Abstract
The inflammatory response to prosthetic implant-derived wear particles is the primary cause of bone loss and aseptic loosening of implants, but the mechanisms by which macrophages recognize and respond to particles remain unknown. Studies of innate immunity demonstrate that Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPS). All TLRs signal through myeloid differentiation factor 88 (MyD88), except TLR3 which signals through TIR domain containing adapter inducing interferon-beta (TRIF), and TLR4 which signals through both MyD88 and TRIF. We hypothesized that wear-debris particles may act as PAMPs/DAMPs and activate macrophages via TLRs. To test this hypothesis, we first demonstrated that inhibition of MyD88 decreases polymethylmethacrylate (PMMA) particle-induced production of TNF-α in RAW 264.7 macrophages. Next we compared particle-induced production of TNF-α among MyD88 knockout (MyD88−/−), TRIF knockout (TRIF−/−), and wild type (WT) murine macrophages. Relative to WT, disruption of MyD88 signaling diminished, and disruption of TRIF amplified the particle-induced production of TNF-α. Gene expression data indicated that this latter increase in TNF-α was due to a compensatory increase in expression of MyD88 associated components of the TLR pathway. Finally, using an in vivo model, MyD88−/− mice developed less particle-induced osteolysis than WT mice. These results indicate that the response to PMMA particles is partly dependent on MyD88, presumably as part of TLR signaling; MyD88 may represent a therapeutic target for prevention of wear debris-induced periprosthetic osteolysis.
Keywords: Osteolysis, Polymethylmethacrylate, Wear debris, Immune response
1. Introduction
Aseptic loosening of total joint replacements is the most common cause of revision surgery [1]. One potential contributing factor is implant wear resulting in the release of numerous particles into the joint space [2]. Macrophages phagocytose the wear debris and synthesize cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), IL-6 and others, which, among other actions, lead to osteoclast activation and breakdown of the surrounding bone, compromising the long-term outcome of the prosthesis [3,4]. Although it is widely accepted that aseptic loosening of joint replacements is largely driven by wear particles [4], the mechanism by which particles are recognized and activate macrophages remains largely undetermined.
Binding of pathogens to macrophages can induce responses of the innate immune system and lead to the induction of adaptive immunity [5]. The Toll-Like Receptors (TLR) are a group of evolutionarily conserved transmembrane receptors that function as signaling receptors. Each member of the TLR family is activated by a different stimulus and subsequently activates a response to that type of pathogen [6]. For example, TLR-4 signals the presence of lipopolysaccharide (LPS) on the cell membrane of gram-negative bacteria and activates an inflammatory response [7]. When the majority of TLR’s are stimulated, they interact with an adapter protein called Myeloid Differentiation primary response gene 88 (MyD88), which couples the TLR to downstream signaling kinases, eventually culminating in the activation (by translocation from the cytoplasm to the nucleus) of the transcription factor nuclear factor κB (NF-κB) [8] (Supplementary Fig. 1). However, there exist MyD88-independent signaling pathways for TLR3 and TLR4. TLR4 possesses two options for signal transduction, either to signal through MyD88 or through TIR domain containing adapter inducing interferon-beta (TRIF), while TLR3 can signal only through TRIF [6]. Although the MyD88-dependent and MyD88-independent pathways utilize distinct adapter proteins, both signaling pathways involve the activation and nuclear translocation of NF-κB which will lead to the expression of numerous proinflammatory cytokines. Osteoclastogenesis is also related to activation of the NF-κB pathway, and previous studies have demonstrated that wear debris-induced inflammatory osteoclastogenesis occurs in part by an increase in cytokine production and osteoclast differentiation, both of which involve the NF-κB pathway [9,10].
Considering the relationship between TLR signaling, NF-κB signaling and the known proinflammatory cytokines associated with aseptic loosening of joint implants, we proposed that TLRs play a critical role in the recognition of wear particles.
2. Materials and methods
2.1. Preparation of PMMA particles
PMMA particles (Polysciences) 1–10 μm in diameter, were washed five times with 70% ethanol and incubated overnight with shaking at 4 °C. The particles were then washed extensively with phosphate-buffered saline (PBS) and resuspended to make a concentrated 16% v/v stock solution. The particles were free of endotoxin using a high-sensitivity Limulus amebocyte lysate assay (BioWhittaker).
2.2. Quantification of TNF-α release
Macrophages were exposed to PMMA particles and samples from the culture media were collected at the indicated time points post challenge. TNF-α levels were quantified using commercially available ELISA kits per the manufacturer’s instructions (R & D systems).
2.3. Polymyxin B co-incubation experiments
Polymyxin B (Sigma–Aldrich) was prepared fresh before each use and added to cell cultures at a concentration of 10 μg/ml. This concentration has been shown to decrease LPS-induced inflammatory reactions without causing significant toxicity. The murine monocyte/macrophage cell line RAW 264.7 was cultured in DMEM (Invitrogen-Gibco) containing 10% (v/v) fetal bovine serum (FBV) and maintained in a 5% CO2 atmosphere at 37 °C. Cells were plated in 24-well tissue culture plates at 1 × 105 cells/well in 1 ml of media with serum and allowed to adhere for 24 h. The media was then replaced with 1 ml of media containing one of the following treatments: 1) PMMA particles (0.30% v/v); 2) PMMA particles and Polymyxin B; 3) 100 ng/ml LPS (E. coli 055:B5, Sigma–Aldrich); 4) LPS and Polymyxin B.
2.4. MyD88 inhibitory peptide experiments
RAW 264.7 were cultured as described above. Following adherence, cells were then preincubated for 24 h with 1 ml of fresh media containing one of the following treatments: 1) 100 μM MyD88 homodimerization inhibitory peptide (Imgenex), which binds the MyD88 monomer, blocking MyD88 activation; 2) 100 μM control peptide, which crosses the cell membrane but does not interact with MyD88; 3) no peptide. Following the preincubation period in each group, PMMA particles were added at a dose of 0.30% v/v.
2.5. Primary murine macrophage experiments
Bone marrow derived macrophages (BMDM) were isolated from the femurs of C57BL/6 wild type (WT), MyD88−/− and TRIF−/− knockout mice as previously described [11]. Briefly, stem cells were flushed from femurs and incubated in differential medium consisting of RPMI 1640 media (Invitrogen), 13% FBS, 5% horse serum, 30% macrophage-colony stimulating factor and 0.5% glucose for 7 days to allow proliferation and differentiation. BMDMs were cultured in 24-well plates at a density of 8 × 105 cells/well in 1 ml of RPMI-1640 media with 10% FCS, 1% HEPES, 1% penicillin/streptomycin and 1% sodium pyruvate (Invitrogen-Gibco) for 24 h to allow adherence. The media was then exchanged with 1 ml of fresh media containing PMMA particles at a dose of 0.30% v/v.
2.6. Reverse transcription PCR
WT, MyD88−/−, TRIF−/− BMDMs were cultured as described above. Following PMMA particle exposure for the indicated time periods, cells were lysed with Trizol Reagent (Invitrogen-Gibco) and RNA was purified using RNeasy Kit (Qiagen) per the manufacturer’s protocol. The total amount of RNA was quantified using spectro-photometric OD260 readings. RNA quality was assessed by light absorbance at 260 and 280 nm. To prepare RNA for polymerase chain reaction (PCR) analysis, RNA was converted to cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed using probes for TNF-α (Applied Biosystems) or probes included in the TLR RT2 Profiler™ PCR Array (SA Biosciences). Relative quantification was measured with the delta comparative threshold (CT) method after determining the CT values for reference and target genes [12].
2.7. Particle-induced osteolysis
PMMA-induced calvarial osteolysis was performed as previously described with slight modifications [13]. Briefly, C57BL/6 WT and MyD88−/− male mice 10–14 weeks old were sedated with isoflurane gas. A 10-mm middle sagittal incision was made over the midline suture of the calvarium and 1.0 × 1.0 cm area of periosteum was exposed. The exposed periosteum was uniformly covered with 20 μl of a 0.30% v/v PMMA particle suspension and the incision was sutured.
2.8. Micro-computed tomography
All mice were scanned using a GE explore RS Micro CT system (Fairfield, CT, USA) at a 45 micron resolution. The scans were individually corrected to a calibration piece placed in each scan to control for any inter-scan variability. The resultant CT data was normalized to Hounsfield units (HU), and the calibrated CT data was analyzed using MicroView imaging software, an open source 3D Volume Viewer and Analysis Software package. Calculations of Bone Volume were done on a standardized region of interest using the posterior coronal suture and sagittal suture as standard landmarks to maintain consistency (Fig. 3c). Data were analyzed with a global fixed threshold, according to the method previously described [14]. The bone volume was outputted and analyzed in cm3 of hydroxyapatite.
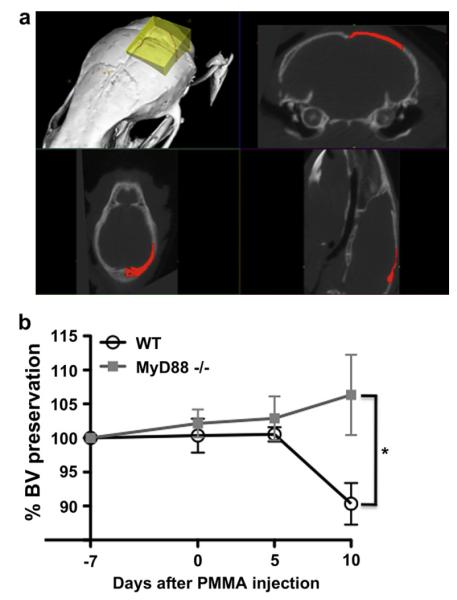
Fig. 3.
Disruption of MyD88-dependent signaling diminishes PMMA particle-induced osteolysis (a) PMMA particle-induced osteolysis in WT and MyD88−/− mice as assessed within the volume of interest by longitudinal 3D micro-computed tomography (μCT). The volume of interest is indicated by the yellow shaded region (top left panel), with the 2-dimensional borders indicated in the coronal plane (top right panel), axial plane (bottom left panel) and sagittal plane (bottom right panel). (b) Graphical representation of micro-computed tomography quantifying the percent change in bone volume (BV) induced by injecting PMMA particles onto the calvarium of WT (n = 7) and MyD88−/− mice (n = 5). * = P < 0.05. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.9. Statistical analysis
A two-way analysis of variance with Bonferroni post-tests or two-tailed t-test was conducted where appropriate. A P-value < 0.05 was chosen as the threshold for significance.
3. Results
3.1. Dose-dependent relationship between PMMA particle concentration and TNF-α
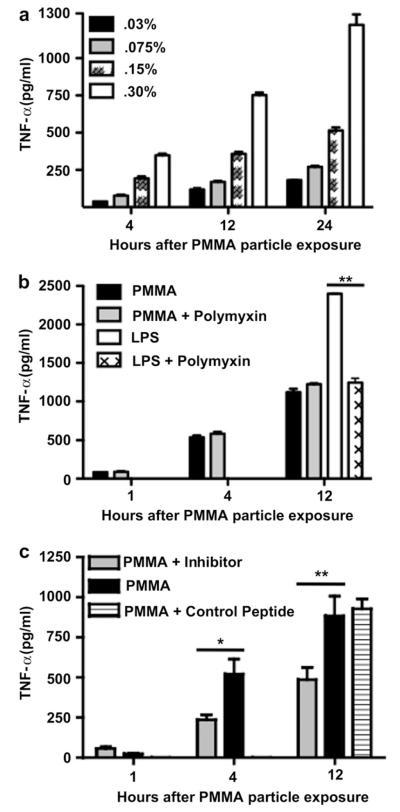
To investigate whether the TLR pathway contributes to the recognition of PMMA particles we identified an optimal particle concentration which stimulates robust production of TNF-α by the phagocytic cells. To simulate the in vivo scenario of macrophages being exposed to orthopedic implant wear-debris particles, the macrophage cell line RAW 264.7 was incubated in vitro with PMMA particles. At 4, 12 and 24 h post-particle exposure we observed a linear correlation between PMMA particle dose and TNF-α production (r2 = 0.99, 0.99, 0.98 respectively, Fig. 1a, Supplementary Fig. 2). For the PMMA particle concentrations tested, the greatest TNF-α production was observed at a particle concentration of 0.30%. This concentration was therefore chosen for all future experiments in this study.
Fig. 1.
Macrophage recognition of PMMA particles involves MyD88-dependent TLRs and is independent of adherent endotoxin TNF-α production by RAW 264.7 murine macrophages cultured with (a) PMMA particles at a concentration of 0.03, 0.075, 0.15 and 0.30% v/v (n = 3); (b) PMMA particles, PMMA particles and Polymyxin B, LPS, LPS and Polymyxin B (4 independent trials, n = 3–6 per trial); (c) PMMA particles, PMMA particles plus a MyD88 inhibitory peptide, PMMA particles plus a control peptide. (2 independent trials, n = 3–6 per trial). % v/v = volume of particle (mL) per 100 mL of solution. * = P < 0.05, ** = P < 0.01.
3.2. TNF-α production independent of adherent endotoxin
Within the literature investigating prosthetic implant loosening, controversy exists regarding the role that endotoxin, the lipopolysaccharide (LPS) from the cell wall of gram-negative bacteria, may play in the biological activity of wear-debris particles. Some investigators have claimed that treatments which remove adsorbed endotoxin markedly decrease or completely reverse the biological activity of the particles [15,16]; others have argued that the impact of these treatments is due to alterations in the particles themselves, specifically by altering the surface chemistry or anatomy, rather than the removal of endotoxin. For our study it is critical to address the inflammatory contribution of endotoxin because one of the ligands for TLR4 is LPS. To remove adherent endotoxin, we subjected commercially available PMMA particles to a series of ethanol washes. We then tested the PMMA particles for the presence of endotoxin using the limulus amebocyte lysate (LAL) assay. We were unable to detect the presence of any endotoxin with this assay which has a minimum sensitivity of detection of 0.1EU/ml. This traditional approach provides an indicator for the presence of LPS, however, it does not address the significance of endotoxin levels which may exist below the already very low sensitivity of detection of the assay. Polymyxin B is an antibiotic used for treating gram-negative septic shock and works by binding to and neutralizing LPS [17]. To determine the presence and significance of endotoxin which potentially could exist at a concentration below the sensitivity of the LAL assay, we compared the inflammatory response of macrophages incubated with PMMA particles to that of macrophages incubated with both PMMA particles and Polymyxin B. Macrophages incubated with PMMA particles alone or with PMMA particles plus Polymyxin B exhibited similar particle-induced TNF-α release at both 4 and 12 h (P > 0.05, Fig. 1b). This is unlikely to reflect ineffective neutralization of endotoxin by Polymyxin B because macrophages incubated with LPS alone produced TNF-α levels which saturated the upper level of detection of the assay. The inclusion of Polymyxin B in the culture significantly decreased the LPS-induced TNF-α production by at least 50% (P < 0.01 LPS vs. LPS and Polymyxin B) (Fig. 1b). The exquisite sensitivity of macrophages to LPS (marked activation at LPS concentration of 500 pg/ml) is consistent with studies which suggest that LPS contamination may be responsible for macrophage activation in some particle experiments. However, by neutralizing LPS without the use of harsh treatments potentially altering the surface chemistry of the particles, our results demonstrate that particles alone, without residual LPS, are sufficient to produce intense macrophage activation
3.3. MyD88 inhibitory peptide
To investigate whether the TLR pathway is involved in the recognition of PMMA particles, we disrupted cell signaling through the adapter protein MyD88. This was accomplished by applying a small peptide inhibitor which binds to MyD88 monomers and prevents the homodimerization necessary for the recruitment and activation of the downstream signaling kinase IRAK [18]. After allowing the macrophages 24 h to adhere to the culture plate and in the absence of PMMA particles the concentration of TNF-α was 38 ± 9.0 pg/ml (data not shown). When exposed to PMMA particles, macrophages exhibited a time-dependent increase in TNF-α release as early as 4 h post-particle exposure (P < 0.001, media only vs. PMMA exposure) (Fig. 1c). Addition of the MyD88 inhibitory peptide significantly decreased PMMA particle-induced TNF-α release at 4 h (PMMA, 522.1 ± 82.3; PMMA + MyD88-inhibitor, 237.3 ± 26.5 pg/ml; P < 0.05 PMMA vs. PMMA + MyD88-inhibitor) and 12 h post-particle exposure (PMMA, 884.0 ± 106.7; PMMA + MyD88-inhibitor, 486.6 ± 62.5 pg/ml; P < 0.01 PMMA vs. PMMA + MyD88-inhibitor) (Fig. 1c). Finally, there was no significant difference in TNF-α production between macrophages incubated with either PMMA particles or PMMA particles plus control peptide (Fig. 1c). The control peptide contains only the protein transduction sequence of the inhibitory peptide, so the cell is permeable to both peptides, but only the inhibitory peptide binds specifically to the MyD88 monomer. This demonstrates that the decrease in PMMA particle-induced TNF-α production was because of specific inhibition of MyD88 rather than a decrease in the ability of macrophages to produce TNF-α.
3.4. MyD88 knock out macrophages
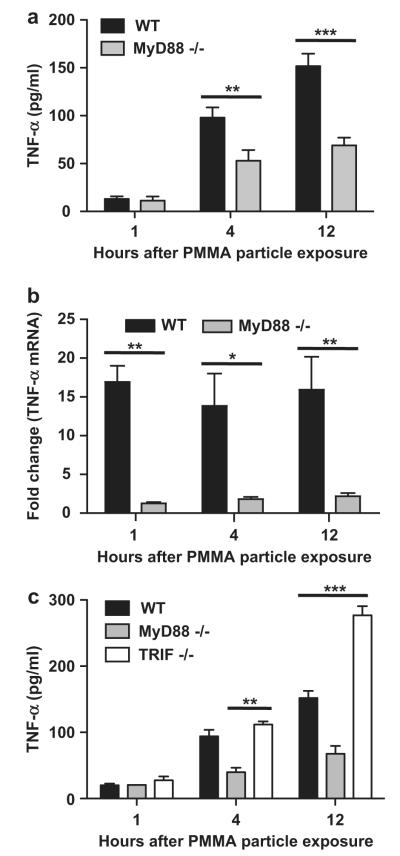
Having observed that disruption of MyD88 signaling in a macrophage cell line diminishes the PMMA particle-induced inflammatory response, we next asked whether similar results would be observed in primary bone marrow derived macrophages (BMDM). Macrophages were isolated from wild type (WT) and MyD88 knock out (MyD88−/−) mice. Similar to the results observed using a macrophage cell line, primary WT macrophages exposed to PMMA particles exhibited a time dependent increase in the release of TNF-α that was significant at 4 and 12 h post-particle exposure (P < 0.001, media only vs. PMMA exposure). Relative to WT macrophages, disruption of MyD88 signaling significantly decreased the PMMA particle-induced production of TNF-α at 4 h (WT, 98.0 ± 10.5; MyD88−/−, 52.8 ± 11.3 pg/ml; P < 0.01) and 12 h (WT, 151.8 ± 13.1; MyD88−/−, 65.3 ± 7.7 pg/ml; P < 0.001) (Fig. 2a). The production of TNF-α is known to be regulated at numerous levels of control, including the transcription of the gene [19], stability of the mRNA transcript [20], translation of the mRNA and secretion of the protein [21,22]. To gain greater insight into the role which MyD88-dependent TLRs play in the inflammatory response to PMMA particles we compared the PMMA-induced increase in TNF-α mRNA expression between WT and MyD88−/− macrophages. Disruption of MyD88 signaling significantly mitigated the PMMA-induced increase in TNF-α expression at 1 (P < 0.01), 4 (P < 0.05) and 12 (P < 0.01) hours post PMMA particle exposure (Fig. 2b).
Fig. 2.
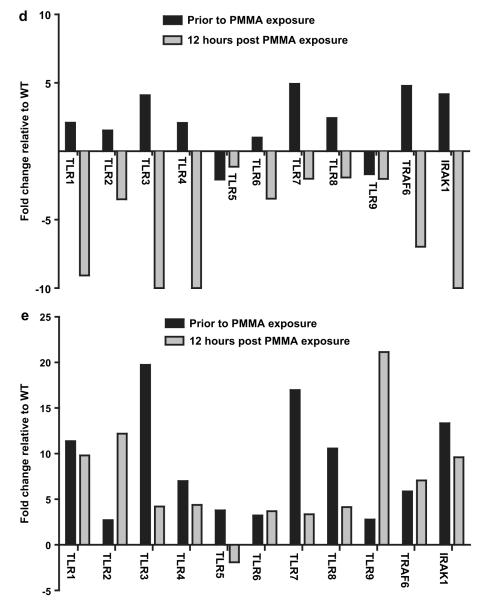
Inflammatory response to PMMA particles is mediated by MyD88-dependent and TRIF-independent TLRs The inflammatory response toward PMMA particles is diminished in MyD88 knockout (MyD88−/−) relative to wild type (WT) bone marrow derived macrophages at the both the level of TNF-α (a) protein production (4 independent trials, n = 3–4 per trial); and (b) mRNA expression (2 independent trials, n = 3 per trial). Fold change is relative to the mRNA expression level of each cell type prior to PMMA particle exposure. (c) TRIF knockout (TRIF−/−) macrophages demonstrate enhanced TNF-α production relative to WT bone marrow derived macrophages in response to PMMA particles (2 independent trials, n = 3–4 per trial). (d) RT-PCR comparing the expression of numerous genes involved in the TLR signaling pathway by MyD88−/− and (e) TRIF−/− BMDMs prior to and following 12 h of PMMA particle exposure. Fold change is expressed relative to WT cells. * = P < 0.05, ** = P < 0.01, *** = P < 0.001.
3.5. TRIF knock out macrophages
The TLR signaling cascade contains TLRs which signal through either MyD88, TRIF or both. To address the involvement of TLRs which interact with TRIF, we compared the PMMA particle-induced inflammatory response by macrophages isolated from WT and TRIF knock out (TRIF−/−) mice. Relative to WT macrophages, disruption of TRIF signaling did not significantly alter the PMMA particle-induced production of TNF-α at 4 h (WT, 94.2 ± 9.6; TRIF−/−, 111.8 ± 4.9 pg/ml TNF-α; P > 0.05). At 12 h following PMMA particle exposure, TRIF−/− macrophages produced significantly more TNF-α than WT macrophages (WT, 152.0 ± 10.8; TRIF−/−, 276.8 ± 13.8 pg/ml TNF-α; P < 0.001 WT vs. TRIF−/−) (Fig. 2c).
3.6. Gene expression analysis of WT, TRIF−/− and MyD88−/− macrophages
To understand the mechanism by which disrupting TRIF signaling increases the inflammatory response to PMMA particles, we assayed the gene expression of numerous components of the TLR signaling pathway. Prior to PMMA particle exposure, both TRIF−/− and MyD88−/− macrophages demonstrated increased expression of Interleukin-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) as well as numerous TLRs (Fig. 2d and e). Relative to WT cells, TRIF−/− macrophages have increased expression of TLRs 1–9 (Fig. 2e) and MyD88−/− macrophages exhibit increased expression of TLRs 1–4, 7, 8 (Fig. 2d). This gene expression data indicates that overall, prior to PMMA particle exposure, both MyD88−/− and TRIF−/− macrophages demonstrate increased expression of numerous TLRs and TLR-associated signaling proteins relative to WT macrophages.
Exposing macrophages isolated from MyD88−/− or TRIF−/− mice to PMMA particles for 12 h affects the expression of TLRs and TLR-related signaling components by each knock out cell type in the opposite manner. Relative to WT, TRIF−/− macrophages demonstrate increased expression of TRAF6, IRAK1, TLRs 1–4 and 6–9 (Fig. 2e). In contrast, MyD88−/− macrophages now demonstrate decreased expression relative to WT cells of TRAF6, IRAK1 and TLRs 1–9 (Fig. 2d).
3.7. In vivo osteolysis of WT and MyD88−/− mice
We next asked whether the decreased in vitro inflammatory response observed in MyD88−/− macrophages would translate into decreased PMMA particle-induced osteolysis in MyD88−/− mice. PMMA particles were placed on WT and MyD88−/− murine calvaria and particle-induced bone resorption was quantified at 5 and 10 days post-particle exposure using micro-computed tomography (μCT) (Fig. 3a). Since the calvaria bone is not a fixed but rather a dynamic shape, size and volume, we performed a μCT scan of the calvarium of each mouse prior to PMMA particle exposure and set this bone volume (BV) as 100%. We then compared the BV values at 5 and 10 days following PMMA particle exposure to this baseline value and expressed the data as percentage change in bone volume. Prior to particle implantation, the BV of WT mice was not significantly different from MyD88−/− mice (WT, 5.07 ± 0.11; MyD88−/−, 5.12 ± 0.23 cm3 hydroxyapatite. P = 0.84) (Fig. 3b). At 5 days following PMMA particle exposure, the percent change in BV was not significantly different between the groups (WT, 0.53 ± 1.05%; MyD88−/−, 2.89 ± 3.22%. P = 0.52). At 10 days post-particle exposure, the change in BV was significantly greater in the WT than the MyD88−/− mice (WT, −9.67 ± 3.06%; MyD88−/−, 6.33 ± 5.90%. P < 0.05) (Fig. 3b). This suggests that similar to the decreased production of TNF-α in response to PMMA particles observed with MyD88−/− macrophages in vitro, disruption of MyD88 signaling significantly decreases PMMA particle-induced osteolysis in vivo.
4. Discussion
The aim of this study was to investigate whether the inflammatory response induced by prosthetic implant wear-debris particles is mediated by components of the TLR signaling pathway. To address this question we first challenged a murine macrophage cell line with PMMA particles and compared the expression of TNF-α. Macrophages treated with a small peptide inhibitor of MyD88 demonstrated significantly diminished production of TNF-α compared to untreated control macrophages. We next tested whether disruption of MyD88 signaling in primary murine macrophages would affect the inflammatory response toward PMMA particles. Similar to the results observed with the macrophage cell line, primary MyD88−/− macrophages produced significantly less TNF-α mRNA and protein in response to PMMA particles than WT macrophages. This suggests that the inflammatory response to PMMA particles is partly dependent on signaling through MyD88 and therefore one or multiple TLRs.
The majority of TLR signaling is conducted through the adapter protein MyD88, however, TLR3 and TLR4 can utilize a MyD88-independent signaling pathway through the adapter protein TRIF. To investigate the role of TRIF-dependent TLR signaling, the PMMA particle-induced production of TNF-α by TRIF−/− primary murine macrophages was compared to control WT macrophages. TRIF−/− macrophages exposed to PMMA particles for 12 h produced significantly more TNF-α than WT macrophages. If TRIF were critical to the inflammatory response stimulated by PMMA particles, a decrease in TNF-α production would be anticipated. Therefore, these results suggest that TRIF-dependent signaling is not involved in the inflammatory response toward PMMA particles. Similarly because TLR 3 is entirely TRIF-dependent it is improbable that PMMA particles act as a ligand for this receptor.
To study the effect which disruption of MyD88 or TRIF signaling exerts on the other components of the TLR signaling pathway, the gene expression of multiple TLRs and TLR-associated signaling proteins was analyzed. Macrophages isolated from both TRIF−/− and MyD88−/− mice demonstrated increased expression of IRAK1 compared to WT cells. The role of IRAK1 in the TLR signaling pathway is that following TLR activation, IRAK1 is recruited to the receptor-signaling complex and has been implicated in TLR 2, 4, 7 and 9 mediated inflammatory responses [23,24]. In the MyD88-dependent pathway of TLR signaling, IRAK1 is recruited and phosphorylates TRAF6 which leads to the activation of IκB kinase. IκB kinase accelerates the degradation of IκB which then permits NF-κB to translocate to the nucleus [25,26]. Both TRIF−/− and MyD88−/− primary macrophages also demonstrated increased expression of TRAF6. TRAF6 has previously been implicated in the maintenance of normal bone architecture, as TRAF6−/− mice have defects in NF-κB signaling and develop osteopetrosis [27]. In addition to increased expression of IRAK1 and TRAF6, prior to PMMA particle exposure both MyD88−/− and TRIF−/− macrophages demonstrate increased expression of numerous TLRs relative to WT cells. This may indicate why TRIF−/− macrophages demonstrate an exaggerated PMMA particle-induced inflammatory response relative to WT macrophages. We propose that there exists a basal or homeostatic level of TLR signaling in a cell. Disruption of TRIF signaling will remove the TRIF-mediated contribution to the overall level of TLR signaling. In an attempt to compensate, the cell increases the expression of other TLR signaling components, many of which may be MyD88-dependent. This results in an exaggerated inflammatory response toward MyD88-dependent ligands. Similarly, MyD88−/− cells compensate by increasing the expression of TLR signaling components, however, the inflammatory response to MyD88-dependent ligands is still diminished relative to WT cells because of the lack of MyD88.
PMMA particle exposure had an opposite effect on the expression of TLR-related genes by MyD88−/− compared to TRIF−/− macrophages. Specifically, MyD88−/− macrophages demonstrate decreased expression, whereas TRIF−/− macrophages demonstrate increased expression of numerous TLRs as well as TRAF6 and IRAK1 relative to WT cells. The increased expression of numerous genes related to TLR signaling by TRIF−/− and WT cells relative to MyD88−/− macrophages may indicate a positive feedback process induced by PMMA particles. Previous studies have demonstrated that exposure to T-helper cell type 1 (Th1) proinflammatory cytokines such as TNF-α, transcriptionally upregulates the expression of numerous TLRs [28,29]. This may explain the TLR transcriptional pattern of each cell type because in response to PMMA particles, TRIF−/− macrophages produce more TNF-α than WT macrophages, which produce more TNF-α than MyD88−/− macrophages. The relative levels of TNF-α expression by each of these cell types matches the relative expression levels of various TLR pathway components.
An aim of this study was to investigate the relationship between PMMA particles, the TLR family and PMMA particle-induced inflammation. Disruption of MyD88-dependent signaling decreased the PMMA-induced production of TNF-α mRNA and protein production. Similarly, MyD88−/− macrophages exposed to PMMA particles demonstrate a transcriptional pattern suggestive of decreased TLR signaling pathway activation compared to WT and TRIF−/− macrophages. A limitation of this approach is that assaying changes in gene expression or production of proinflammatory cytokines is an indirect measure of TLR activation. A more accurate assay would involve direct measurement of NF-κB activation. An electrophoretic mobility shift assay (EMSA) is one approach to demonstrate a relationship between a ligand, a receptor complex and subsequent NF-κB activation. This approach has successfully been applied in the diabetes literature where EMSAs have been used to demonstrate that binding of advanced glycosylated end products (AGE) to the transmembrane receptor for AGE (RAGE) directly converts short-lasting NF-κB activation into prolonged activation [30]. Similarly, a modified EMSA has been used to demonstrate a relationship between hyperglycemia and increased activation of NF-κB [31]. Prior to this study a relationship between NF-κB activation and many of the late complications of diabetes had been suggested [32], however, a direct in vivo relationship between hyperglycemia and NF-κB activation in patients had not been demonstrated. They found that monocytes isolated from patients with poor glycemic control demonstrated significantly higher NF-κB binding by EMSA than patients with well controlled diabetes [31]. Future experiments could use a similar approach as these studies referenced from the diabetes literarure to investigate whether interfering with MyD88 or TRIF signaling alters the wear-debris induced activation of NF-κB.
One of the advantages of disrupting MyD88 or TRIF signaling is the ability to simultaneously investigate the involvement of numerous TLRs in the recognition of PMMA particles. However, this is also a limitation of the study because it is unclear which of the TLRs are activated by exposure to PMMA particles. This study provides gene expression evidence indicating differential expression of certain TLRs following exposure to PMMA particles, however, at this time it is not clear which TLR(s) are involved. It is unlikely that TLR3 is involved in the recognition of PMMA particles because TLR3 is TRIF-dependent and disruption of TRIF did not decrease the inflammatory response toward PMMA particles. Which specific TLR(s) are critical to the recognition of PMMA particles is a question which will warrant future investigation.
The purpose of the murine calvaria model was to address whether the diminished expression and production of TNF-α in response to PMMA particles by MyD88−/− macrophages in vitro, would translate into a difference in the degree of osteolysis. At 5 days following PMMA particle exposure the percent change in BV was not significantly different between the WT and MyD88−/− mice. At this time point the changes in BV were positive for both groups, this indicates that the BVs are constantly increasing in these mice. Additionally, this indicates that either 5 days is too early of a time point post-particle exposure to detect significant osteolyis or that the steady increase in BV is more significant than the loss of BV at this time point. However, by 10 days following particle exposure the change in BV was significantly greater in the WT than the MyD88−/− mice. The reduced osteolysis in MyD88−/− relative to WT mice, supports prior studies demonstrating that the combination of TNF-α and activation of MyD88-dependent TLRs on osteoclast precursor cells results in increased osteoclastogenesis and osteoclast survival [33,34].
This in vivo experiment is subject to several limitations, one being that TLRs are expressed on macrophages as well as osteoclasts. This makes it difficult to determine whether the decrease in osteolysis results from diminished PMMA particle-mediated stimulation of TLRs on MyD88−/− macrophages or decreased differentiation and survival of MyD88−/− osteoclasts. In addition, the quality of the CT data was limited to 45 microns due to radiation limits on in vivo imaging, precluding the use of higher resolution protocols. Additionally, it is important to study the same Region of Interest (ROI) on all subjects. To help minimize variability, the ROI size was kept constant, and anatomical landmarks of the coronal and sagittal sutures were used to standardize the analysis. Finally, this experimental model is not identical to the clinical scenario of particle-induced implant loosening because the model does not incorporate an implant, mechanical load, or fluid pressure, the PMMA particles were given as a single bolus, and the osteolytic process was studied for only a period of 10 days. However the experimental studies reported facilitate a mechanistic understanding of basic biological processes relevant to particle-induced recognition and signaling pathways.
5. Conclusions
This study demonstrates that the response to PMMA particles is dependent in part on MyD88, as part of the TLR signaling pathway. This conclusion is supported by evidence demonstrating that in both a murine macrophage cell line and primary mouse macrophages, disruption of MyD88 signaling reduces PMMA particle-induced production of TNF-α. In contrast, the inflammatory response to PMMA particles by TRIF−/− BMDM was enhanced, possibly because of a compensatory increase in the expression of numerous components of the TLR signaling pathway. Using an in vivo model of particle-induced osteolysis, MyD88−/− mice demonstrated significantly less osteolysis than WT mice at 10 days. These results indicate that MyD88 may represent a therapeutic target for the prevention of wear-debris particle-induced periprosthetic osteolysis.
Supplementary Material
Acknowledgments
We thank Dr. Judith Hellman (University of California San Francisco) for generously providing us with the wild type, MyD88 knockout and TRIF knockout bone marrow derived macrophages. This work was supported in part by the Robert L and Mary Ellenburg Chair in Surgery (S.B.G), the Stanford Medical Scholars Program (J.I.P) and the Orthopaedic Research and Education Foundation (J.I.P).
Footnotes
Appendix. Supplementary material Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.biomaterials.2011.04.046
References
- [1].Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001:66–70. doi: 10.1097/00003086-200112000-00007. [DOI] [PubMed] [Google Scholar]
- [2].Day MJ, Butterworth SJ, Palmer MR, Case CP. Characterization of wear debris associated with aseptic loosening of a canine hip prosthesis. J Comp Pathol. 1998;119:89–93. doi: 10.1016/s0021-9975(98)80075-3. [DOI] [PubMed] [Google Scholar]
- [3].Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–86. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- [4].Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001:71–7. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- [5].Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- [6].Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- [7].Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- [8].Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–63. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- [9].Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–9. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- [10].Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–95. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schilling D, Thomas K, Nixdorff K, Vogel SN, Fenton MJ. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J Immunol. 2002;169:5874–80. doi: 10.4049/jimmunol.169.10.5874. [DOI] [PubMed] [Google Scholar]
- [12].Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- [13].Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154:203–10. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Muller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, et al. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998;23:59–66. doi: 10.1016/s8756-3282(98)00068-4. [DOI] [PubMed] [Google Scholar]
- [15].Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, et al. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–91. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- [16].Daniels AU, Barnes FH, Charlebois SJ, Smith RA. Macrophage cytokine response to particles and lipopolysaccharide in vitro. J Biomed Mater Res. 2000;49:469–78. doi: 10.1002/(sici)1097-4636(20000315)49:4<469::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [17].Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Loiarro M, Sette C, Gallo G, Ciacci A, Fanto N, Mastroianni D, et al. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-{kappa}B. J Biol Chem. 2005;280:15809–14. doi: 10.1074/jbc.C400613200. [DOI] [PubMed] [Google Scholar]
- [19].Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- [20].Spriggs DR, Deutsch S, Kufe DW. Genomic structure, induction, and production of TNF-alpha. Immunol Ser. 1992;56:3–34. [PubMed] [Google Scholar]
- [21].Anderson P, Phillips K, Stoecklin G, Kedersha N. Post-transcriptional regulation of proinflammatory proteins. J Leukoc Biol. 2004;76:42–7. doi: 10.1189/jlb.1103536. [DOI] [PubMed] [Google Scholar]
- [22].Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, et al. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–63. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Swantek JL, Tsen MF, Cobb MH, Thomas JA. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J Immunol. 2000;164:4301–6. doi: 10.4049/jimmunol.164.8.4301. [DOI] [PubMed] [Google Scholar]
- [24].Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, et al. Interleukin-1 receptor-associated kinase-1 p.ays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–23. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–7. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- [26].Arch RH, Gedrich RW, Thompson CB. Tumor necrosis factor receptor-associated factors (TRAFs)–a family of adapter proteins that regulates life and death. Genes Dev. 1998;12:2821–30. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- [27].Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Covacu R, Arvidsson L, Andersson A, Khademi M, Erlandsson-Harris H, Harris RA, et al. TLR activation induces TNF-alpha production from adult neural stem/progenitor cells. J Immunol. 2009;182:6889–95. doi: 10.4049/jimmunol.0802907. [DOI] [PubMed] [Google Scholar]
- [29].Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, et al. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277:20431–7. doi: 10.1074/jbc.M110333200. [DOI] [PubMed] [Google Scholar]
- [30].Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- [31].Hofmann MA, Schiekofer S, Kanitz M, Klevesath MS, Joswig M, Lee V, et al. Insufficient glycemic control increases nuclear factor-kappa B binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care. 1998;21:1310–6. doi: 10.2337/diacare.21.8.1310. [DOI] [PubMed] [Google Scholar]
- [32].Bierhaus A, Illmer T, Kasper M, Luther T, Quehenberger P, Tritschler H, et al. Advanced glycation end product (AGE)-mediated induction of tissue factor in cultured endothelial cells is dependent on RAGE. Circulation. 1997;96:2262–71. doi: 10.1161/01.cir.96.7.2262. [DOI] [PubMed] [Google Scholar]
- [33].Sato N, Takahashi N, Suda K, Nakamura M, Yamaki M, Ninomiya T, et al. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1alpha. J Exp Med. 2004;200:601–11. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zou W, Amcheslavsky A, Bar-Shavit Z. CpG oligodeoxynucleotides modulate the osteoclastogenic activity of osteoblasts via Toll-like receptor 9. J Biol Chem. 2003;278:16732–40. doi: 10.1074/jbc.M212473200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.