Abstract
T-DNA insertion mutants have been widely used to define gene functions in Arabidopsis and in other plants. Here, we report an unexpected phenomenon of epigenetic suppression of T-DNA insertion mutants in Arabidopsis. When the two T-DNA insertion mutants, yuc1-1 and ag-TD, were crossed together, the defects in all of the ag-TD plants in the F2 population were partially suppressed regardless of the presence of yuc1-1. Conversion of ag-TD to the suppressed ag-TD (named as ag-TD*) did not follow the laws of Mendelian genetics. The ag-TD* could be stably transmitted for many generations without reverting to ag-TD, and ag-TD* had the capacity to convert ag-TD to ag-TD*. We show that epigenetic suppression of T-DNA mutants is not a rare event, but certain structural features in the T-DNA mutants are needed in order for the suppression to take place. The suppressed T-DNA mutants we observed were all intronic T-DNA mutants and the T-DNA fragments in both the trigger T-DNA as well as in the suppressed T-DNA shared stretches of identical sequences. We demonstrate that the suppression of intronic T-DNA mutants is mediated by trans-interactions between two T-DNA insertions. This work shows that caution is needed when intronic T-DNA mutants are used.
Key words: epigenetics, T-DNA mutant, genetic suppression, trans-interaction, YUC.
INTRODUCTION
Agrobacterium-mediated plant transformation is achieved when the T-DNA (Transfer DNA) fragment from the modified Ti plasmids is integrated into chromosomes in plant cells. T-DNA transformation can be used as a tool for insertional mutagenesis and also serves as an efficient vehicle for delivering target genes into plant cells. T-DNA fragments randomly insert into a plant genome during transformation and, when a T-DNA insertion is inserted in an exon or an intron, it often leads to the inactivation of the gene. As part of the different functional genomic initiatives in Arabidopsis, a number of T-DNA insertional mutagenesis have been conducted and currently we have access to large libraries of sequence-indexed T-DNA insertion lines in Arabidopsis (Samson et al., 2002; Sessions et al., 2002; Alonso et al., 2003). The T-DNA insertion mutants are tremendous resources for the determination of gene function and the elucidation of metabolic/signaling pathways. T-DNA mutants in Arabidopsis have become the first choice for many scientists because (1) the mutants are easily accessible through the Arabidopsis stock centers and (2) the mutants are often null alleles. T-DNA insertion mutants have been extensively used in reverse genetics and in studies of genetic interactions in Arabidopsis.
Studies on genetic interactions between two non-allelic mutants often provide insights into the functions of the two genes and the relative positions of the genes in a genetic pathway (Guarente, 1993; Hodgkin, 2005). Phenotypes of a mutant can be suppressed or enhanced by mutations in other genes. Synergistic genetic enhancement between two mutants often suggests that the two genes have overlapping functions or participate in parallel pathways (Guarente, 1993). If the two mutants are not null alleles and the two genes have no sequence homology, synergistic enhancement can also suggest that the two genes function in the same pathway (Cheng et al., 2008). Genetic suppression of the phenotypes of one mutant by a mutation in another gene could be achieved through several mechanisms (Hodgkin, 2005). A mutant could be rescued if the general machinery of transcription and/or translation is altered. For example, a mutation that converts a sense codon to the stop codon UAG in a gene can be suppressed if the anti-codon in Trp-tRNA is mutated from CCA to CUA. Additionally, genetic suppression could also take place if the suppressor removes toxic proteins or metabolic intermediates. A mutant can also be suppressed if protein interactions or gene dosages are altered. Genetic screens for enhancers and suppressors for mutants have led to the discoveries of the regulatory mechanisms of major signaling and metabolic pathways.
In general, the phenotypes of a mutation are suppressed when an extragenic suppressor is present. Removal of the suppressor leads to the restoration of the original mutant phenotypes. In this paper, we report an unexpected phenomenon that phenotypes of a T-DNA insertion mutant are partially suppressed by another T-DNA insertion at another locus. Remarkably, the suppressed phenotypes could be stably transmitted for generations even in the absence of the suppressor T-DNA insertion. We crossed an auxin biosynthesis mutant yuc1-1 to a floral mutant ag-TD in order to generate the yuc1-1ag-TD double mutants for analyzing the roles of auxin in flower development. Both yuc1-1 and ag-TD are T-DNA insertion mutants (Figure 1A) and both are loss-of-function, recessive mutants. The YUC1 gene encodes a flavin-containing monooxygenase involved in auxin biosynthesis (Zhao et al., 2001; Cheng et al., 2006, 2007). The yuc1-1 mutant has no obvious developmental defects because of the existence of other homologous YUC genes in Arabidopsis (Cheng et al., 2006). AGAMOUS (AG) is an essential gene for reproductive organ formation in Arabidopsis (Yanofsky et al., 1990). The ag-TD mutant displays the characteristic ag loss-of-function phenotypes including the transformation of stamens into petals, loss of floral meristem determinacy, and a lack of carpels and stamens (Yanofsky et al., 1990). Surprisingly, none of the ag-TD plants in the F2 population displayed the typical ag-TD phenotypes, regardless of the presences of yuc1-1. We demonstrate that suppression of ag-TD is mediated by trans-interaction between the T-DNA insertions in yuc1-1 and ag-TD. Although gene silencing mediated by trans-interaction between two T-DNA insertions has been well documented (Daxinger et al., 2008), it has never been reported previously that such a trans-interaction among T-DNA insertions can lead to the restoration of gene functions inactivated by the same T-DNA insertions. We show that suppression of intronic T-DNA insertional mutants is frequently induced by other T-DNA insertions, suggesting that caution is needed when intronic T-DNA mutants are used in Arabidopsis.
Figure 1.
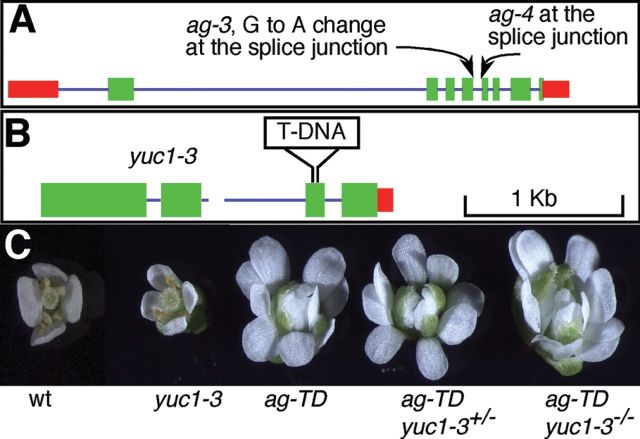
Suppression of ag-TD by Crossing ag-TD +/- to yuc1-1.(A) A diagrammatic presentation of the two T-DNA insertion mutants: yuc1-1 and ag-TD.(B) The crossing scheme. The ag-TD +/+ yuc1-1 +/- F1 plants were discarded.(C) Suppression of the floral defects in ag-TD in the F2 population. From left to right: WT, yuc1-1, ag-TD, and ag-TD*.(D) Phenotypic difference between ag-TD and ag-TD* inflorescence.(E) Production of petal-like stamens in ag-TD*. (F) Normal floral organs in ag-TD*.
RESULTS
Suppression of an agamous T-DNA Insertion Mutant by yuc1-1
To investigate the mechanisms of local auxin biosynthesis in specifying flower development, we combined the auxin biosynthesis mutant yucca1 (yuc1) (Cheng et al., 2006) with a known floral homeotic mutant agamous (ag) (Yanofsky et al., 1990). We chose the recessive T-DNA insertion mutants ag-TD and yuc1-1 because both mutants are in the Columbia background. In both ag-TD and yuc1-1, the T-DNA is inserted in an intronic region (Figure 1A). The yuc1-1 does not show obvious developmental defects (Cheng et al., 2006), but ag-TD fails to produce any stamens and carpels (Figure 1). We crossed ag-TD +/- to yuc1-1 and genotyped the F1 plants to select the ag-TD +/- yuc1-1 +/- plants (Figure 1B), which did not have obvious defects as expected. We let the F1 ag-TD +/- yuc1-1 +/- plants self-pollinate and collected the F2 seeds (Figure 1B). We analyzed the F2 population in order to identify the ag-TD yuc1-1 double mutants. Unexpectedly, none of the ag-TD plants displayed the typical ag phenotypes, indicating that ag-TD phenotypes were partially suppressed (Figure 1C). We named the plants with ag-TD genotype but without ag flower phenotypes as ag-TD* (Figure 1C). Note that ag-TD* is still homozygous for the T-DNA insertion as shown in Figure 1A, but the AG function is no longer inactivated by the T-DNA insertion in ag-TD*. The ag-TD* plants were fertile and produced viable seeds (Figure 1D). The suppression of ag-TD was only partial, because some ag-TD* flowers still contained petal-like stamens (Figure 1E) and indeterminate flowers (Figure1D). However, the majority of ag-TD * flowers had flower with four sepals, four petals, and one gynoecium consisting of two fused carpels (Figure 1F).
The yuc1-1 Is Not Required in the F2 Population for ag-TD * Phenotypes
We genotyped the F2 plants from the cross between ag-TD and yuc1-1 for the presence of ag-TD and yuc1-1. Among the 176 F2 individual plants, 56 were ag-TD, indicating that the T-DNA insertion at the AG locus segregated normally. Among the ag-TD plants, 43 did not contain T-DNA insertion at YUC1, 13 were yuc1-1 +/-, and zero were yuc1-1. Because both AG and YUC1 are on chromosome IV and they are about 15cM apart, it was expected that very few ag-TD yuc1-1 would be observed in the F2 population. Floral defects in all of the ag-TD plants in the F2 population were partially suppressed. Overall, 80% of the ag-TD YUC1 plants were suppressed well enough to be fertile. We noticed that all of the ag-TD -/- yuc1-1 +/- plants were able to set seeds, suggesting that the presence of the yuc1-1 mutation enhanced the suppression. However, the continued presence of the yuc1-1 mutation was not required to suppress ag-TD.
The ag-TD* Is Genetically Stable
To test whether ag-TD* phenotypes could be stably transmitted, we let the ag-TD* plants self-fertilize and studied the progeny for five generations. All of the progeny of ag-TD* was fertile in every generation and set a good number of seeds. We also noticed that the later generation of ag-TD* produced more seeds than the earlier generation of ag-TD* (Figure 2). We concluded that, once ag-TD was converted to ag-TD*, the ag-TD* does not spontaneously revert to ag-TD over generations (Figure 2).
Figure 2.
Inheritability of ag-TD*. The ag-TD* has been transmitted for five generations. Note that the fifth generation of ag-TD* produced more seeds than the earlier generations of ag-TD*.
We crossed ag-TD* to wild-type (WT) Columbia (Col) and let the F1 plants self-pollinate to generate an F2 population for analysis of the genotypes and phenotypes. Among the 98 plants analyzed, 23 were homozygous for the T-DNA insertion at the AG locus and all of the ag-TD plants displayed the ag-TD* phenotypes, indicating that ag-TD* is very stable.
The ag-TD* Is Able to Convert ag-TD to ag-TD*
We tested whether ag-TD* could induce similar changes in ag-TD. We crossed ag-TD* to ag-TD +/- plants and half of the resulting F1 plants were homozygous with the T-DNA insertion as expected. The F1 plants that presumably had the ag-TD*/ag-TD genotype were fertile and set a good number of seeds. We further analyzed the F2 plants generated from ag-TD*/ag-TD selfing. Among the 68 F2 plants analyzed, 66 plants behaved like ag-TD*. Two plants had weak ag-TD phenotypes and did not set seeds. Our data suggest that ag-TD* has the capacity to convert ag-TD into ag-TD*.
The ag-TD* Cannot Suppress Non-T-DNA ag Alleles
We have shown that ag-TD* allele induced the conversion of ag-TD into ag-TD*. We investigated whether ag-TD* could also restore the AG functions in other non-T-DNA ag mutant alleles. We used the strong ag-3 mutant and the weak ag-4 mutant alleles for the experiments (Figure 3A). Both ag-3 and ag-4 carried point mutations at splice junction sites (Figure 3A) (Jack et al., 1997; Chen and Meyerowitz, 1999). We crossed ag-TD* to ag-3 +/-, and the resulting F1 ag-TD*/ag-3 plants still displayed the typical ag mutant phenotypes and were sterile, suggesting that ag-TD* could not rescue ag-3. When we crossed ag-TD* to the weak ag-4 +/- plants, the ag-TD*/ag-4 plants were partially fertile. Normally, the ag-4 plants produce some stamens and carpel-like structures, but are sterile in our growth conditions. The ag-TD*/ag-4 plants could set seeds and their phenotypes were intermediate when compared to ag-TD* and ag-4 plants. We further analyzed the F2 population produced from selfing the ag-TD*/ag-4 plants. All of the homozygous ag-TD plants from the F2 population displayed the same phenotypes as those of ag-TD*. The ag-TD*/ag-4 plants in the F2 population were fertile, but all of the ag-4 plants were sterile. Our data indicate that ag-TD* could not rescue non-T-DNA ag mutants.
Figure 3.
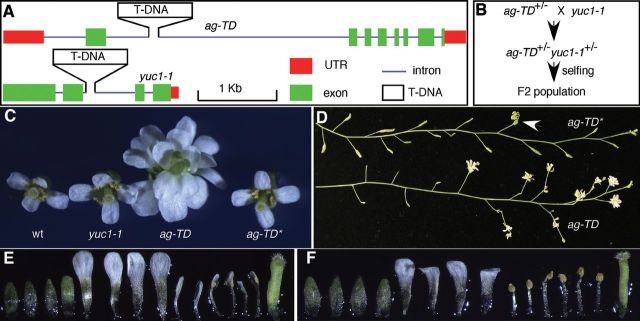
Suppression of ag Involves Special Alleles of ag and yuc.(A) The two non-T-DNA alleles of ag used in this study.(B) Allele of yuc1-3. yuc1-3 has a T-DNA insertion in the third exon.(C) The ag-TD was not suppressed by yuc1-3.
Conversion of ag-TD to ag-TD* Depends on a Specific yuc1 T-DNA Allele
The ag-TD mutant was rescued when it was crossed to yuc1-1 (Figure 1). We tested whether other T-DNA insertion mutants in yuc1 could also convert ag-TD to ag-TD*. We crossed ag-TD to yuc1-3 (Figure 3B). The yuc1-3 contained a T-DNA insertion at the third exon in the YUC1 gene (Figure 3B). Although both yuc1-1 and yuc1-3 were T-DNA insertion lines, they were generated using two different plasmids. The yuc1-1 was generated using the plasmid pROK2, which renders kanamycin resistance in Arabidopsis. The yuc1-3 was produced using a different plasmid that contains the SPM transposase gene and the BAR gene.
We genotyped the F2 population generated from selfing ag-TD +/- yuc1-3 +/- to identify ag-TD plants. All of the ag-TD plants in the F2 population displayed the typical ag-TD phenotypes regardless of the existence of yuc1-3 mutation and none of the ag-TD plants set any seeds (Figure 3C). We also isolated ag-TD yuc1-3 plants from the progeny of a single ag-TD +/- yuc1-3 plant and the double mutants behaved like ag-TD. These results suggest that inactivation of YUC1 is not sufficient to trigger the suppression of ag-TD and that the suppressor and the suppressed T-DNA mutants need to be generated from similar plasmids.
Production of Full-Length AG cDNA Using mRNAs from ag-TD and ag-TD*
We investigated whether the ag-TD to ag-TD* conversion is caused by an increased expression of AG in ag-TD*. We designed PCR primers to amplify the portion of AG cDNA starting from the start codon to the stop codon. To our surprise, ag-TD produced the full-length AG cDNA, suggesting that ag-TD is a partial loss-of-function mutant. We sequenced the AG cDNA from WT plants, ag-TD, and ag-TD*, and discovered that there were no structural differences among the cDNAs from the analyzed genotypes. It is difficult to compare the expression levels of AG in WT and in ag-TD using RT–PCR or Northern blot because the floral structures are quite different for the two genotypes. We used RNA in situ hybridization to detect the expression levels of AG in WT, ag-TD, and ag-TD*. The AG expression in ag-TD was weaker than that in WT, but ag-TD* clearly had more AG expression than ag-TD, suggesting that the conversion of ag-TD to ag-TD* correlates with an increased AG mRNA level in ag-TD* (Figure 4A).
Figure 4.
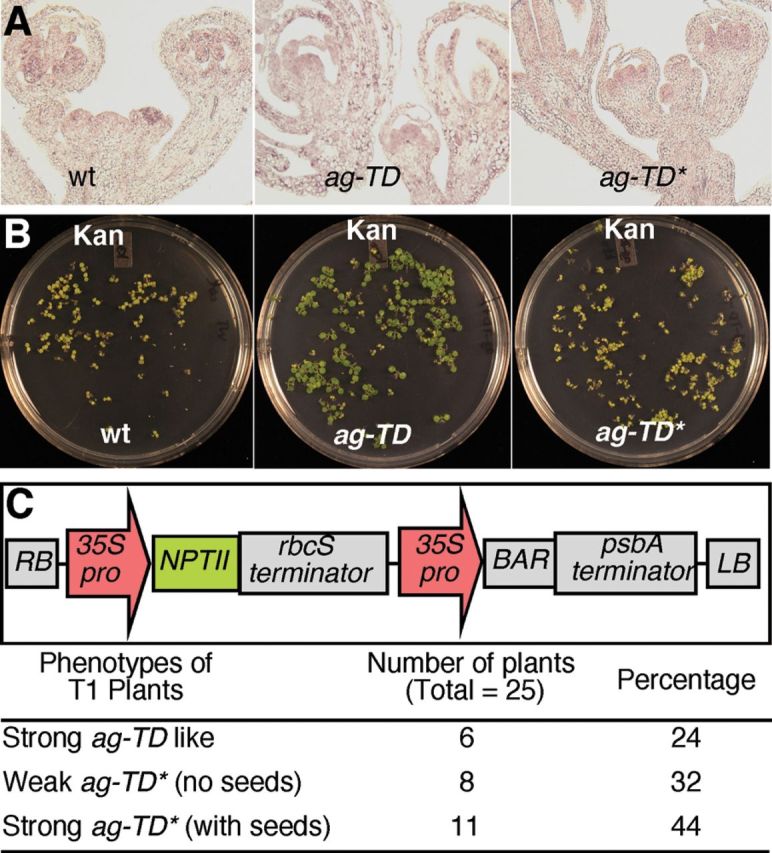
Suppression of ag-TD Is Probably Mediated by Trans- Interaction between Two -DNA Insertions.(A) In situ analysis of AG expression in WT, ag-TD, and ag-TD*.(B) Conversion of ag-TD to ag-TD* correlates with the loss of kanamycin resistance.(C) Conversion of ag-TD to ag-TD* can be achieved by the introduction of a T-DNA fragment that expresses the NPT II gene. About 75% of the T1 plants with ag-TD genotype did not show the typical ag phenotypes.
Kanamycin Resistance Gene Is Silenced in ag-TD*
We hypothesized that perhaps the partial restoration of AG function in ag-TD* might be caused by structural changes in DNA/chromatin in or near the T-DNA insertion. Such DNA/chromatin structural modifications might also alter the expression of the Neomycin phosphotransferase II (NPT II) gene, which renders plants resistant to kanamycin, within the T-DNA fragment. The NPT II gene in the T-DNA insertion made ag-TD plants resistant to kanamycin (Figure 4B) and, accordingly, about 25% of the progeny from ag-TD +/- plants were kanamycin-sensitive, suggesting that ag-TD contains a single T-DNA insertion. In contrast, all of the ag-TD* plants were kanamycin-sensitive (Figure 4B), although the NPT II gene still existed in ag-TD*. These data suggest that transcripts from the T-DNA fragment are also affected by the epigenetic modifications that suppressed ag-TD.
Suppression of ag-TD by Trans-Interactions between T-DNA Loci
The observation that kanamycin resistance was lost in ag-TD* suggested that trans-interactions between the T-DNA fragment in yuc1-1 locus and the T-DNA in ag-TD may be responsible for the suppression of ag-TD. To test this hypothesis, we transformed ag-TD +/- plants with a construct that expressed both the NPT II and the BAR gene (Figure 4C). Transformants were selected on basta-containing media. Among the 26 T1 plants with ag-TD genotype, 76% were partially suppressed and 44% were fertile (Figure 4C), demonstrating that introduction of another T-DNA insertion that expresses NPT II gene is sufficient to suppress ag-TD. The suppression of ag-TD is likely mediated by trans-interactions among T-DNA insertions.
Suppression of T-DNA Mutants by Other T-DNA Insertions Is Not Rare
We have demonstrated that ag-TD is suppressed by yuc1-1 and also by transforming a T-DNA fragment into ag-TD. We investigated whether other T-DNA insertion mutants can also be suppressed by similar T-DNA interactions. We crossed yuc1-1 to cob-TD, which also contains a T-DNA insertion in the large intron (Figure 5A). The COB gene encodes a glycosylphosphatidylinositol (GPI) anchored protein and plays an important role in cellulose microfibril orientation in Arabidopsis (Schindelman et al., 2001; Ko et al., 2006). Inactivation of COB by the T-DNA insertion led to very short roots and other defects (Figure 5B). However, all of the cob-TD plants in the F2 plants from the cross between yuc1-1 and cob-TD had longer roots than the original cob-TD lines, indicating that yuc1-1 also converted cob-TD to cob-TD*, which was partially suppressed (Figure 5). The presence of yuc1-1 made the suppression of cob-TD better (Figure 5B). This result is consistent with the observation that the ag phenotypes in ag-TD yuc1-1 +/- were better suppressed than those in ag-TD alone. Interestingly, the conversion of cob-TD to cob-TD* also led to the loss of kanamycin resistance (Figure 5D).
Figure 5.
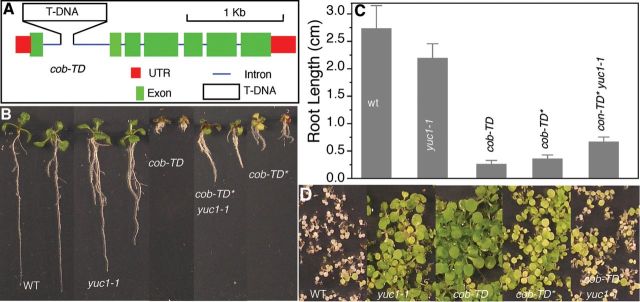
Partial Suppression of cob-TD by yuc1-1.(A) The cob-TD mutant with a T-DNA insertion in the large intron.(B) Suppression of cob-TD in the F2 population from a cross between cob-TD and yuc1-1.(C) Increased root length in cob-TD*.(D) The conversion of cob-TD to cob-TD* correlated with the loss of kanamycin resistance.
DISCUSSION
In this paper, we presented the analyses of an unexpected epigenetic phenomenon in Arabidopsis. We showed that some Arabidopsis T-DNA mutants were stably suppressed by T-DNA insertions in other non-homologous loci. We proposed that a T-DNA insertion in one locus could trigger undefined epigenetic modifications at a different T-DNA insertion site. The epigenetic modifications in the T-DNA mutants were heritable in the absence of the T-DNA suppressor. Because T-DNA mutants have been widely used in reverse genetics and in analyzing genetic interactions in Arabidopsis, this work suggests that we should be cautious about intronic T-DNA mutants.
Suppression Reported in this Work Violates Rules of Mendelian Genetics
When ag-TD was crossed to yuc1-1, all of the ag-TD plants in the F2 population were partially suppressed no matter whether yuc1-1 was present or not, although the presence of yuc1-1 rendered better suppression (Figure 1). The ag-TD* not only could be stably transmitted for many generations in the absence of yuc1-1 (Figure 2), but also had the ability to trigger new epigenetic suppressions in ag-TD. There are many similarities between the epigenetic suppression of T-DNA mutants and paramutation, a well-studied epigenetic phenomenon in Maize (Brink et al., 1968; Wolffe and Matzke, 1999; Erhard et al., 2009; Chandler, 2010). In paramutation, one allele (B’) causes heritable changes in another allele (B-I) of the same locus. Both the conversions of B-I to B′ and ag-TD to ag-TD* were triggered by a cross. Once B-I is converted to B′, the new B′ from B-I can be stably transmitted. We have shown that ag-TD* could also be stable for many generations (Figure 2). The newly converted B′ can convert B-I to B′ and we showed that ag-TD* can convert ag-TD to ag-TD*. The end results of paramutation appear to be unidirectional because it is always that B-I is converted to B′. In the cases of T-DNA suppression that we have analyzed, the suppression appeared to be one-directional as well. When ag-TD* was crossed with ag-TD, ag-TD was always converted to ag-TD*.
Epigenetic Suppression of T-DNA Mutants Is Triggered by Trans-Interaction between T-DNA Insertions
Several lines of evidence support the hypothesis that the conversion of ag-TD to ag-TD* is probably caused by trans-interaction between T-DNA insertions. First, ag-TD mutant displays strong kanamycin resistance whereas ag-TD* is kanamycin-sensitive (Figure 4). It has been demonstrated that trans-inactivation between homologous genes causes the loss of antibiotic resistance in T-DNA insertion mutants (Daxinger et al., 2008). The T-DNA insertions in both yuc1-1 and ag-TD are from the same plasmid; therefore, the NPT II transcripts from the T-DNA insertion at the yuc1-1 locus have the capacity to induce the silencing of the NPT II gene in the T-DNA fragment at the ag-TD locus. Second, the conversion of ag-TD to ag-TD* could also be achieved by transforming ag-TD +/- plants with a construct that expresses NPT II from the 35S promoter (Figure 4C).
We propose that transcripts such as the NPT II mRNA from the T-DNA insertions in yuc1-1 and ag-TD interact in trans to cause the silencing of NPT II. It has been well documented that trans T-DNA interactions can lead to the silencing of homologous genes (Daxinger et al., 2008). What is very unusual is that the silencing of the genes located in the T-DNA fragments such as NPT II is correlated with the restoration of the gene function inactivated by the T-DNA insertion.
It is often hypothesized that intronic T-DNA insertions disrupt gene function because transcripts cannot be properly spliced. However, some genes have very large introns that are spliced out properly from the primary transcripts, suggesting that other factors may also contribute to the inactivation of gene function by intronic T-DNA insertions. When a T-DNA fragment is inserted into an intron of a gene, the primary transcript from the gene contains the entire intron plus the T-DNA fragment if the transcription is not prematurely terminated within the T-DNA region. Therefore, it is conceivable that transcripts from the T-DNA fragment such as the NPT II transcript may be able to form a partial duplex with the primary transcript when the NPT II gene is transcribed from the opposite direction. Such a duplex may affect proper processing of the primary transcript or even lead to degradation of the transcript. When those T-DNA-generated reverse transcripts are silenced by transcripts from another homologous T-DNA insertion (a process very similar to co-suppression), the duplex between the NPT II transcript and the primary transcript would be resolved. Consequently, the intronic T-DNA mutants are partially suppressed and the NPT II gene is silenced. We recognize that the NPT II transcript from the T-DNA insertion is similar to long intronic non-coding RNA, which causes epigenetic changes and affects gene expression levels (Heo and Sung, 2010)
Our findings indicated that intronic T-DNA insertion mutants can be easily suppressed by trans-interaction with another T-DNA. Therefore, the use of intronic T-DNA insertion mutants sometimes may lead to incorrect interpretations. We would like to point out that trans-interaction with another T-DNA insertion may not be the only trigger that is capable of causing the suppression of the phenotypes of an intronic T-DNA mutant. Environmental factors may also be able to cause the suppression of T-DNA mutants. For example, the intronic T-DNA insertion mutant opr3 has long been recognized as a null allele and it produced no detectable Jasmonic acids (JAs) following wounding and looper infestation (Chehab et al., 2011). However, recently it was shown that the same opr3 mutant became activated upon fungal infection and accumulated substantial levels of JAs. It was suggested that splicing of the T-DNA-containing intron might be responsible for the reactivation of OPR3 (Chehab et al., 2011). In light of our findings, it is also possible that epigenetic modifications induced by fungal infection may play a role. Our study indicates that we should be careful about the use of intronic T-DNA mutants because some intronic T-DNA insertion mutants may undergo epigenetic changes that complicate interpretations of genetic interactions in Arabidopsis.
METHODS
The T-DNA insertion mutants cob-TD and yuc1-3 were obtained from the ABRC at Ohio. The ag-TD was from Dr Yanofsky. The yuc1-1 was previously described (Cheng et al., 2006; Won et al., 2011). For genotyping T-DNA mutants, we used PCR-based methods as previously described (Alonso et al., 2003). The gene-specific primers for genotyping ag-TD were 5′-ACGGCGTACCAATCGGAGCTAGGAGGA-3′ and 5′-TCTAGCTAGTTTCACCTTATTCACTCTC-3′. Primers for genotyping yuc1-1 and yuc1-3 were 5′-GGTTCATGTGTTGCCAAGGGA-3′ and 5′-CCTGAAGCCAAGTAGGCACGTT-3′. Gene-specific primers for cob-TD were 5′-TCCACTCCTCCTTCAAGCAAAGC-3′ and 5′-CCATTTCATTGTAATGTTGCCTTC-3′. The T-DNA specific primer for genotyping ag-TD, cob-TD, and yuc1-1 was JMLB1 (5′-GGCAATCAGCTGTTGCCCGTCTCACTGGTG-3′). T-DNA primer for yuc1-3 was Spm32 (5′-TACGAATAA GAGCGTCCATTTTAGAGTGA-3′). RNA in situ hybridization was performed as described previously (Cheng et al., 2006).
FUNDING
This work is partially supported by the N.I.H. R01GM068631 to Y.Z. and the N.S.F. Plant Genome DBI-0820729 to Y.Z.
ACKNOWLEDGMENTS
We would like to thank Drs Marty Yanofsky, Juan Jose Ripoll, and Bing Ren for valuable discussions. We thank Dr Marty Yanofsky, Dr Juan Jose Ripoll, Miss Allison Zhao, and members of the Zhao lab for critical reading of the manuscript. No conflict of interest declared.
REFERENCES
- Alonso J.M, et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana . Science. 301, 653–657 [DOI] [PubMed] [Google Scholar]
- Brink R.A, Styles E.D, Axtell J.D. (1968). Paramutation: directed genetic change: paramutation occurs in somatic cells and heritably alters the functional state of a locus. Science. 159, 161–170 [DOI] [PubMed] [Google Scholar]
- Chandler V.L. (2010). Paramutation’s properties and puzzles. Science. 330, 628–629 [DOI] [PubMed] [Google Scholar]
- Chehab E.W, Kim S, Savchenko T, Kliebenstein D, Dehesh K, Braam J. (2011). Intronic T-DNA insertion renders Arabidopsis opr3 a conditional jasmonic acid-producing mutant. Plant Physiol. 156, 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Meyerowitz E.M. (1999). HUA1 and HUA2 are two members of the floral homeotic AGAMOUS pathway. Mol. Cell. 3, 349–360 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. (2006). Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis . Genes Dev. 20, 1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. (2007). Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis . Plant Cell. 19, 2430–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y. (2008). NPY genes and AGC kinases define two key steps in auxin-mediated organogenesis in Arabidopsis . Proc. Natl Acad. Sci. U S A. 105, 21017–21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daxinger L, et al. (2008). Unexpected silencing effects from T-DNA tags in Arabidopsis . Trends Plant Sci. 13, 4–6 [DOI] [PubMed] [Google Scholar]
- Erhard K.F, Stonaker J.L, Parkinson S.E, Lim J.P, Hale C.J, Hollick J.B. (2009). RNA polymerase IV functions in paramutation in Zea mays . Science. 323, 1201–1205 [DOI] [PubMed] [Google Scholar]
- Guarente L. (1993). Synthetic enhancement in gene interaction: a genetic tool come of age. Trends Genet. 9, 362–366 [DOI] [PubMed] [Google Scholar]
- Heo J.B, Sung S. (2010). Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 331, 76–79 [DOI] [PubMed] [Google Scholar]
- Hodgkin J. (2005). Genetic suppression. WormBook. 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T, Sieburth L, Meyerowitz E. (1997). Targeted misexpression of AGAMOUS in whorl 2 of Arabidopsis flowers. Plant J. 11, 825–839 [DOI] [PubMed] [Google Scholar]
- Ko J.H, Kim J.H, Jayanty S.S, Howe G.A, Han K.H. (2006). Loss of function of COBRA, a determinant of oriented cell expansion, invokes cellular defence responses in Arabidopsis thaliana . J. Exp. Bot. 57, 2923–2936 [DOI] [PubMed] [Google Scholar]
- Samson F, et al. (2002). FLAGdb/FST: a database of mapped flanking insertion sites (FSTs) of Arabidopsis thaliana T-DNA transformants. Nucleic Acids Res. 30, 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelman G, et al. (2001). COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis . Genes Dev. 15, 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, et al. (2002). A high-throughput Arabidopsis reverse genetics system. Plant Cell. 14, 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A.P, Matzke M.A. (1999). Epigenetics: regulation through repression. Science. 286, 481–486 [DOI] [PubMed] [Google Scholar]
- Won C, et al. (2011). Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis . Proc. Natl Acad. Sci. U S A. 108, 18518–18523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky M.F, Ma H, Bowman J.L, Drews G.N, Feldmann K.A, Meyerowitz E.M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 346, 35–39 [DOI] [PubMed] [Google Scholar]
- Zhao Y, et al. (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 291, 306–309 [DOI] [PubMed] [Google Scholar]