Abstract
Purpose
The contribution of axonal injury to brain damage following aneurysmal subarachnoid hemorrhage (aSAH) is unknown. Neurofilament light chain (NF-L), a component of the axonal cytoskeleton, has been shown to be elevated in the cerebrospinal fluid of patients with many types of axonal injury. We hypothesized that patients with aSAH would have elevated CSF NF-L levels, and sought to explore the clinical correlates of CSF NF-L dynamics.
Methods
Serial ventricular cerebrospinal fluid (vCSF) samples were collected from 35 aSAH patients for up to 15 days. vCSF NF-L measurements were determined by enzyme-linked immunosorbent assay. NF-L levels were analyzed in relation to acute clinical status, radiological findings, and 6-month outcomes.
Results
vCSF NF-L concentrations were elevated in all aSAH patients. Patients with early cerebral ischemia (ECI), defined as a CT hypodense lesion visible within the first 3 days, had higher acute vCSF NF-L levels than patients without ECI. These elevated NF-L levels were similar in patients with ECI associated with intracranial hemorrhage and ECI associated with surgical/endovascular complications. vCSF NF-L levels did not differ as a function of acute clinical status, clinical vasospasm, delayed cerebral ischemia, or 6-month Glasgow Outcome Scale.
Conclusions
Elevated vCSF NF-L levels may in part reflect increased injury to axons associated with early cerebral ischemia. However our results suggest that axonal injury following aSAH as reflected by release of NF-L into the CSF may not play a major role in either secondary adverse events or long term clinical outcomes.
Keywords: cerebral aneurysm, subarachnoid hemorrhage, biomarker, neurofilament, axonal injury
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease associated with long-term cognitive impairment in nearly 50% of survivors.[1] Key factors associated with unfavorable outcome include older age, poor presenting neurological condition, extent of aSAH on head CT, intraparenchymal hemorrhage (IH), vasospasm and cerebral infarction.[2] Earlier and more refined methods for recognition of patients at high risk of the secondary sequelae would permit early and more aggressive initiation of appropriate therapy, while identification of patients likely to suffer poor outcomes would allow for more informed discussions with family regarding patient prognosis. Aside from clinical examination and radiographic imaging, no diagnostic study is currently used for the early recognition of patients susceptible to ischemic secondary events.
In recent years there has been considerable effort towards identifying biomarkers (i.e. Cardiac troponin, S100-B, alpha-II spectrin) that might prove useful in assessing brain injury following aSAH;[3-5] however, these biomarkers have not been widely used in clinical practice to date.
Neurofilaments constitute a major component of the axonal cytoskeleton and are composed of neurofilament heavy chain (NF-H 190-210 kDa), neurofilament medium chain (160 kDa), neurofilament light chain (NF-L 68 kDa), and alpha-internexin (66 kDa).[6,7] Their function is to maintain axonal structural integrity.[8]
Neurofilaments are normally restricted to intracellular compartments. Disruption of axonal membrane integrity could result in neurofilament proteins being released into the extracellular space, from which they may diffuse into the CSF. As such, neurofilament subunits are candidate CSF biomarkers of axonal injury.
Petzold et al. reported a correlation between CSF levels of NF-H and outcome in 17 aSAH patients.[9] However they did not specify whether patients developed secondary insults. More recently, Lewis et al. demonstrated that elevated CSF and blood NF-H levels in aSAH patients were associated with poor outcome and that patients with vasospasm had higher CSF NF-H levels compared to those without vasospasm[10] suggesting that NF-H may be a useful marker of axonal injury in aSAH.[9,11]
NF-L may be a complementary marker of axonal injury as it has been detected in the CSF of patients with traumatic brain injury, HIV infection, and neurodegenerative disease. It has also been detected in the lumbar CSF of aSAH patients.[6,8,12,13] However no study has investigated vCSF levels of NF-L after aSAH. The aims of this study were to examine the relationship between vCSF NF-L dynamics and relevant clinical outcomes.
Materials and Methods
Patients
The study was approved by the research ethics committees of the Ospedale Maggiore Policlinico, Milano and Washington University, Saint Louis. 35 aSAH patients were enrolled (Supplementary Table 1). Written informed consent was obtained from patients or, in the comatose patients, from the next of kin. Clinical management was performed as previously described;[14] clinical outcome was assessed at six months post-injury using Glasgow Outcome Scoring (GOS) system.[15]
Neuroradiologic monitoring
All patients received a first head CT on admission. A second CT was performed within 48h after aneurysm treatment to identify procedural complications, and a later CT was performed between day 21 and 28 following aSAH for follow-up. Additional scans were performed in cases of neurological deterioration such as the loss of one point of the Glasgow Coma Scale motor component (mGCS) and/or the presence of new focal deficits.
All CTs were reviewed by two investigators blinded to the clinical history who independently assessed the occurrence of ischemic events. Early cerebral ischemia (ECI) was defined as a hypodense lesion that was visible on the CT performed within the first 48h after aneurysm treatment. Delayed cerebral ischemia (DCI) was defined as the appearance of a new hypodense lesion detectable on the 21-28 day follow-up CT that was not present on the CT performed within 48h after aneurysm treatment.
Criteria for evidence of vasospasm
Clinical vasospasm was defined as neurological deterioration associated with angiographic confirmation of vasospasm, defined as an arterial diameter narrowing >20% from baseline, by a neuroradiologist blinded to the clinical history.
CSF sampling
CSF was collected twice daily from an external ventricular drain in all patients. The median number of samples per patient was 9, range (1-21). CSF samples were immediately treated with 10 mM ethylenediaminetetraacetic acid (EDTA) and 0.125% polybrene (Sigma) to prevent admixed blood from clotting. Superrnatant was separated by centrifugation (10 minutes at 2500× g at 21°C) and stored at −80°C. Additionally CSF samples of 13 patients free of neurological diseases that underwent lumbar puncture as part of a study protocol at Washington University, Saint Louis and Ospedale Maggiore Policlinico, Milano served as negative controls. NF-L levels were analyzed by ELISA. The protocol was adapted from Van Geel et al.[6], for details on analysis and assay procedure, please refer to Supplementary materials.
Statistics
NF-L values did not follow normal distributions. Therefore, Mann-Whitney U-tests were used to assess the relationship between NF-L values and clinical outcomes. Kruskal-Wallis test followed by Dunn's multiple comparison test was used to assess the relationship between NF-L and ECI. The Wilcoxon signed rank test was used to investigate the relationship between changes in NF-L values over time and the occurrence of vasospasm.
Results
All 35 patients with Fisher grade 3-4 aSAH had elevated acute vCSF NF-L levels (median 643 pg/ml, range 60-2688 pg/ml). In 2 of these aSAH patients, lumbar CSF was additionally obtained and also found to have elevated NF-L levels. In contrast, 0 of 13 non-neurological patients (age range: 36-72 years) had detectable lumbar CSF NF-L levels (<12 pg/ml).
To explore the relationship between vCSF NF-L levels and initial clinical status following aSAH we divided the patients into good (1-3) vs. poor (4-5) WFNS grade, as well as good (5-6) vs. poor (1-4) mGCS. Acute NF-L levels did not differ between good vs. poor initial clinical status groups (Supplementary Figure 1). This indicates that vCSF NF-L levels do not reflect initial injury severity as reflected by clinical status.
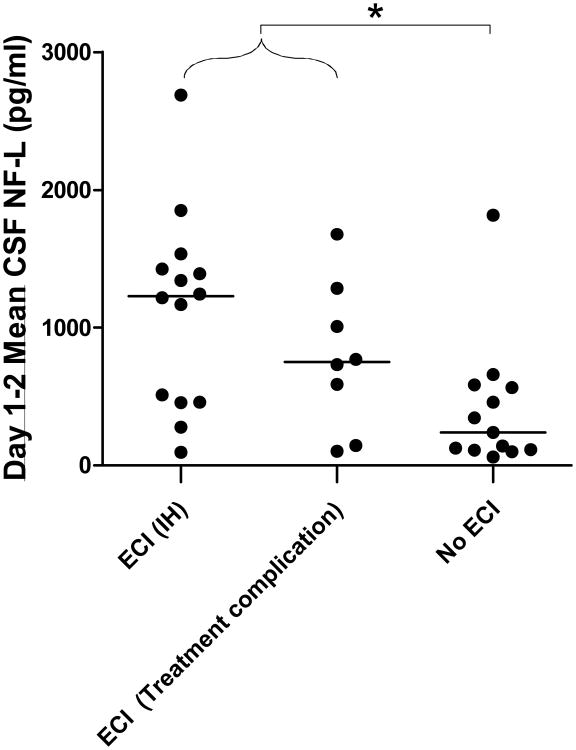
Early cerebral ischemia may cause injury to axons in both gray and white matter. We found that vCSF NF-L levels were significantly higher in patients with ECI compared to patients without ECI (p<0.01), though there was considerable overlap between groups (Figure 1 and Supplementary Figure 2). vCSF NF-L levels on days 1-2 were elevated most prominently in patients with ECI related to intracranial hemorrhage (median 1229 pg/ml, range 93-2688 pg/ml, n=14 patients, p=0.01 vs. no ECI) but also elevated in patients with ECI related to complications of aneurysm treatment (median 749 pg/ml, range 100-1678 pg/ml, n=8 patients. p=0.06 vs. no ECI). Thus higher NF-L levels may reflect increased injury to axons associated with ECI.
Figure 1. Relationship between vCSF NF-L levels and early cerebral ischemia (ECI).
The presence of ECI was associated with higher NF-L levels. (Kruskal-Wallis test *p = 0.02) Patients with ECI related to intraparenchymal hemorrhage (IH) showed higher NF-L levels compared to patients without ECI (p=0.01, Dunn's multiple comparison test). A more modest increase was observed in case of ECI related to treatment complications (p=0.06)
We next analyzed the vCSF NF-L levels in patients who developed clinically significant vasospasm or delayed cerebral ischemia. There were no statistically significant differences in vCSF NF-L levels between groups (Supplementary Figure 3). Neither there were changes in vCSF NF-L levels over time that reflected the development of clinical vasospasm (Supplementary Figure 4).
Finally, we assessed whether acute vCSF NF-L levels would be useful predictors of 6- month clinical outcome. While there were trends towards worse outcomes in patients who had the highest vCSF NF-L levels, there was considerable overlap between groups and no statistically significant differences detected (Supplementary Figure 5).
Discussion
Our results demonstrate that NF-L, a major component of the axonal cytoskeleton, can be detected in vCSF as early as 24h post-aSAH. The levels of NF-L were higher in patients with CT evidence of early cerebral ischemia. This occurred in the context of intracranial hemorrhage or complications of endovascular/surgical aneurysm treatment. However, vCSF NF-L levels were not consistently related to acute clinical status, vasospasm, delayed cerebral ischemia and 6-month outcomes.
What underlies the release of NF-L into the vCSF even in patients without ECI? This has not been determined definitively, but possibilities include axonal shear injury due to a rapid pressure wave occurring at the time of aneurysm rupture, an acute rise in intracranial pressure causing mechanical deformation of axons, transient global ischemia, or ischemic white matter injury due to microvascular dysfunction. All of these would be difficult to detect using current clinical methods. It is not clear whether vCSF NF-L elevations indicate specific axonal injury, as more general brain injury processes such as ischemia that affect axons indiscriminately along with other brain tissues appear also to result in NF-L release into the CSF.
An alternative explanation is that the ventricular drain placement itself was responsible for the high levels of vCSF NF-L measured. The drain insertion through the brain parenchyma could release neurofilament from injured axons in the frontal cortex and white matter.
Conclusion
Ventricular CSF NF-L levels are elevated after aSAH. A relationship between ECI and NF-L levels could be detected suggesting that NF-L levels may reflect increased injury to axons associated with intracranial hemorrhage and/or surgical or endovascular complications.
Supplementary Material
Footnotes
Competing Interests: None Declared
References
- 1.Hackett ML, Anderson CS. Health outcomes 1 year after subarachnoid hemorrhage: An international population-based study. The Australian Cooperative Research on Subarachnoid Hemorrhage Study Group. Neurology. 2000;55(5):658–62. doi: 10.1212/wnl.55.5.658. [DOI] [PubMed] [Google Scholar]
- 2.Rosengart AJ, Schultheiss KE, Tolentino J, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–21. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Pena P, Pereira AR, Sourour NA, et al. S100B as an additional prognostic marker in subarachnoid aneurysmal hemorrhage. Crit Care Med. 2008;36(8):2267–73. doi: 10.1097/CCM.0b013e3181809750. [DOI] [PubMed] [Google Scholar]
- 4.Lewis SB, Velat GJ, Miralia L, et al. Alpha-II spectrin breakdown products in aneurysmal subarachnoid hemorrhage: a novel biomarker of proteolytic injury. J Neurosurg. 2007;107(4):792–6. doi: 10.3171/JNS-07/10/0792. [DOI] [PubMed] [Google Scholar]
- 5.Naidech AM, Kreiter KT, Janjua N, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112(18):2851–6. doi: 10.1161/CIRCULATIONAHA.105.533620. [DOI] [PubMed] [Google Scholar]
- 6.Van Geel WJ, Rosengren LE, Verbeek MM. An enzyme immunoassay to quantify neurofilament light chain in cerebrospinal fluid. J Immunol Methods. 2005;296(1-2):179–85. doi: 10.1016/j.jim.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan MP, Chin SS, Fliegner KH, et al. Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci. 1990;10(8):2735–48. doi: 10.1523/JNEUROSCI.10-08-02735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong D, Jansen RW, Pijnenburg YA, et al. CSF neurofilament proteins in the differential diagnosis of dementia. J Neurol Neurosurg Psychiatry. 2007;78(9):936–8. doi: 10.1136/jnnp.2006.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petzold A, Keir G, Kay A, et al. Axonal damage and outcome in subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77(6):753–9. doi: 10.1136/jnnp.2005.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis SB, Wolper RA, Miralia L, et al. Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2008;28(6):1261–71. doi: 10.1038/jcbfm.2008.12. [DOI] [PubMed] [Google Scholar]
- 11.Petzold A, Rejdak K, Belli A, et al. Axonal pathology in subarachnoid and intracerebral hemorrhage. J Neurotrauma. 2005;22(3):407–14. doi: 10.1089/neu.2005.22.407. [DOI] [PubMed] [Google Scholar]
- 12.Mellgren A, Price RW, Hagberg L, et al. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69(15):1536–41. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]
- 13.Nylen K, Csajbok LZ, Ost M, et al. CSF -neurofilament correlates with outcome after aneurysmal subarachnoid hemorrhage. Neurosci Lett. 2006;404(1-2):132–6. doi: 10.1016/j.neulet.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Zanier ER, Longhi L, Fiorini M, et al. Increased levels of CSF heart-type fatty acid-binding protein and tau protein after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2008;102:339–43. doi: 10.1007/978-3-211-85578-2_65. [DOI] [PubMed] [Google Scholar]
- 15.Pettigrew LE, Wilson JT, Teasdale GM. Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J Head Trauma Rehabil. 2003;18(3):252–8. doi: 10.1097/00001199-200305000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.