inhibit interleukin-7-mediated proliferation and are associated with the age-dependent loss of pre-B cells in autoimmune New Zealand Black Mice. Blood. 1996;87:3289–3296. [PubMed] [Google Scholar]
- Middendorp S, Dingjan GM, Maas A, Dahlenborg K, Hendriks RW. Function of Bruton's tyrosine kinase during B cell development is partially independent of its catalytic activity. J Immunol. 2003;171:5988–5996. doi: 10.4049/jimmunol.171.11.5988. [DOI] [PubMed] [Google Scholar]
- Miller J, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Sieburg HB, Bernitz JM, Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood. 2012;119:3900–3907. doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond DG, Kim N, Manoukian R, Phillips RA, Rico-Vargas SA, Jacobsen K. Dynamics and localization of early B-lymphocyte precursor cells (pro-B cells) in the bone marrow of scid mice. Blood. 1992;79:1695–1703. [PubMed] [Google Scholar]
- Pillai S, Cariappa A. The bone marrow perisinusoidal niche for recirculating B cells and the positive selection of bone marrow-derived B lymphocytes. Immunol Cell Biol. 2008;87:16–19. doi: 10.1038/icb.2008.89. [DOI] [PubMed] [Google Scholar]
- Riley RL, Blomberg BB, Frasca D. B cells, E2A, and aging. Immunol Rev. 2005;205:30–47. doi: 10.1111/j.0105-2896.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Riley RL, Kruger MG, Elia J. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991;59:301–313. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- Rolink A, Karasuyama H, Grawunder U, Haasner D, Kudo A, Melchers F. B cell development in mice with a defective lambda 5 gene. Eur J Immunol. 1993;23:1284–1288. doi: 10.1002/eji.1830230614. [DOI] [PubMed] [Google Scholar]
- Rolink AG, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191:23–32. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov A, Rubtsova K, Fischer A, Meehan R, Gillis J, Kappler J, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen J, Rosenberg N, Burakoff SJ. Expression and ontogeny of CD2 on murine B cells. J Immunol. 1990;144:2925–2930. [PubMed] [Google Scholar]
- Sherwood E, Blomberg B, Xu W, Warner C, Riley R. Senescent BALB/c mice exhibit decreased expression of lambda5 surrogate light chains and reduced development within the pre-B cell compartment. J Immunol. 1998;161:4472–4475. [PubMed] [Google Scholar]
- Sherwood E, Xu W, King A, Blomberg B, Riley RL. The reduced expression of surrogate light chains in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech Ageing Dev. 2000;118:45–59. doi: 10.1016/s0047-6374(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Sherwood E, Xu W, Riley R. B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mech Ageing Dev. 2003;124:147–153. doi: 10.1016/s0047-6374(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Song H, Price PW, Cerny J. Age-related changes in antibody repertoire: contribution from T cells. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 1998;91:75–88. [PubMed] [Google Scholar]
- Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation Controls B Lymphopoiesis by Regulating Chemokine CXCL12 Expression. The Journal of Experimental Medicine. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Broeck A, Cambier J. B cells talk to their progenitors. Blood. 2011;117:2985–2986. doi: 10.1182/blood-2011-02-332544. [DOI] [PubMed] [Google Scholar]
- Van der Put E, Frasca D, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. J Immunol. 2004;173:818–827. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]
- Van der Put E, Sherwood EM, Blomberg BB, Riley RL. Aged mice exhibit distinct B cell precursor phenotypes differing in activation, proliferation and apoptosis. Exp Gerontol. 2003;38:1137–1147. doi: 10.1016/j.exger.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Wilson E, King A, Sherwood E, Riley R. Pre-B cell loss in senescence coincides with preferential development of immature B cells characterized by partial activation and altered Vh repertoire. Exp Gerontol. 2005;40:67–79. doi: 10.1016/j.exger.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz J-D, Matsushita T, Tsubata T, Tedder TF. The Development and Function of Regulatory B Cells Expressing IL-10 (B10 Cells) Requires Antigen Receptor Diversity and TLR Signals. The Journal of Immunology. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]