Abstract
Background
Accurate and reliable assessment of kidney quality before transplantation is needed to predict recipient outcomes and to optimize management and allocation of the allograft. The aim of this study was to systematically review the published literature on biomarkers in two mediums (the perfusate from deceased-donor kidneys receiving machine perfusion and deceased-donor urine) that were evaluated for their possible association with outcomes after kidney transplantation.
Methods
We searched the Ovid Medline and Scopus databases using broad keywords related to deceased-donor biomarkers in kidney transplantation (limited to humans and the English language). Studies were included if they involved deceased-donor kidneys, measured perfusate or urine biomarkers and studied a possible relationship between biomarker concentrations and kidney allograft outcomes. Each included article was assessed for methodological quality.
Results
Of 1430 abstracts screened, 29 studies met the inclusion criteria. Of these, 23 were studies of perfusate (16 biomarkers examined) and 6 were studies of urine (18 biomarkers examined). Only 3 studies (two perfusate) met the criteria of ‘good’ quality and only 12 were published since 2000. Perfusate lactate dehydrogenase, glutathione-S-transferase (GST) and aspartate transaminase were all found to be significantly associated with delayed graft function in a majority of their respective studies (6/9, 4/6 and 2/2 studies, respectively). Urine neutrophil gelatinase-associated lipocalin, GST, Trolox-equivalent antioxidant capacity and kidney injury molecule-1 were found to be significantly associated with allograft outcomes in single studies that examined diverse end points.
Conclusion
Higher quality studies are needed to investigate modern kidney injury biomarkers, to validate novel biomarkers in larger donor populations and to determine the incremental predictive value of biomarkers over traditional clinical variables.
Keywords: biomarkers, deceased donors, delayed graft function, kidney transplantation
Introduction
Kidney transplantation is the treatment of choice for most patients with end-stage renal disease. To help meet the rising demand for deceased-donor organs, the transplant community has increasingly utilized ‘expanded criteria donors’ (ECD) and ‘donors after cardiac death’ (DCD) [1]. Compared to kidneys from standard criteria donors, ECD and DCD kidneys have higher rates of delayed graft function (DGF), while ECD kidney recipients have a 69% higher relative risk for graft failure [2]. In addition to prolonged hospital stays and increased hospital costs, patients with DGF have also been shown to have an increased risk of acute rejection and an increased risk of graft loss at 3 years of follow-up [3, 4]. Furthermore, higher discard rates for ECD kidneys have led some to question whether this precious resource is sub-optimally utilized [5, 6]. Current methods of assessing kidney quality such as patient age, terminal serum creatinine or kidney biopsy have limited accuracy in forecasting long-term allograft outcomes [7, 8].
More efficient and accurate tools to determine deceased-donor kidney quality could help optimize allograft management, decrease the risk of discarding viable organs and avoid transplanting kidneys of inferior quality. Biomarker development to diagnose acute kidney injury (AKI) early and assist with prognosis is an active area of investigation in nephrology [9]. Given the diverse modes of injury in deceased donation (including hemodynamic injury and brain death) and the need for accurate assessment of allograft quality prior to transplantation, the study of biomarkers in this context is an important opportunity [10]. Accurate assessment of structural kidney injury by biomarkers at the time of organ procurement could drive multiple changes in management, including the targeted use of machine perfusion or other allograft treatments. Procured kidneys with evidence of injury may require limited cold ischemia time, machine perfusion, allocation to a recipient with a lower projected lifespan, placement of both lower quality kidneys into a single recipient or protection of the recipient from therapies that cause further injury.
Novel kidney injury biomarkers have yet to be systematically evaluated for potential added utility in prediction models of graft outcomes. Currently, several donor models for kidney graft assessment have been developed to predict transplant outcomes using both donor characteristics and pre-implantation kidney biopsies. Common donor characteristics used for assessment include donor age, hypertension, serum creatinine level, cerebrovascular cause of death and HLA mismatch between donor and recipient [11–15]. A number of investigators have also developed donor quality scores based on renal histopathology [16, 17]. However, urine and perfusate biomarkers have conceptual advantages over renal histology in that they can be collected non-invasively and measured more frequently and objectively (no inter-observer variability). A new examination of perfusate biomarkers is also timely given renewed clinical interest in the use of machine perfusion [18]. Despite its cost and the time investment required, machine perfusion has been increasingly employed to lessen ischemic damage and improve graft outcomes when marginal kidneys (DCD and ECD) have been procured [19, 20]. Non-invasive kidney injury biomarker measurement from perfusate solution could allow assessment at multiple time points during preservation and would likely be much quicker than pre-implantation biopsy preparation and histological examination.
Beginning in the 1970s, a number of human studies have described biomarkers from deceased donors and their allografts for predicting kidney transplant outcomes. These studies measured a wide variety of biomarkers utilizing heterogeneous methods and disparate outcomes. Consequently, it remains unclear whether biomarkers should be used for kidney quality assessment to predict transplant outcomes. We therefore conducted a systematic review to determine the association between biomarkers measured from machine perfusate and donor urine and subsequent outcomes in deceased-donor kidney transplantation.
Materials and methods
Study identification
In consultation with a research librarian, potentially relevant studies were identified through a structured literature search of Ovid Medline (1948 to 05/2011) and Scopus databases using the Medical Subject Headings. We used broad keywords related to deceased-donor biomarkers in kidney transplantation limited to English-language articles on human subjects (Supplementary Digital Content 1). To identify additional relevant articles, we conducted a manual search of references from all eligible studies and relevant review articles and performed a citation analysis of eligible articles using the Web of Knowledge Citation Index. Studies that evaluated any deceased-donor perfusate or urine biomarker with respect to allograft outcomes were further reviewed. Studies were eligible for inclusion if they (i) examined outcomes among recipients of deceased-donor kidneys, (ii) measured perfusate or urine biomarkers, (iii) studied a possible relationship between biomarker concentrations and allograft/recipient outcomes and (iv) reported on a study sample and biomarker outcome that was not redundant with other included studies (when this issue arose, the study with the larger sample size was chosen).
Quality assessment
One reviewer independently searched for potentially relevant trials and assessed methodological quality. Other authors were consulted to decide whether studies met criteria for inclusion. Methodological quality was evaluated with a modified checklist of the Standards for Reporting of Diagnostic Accuracy (STARD) criteria [21]. Since its publication in 2003, the STARD criteria have become a widely implemented tool for evaluating the quality of studies [22]. Of the 25 criteria for assessing the diagnostic accuracy of studies, we limited our quality assessment to the 10 most relevant parameters for this review (Table 1). Studies with a score ≥9 were designated as ‘good’ quality, 7–8 as ‘fair’ quality and ≤6 as ‘poor’ quality.
Table 1.
Study quality scoring systema
| Validity criterion | Explanation | Scoring | Perfusate comments | Urine comments |
|---|---|---|---|---|
| Participant recruitment | Was recruitment based on presenting symptoms, results from previous tests or fact that participants received index testsb? | Presenting symptoms = 1 | All based on presenting symptoms | All based on presenting symptoms |
| Previous tests or index tests = 0 | ||||
| Participant sampling | Was its study population a convenience sample or a consecutive series? | Consecutive series =1 | Based on convenience sample: 12 [23–34] | Based on convenience sample: 3 [35–37] |
| Convenience sample or not stated = 0 | ||||
| Data collection | Was data collection planned before the index test and reference standard were performed prospectively or retrospectively? | Prospective =1 | Planned and performed retrospectively: 6 [24, 38–42] | All prospective |
| Retrospective or not stated = 0 | ||||
| Reference standard | Was the definition for the reference standard statedc? | Stated = 1 | Not stated: 8 [25, 33, 34, 39–43] | Not stated: 1 [36] |
| Not stated = 0 | ||||
| Materials and methods | Were technical specifications of material and methods stated including how and when measurements were taken? | Stated = 1 | Not stated: 9 [24, 25, 32, 33, 39–42, 44] | All stated |
| Not stated = 0 | ||||
| Participant characteristics | Were the clinical and demographic characteristics of the study population stated? | Stated = 1 | Not stated: 17 [23–26, 28–34, 39–42, 44, 45] | Not stated: 3 [35–37] |
| Not stated = 0 | ||||
| Blinding | Were readers of the index test and reference standard blinded? | Blinded = 1 | Not stated: 20 [23–25, 27, 29, 30, 32–34, 38–48] | Not stated: 5 [35–37, 49, 50] |
| Not blinded or not stated = 0 | ||||
| Completion | Was the number of participants that did not undergo index tests stated (no. of tests versus sample size stated)? | Stated = 1 | Completion was always stated | Not stated: 1 [36] |
| Not stated = 0 | ||||
| Diagnostic accuracy and statistical uncertainty | Were estimates of diagnostic accuracy and measures of statistical uncertainty for biomarker results (e.g. 95% confidence intervals) stated? | Stated = 1 | Not stated: 20 [23–34, 39–45, 48] | Not stated: 5 [35–37, 49, 50] |
| Not stated = 0 | ||||
| Clinical applicability | Was the clinical applicability of biomarker findings stated? | Stated = 1 | Not stated: 7 [24, 25, 33, 34, 40, 41, 44] | Stated in all studies |
| Not stated = 0 |
aStudy quality scoring system is modified checklist of the STARD criteria [21].
bIndex test is the biomarker measurement.
cReference standard is each outcome reported in a study.
Data extraction
We extracted data using a prepared standard data extraction form. Variables included publication information, study design, study center, biomarkers measured, other donor characteristics studied, outcomes reported, study population (sample size), donor type, kidneys studied, samples obtained, storage processes, results of biomarkers with respect to specific outcomes, sensitivity/specificity values, area under the receiver-operating characteristic curve (AUCs), correlation coefficients, relative risk values and odds ratios.
Results
Search process
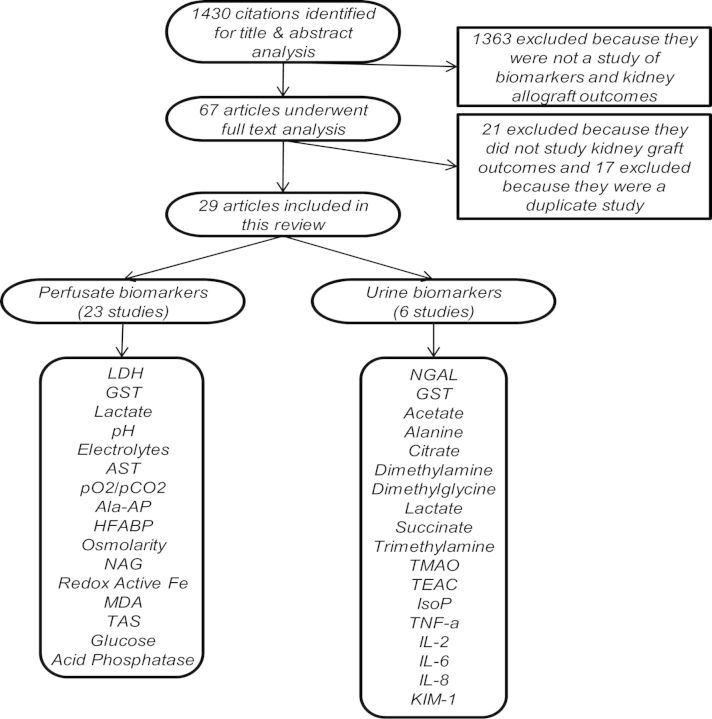
The combined search yielded a total of 1430 citations, of which 1363 were excluded after title and abstract review (Figure 1). After full-text analysis of the remaining 67 articles, 29 met the inclusion criteria. The 38 remaining articles were excluded primarily for duplicate patient populations and/or overlapping results.
Fig. 1.
Flow chart of studies identified, reviewed and selected for inclusion in this systematic review. Ala-AP, alanine aminopeptidase; Fe, iron; IsoP, 8-iso-prostaglandin F2α; TMAO, trimethylamine-N-oxide; TNF-α, tumor necrosis factor-α.
Study characteristics
Of the 29 included articles, 23 studied 16 different perfusate biomarkers in a total of 2578 deceased-donor kidneys (Table 2). Of these 23 studies of perfusate, 7 were published within the last decade. Only 2 perfusate studies were considered ‘good’ quality and the majority, 13 (56%), were ‘poor’ quality.
Table 2.
Study characteristics
| Primary author | Year | Single/multicenter | Location | Kidney Txp'sa | A primary aim to study biomarkers? | Sample medium | Biomarkersb | Quality scorec |
|---|---|---|---|---|---|---|---|---|
| Hollmen et al. [51] | 2011 | Single | Europe | 95d | Yes | Urine | NGAL | 10 |
| Moers et al. [47] | 2010 | Multi | Europe | 306 | Yes | Perfusate | GST, LDH, AST, Ala-AP, NAG and HFABP | 9 |
| Robert et al. [50] | 2010 | Multi | Europe | 48d | Yes | Urine | Lactate, acetate, alanine, citrate, dimethylamine, dimethylglycine, succinate, trimethylamine and TMAO | 8 |
| Nijboer et al. [49] | 2009 | Single | Europe | 20d | Yes | Urine | KIM-1 | 8 |
| de Vries et al. [46] | 2006 | Single | Europe | 231 | Yes | Perfusate | Redox-active Fe/GST/LDH | 9 |
| Asher et al. [41] | 2005 | Single | Europe | 88 | No | Perfusate | GST | 3 |
| Gok et al. [26] | 2003 | Single | Europe | 74 | Yes | Perfusate | GST, Ala-AP and HFABP | 7 |
| Kosieradzki et al. [27] | 2003 | Single | Europe | 50 | Yes | Perfusate | MDA and TAS | 7 |
| Kosieradzki et al. [38] | 2002 | Single | Europe | 234 | Yes | Perfusate | GST, lactate and LDH | 8 |
| Polyak et al. [48] | 2000 | Single | US | 650 | Yes | Perfusate | Na+, Cl−, K+, Ca++, pH | 8 |
| Shoskes et al. [36] | 2000 | Single | US | 29d | Yes | Urine | TEAC and IsoP | 4 |
| Sarvary et al. [35] | 2000 | Single | Europe | 61d | Yes | Urine | GST | 6 |
| Zivna et al. [37] | 1999 | Single | Europe | 22d | Yes | Urine | IL-2, IL-6, IL-8 and TNF-α | 6 |
| Danielewicz et al. [43] | 1997 | Single | Europe | 86 | Yes | Perfusate | Lactate, LDH, Na+, K+, pH, pO2/pCO2, osmolarity | 7 |
| Kootstra et al. [42] | 1997 | Single | Europe | 71 | Yes | Perfusate | GST and LDH | 4 |
| Daemen et al. [29] | 1997 | Single | Europe | 46 | Yes | Perfusate | GST and LDH | 6 |
| Cho et al. [30] | 1981 | Multi | US | 24 | Yes | Perfusate | GST (ligandin) | 6 |
| Newman and Shenton [28] | 1981 | Single | Europe | 23 | Yes | Perfusate | LDH, lactate, pH | 7 |
| Sy et al. [24] | 1980 | Single | US | 50 | Yes | Perfusate | pO2/pCO2, pH, osmolarity, occasional electrolytes | 3 |
| Feinfeld et al. [31] | 1978 | Single | US | 13 | Yes | Perfusate | GST (ligandin) | 7 |
| Horpacsy et al. [32] | 1978 | Single | Europe | 81 | Yes | Perfusate | LDH, lactate, pH | 5 |
| Burleson et al. [33] | 1978 | Single | US | 63 | No | Perfusate | Lactate | 3 |
| Anderson et al. [44] | 1977 | Single | US | 100 | No | Perfusate | LDH and AST | 5 |
| Modgill et al. [45] | 1977 | Single | Europe | 32 | Yes | Perfusate | LDH, lactate, pH | 7 |
| Stephenson et al. [39] | 1975 | Single | Europe | 52 | No | Perfusate | LDH, AST, lactate, glucose, K+ | 4 |
| Johnson et al. [34] | 1974 | Multi | US/Europe | 114 | Yes | Perfusate | Lactate | 4 |
| Johnson et al. [23] | 1973 | Single | Europe | 26 | Yes | Perfusate | LDH, lactate, pH, pO2/pCO2 | 6 |
| Kiser et al. [40] | 1972 | Multi | US | 130 | No | Perfusate | LDH | 3 |
| Sterling et al. [25] | 1972 | Multi | US | 34 | No | Perfusate | LDH, acid phosphatase, electrolytes | 3 |
aTxp's, transplants.
bBiomarker abbreviations (Ala-AP, alanine aminopeptidase; Fe, iron; IsoP, 8-iso-prostaglandin F2α; TMAO, trimethylamine-N-oxide; TNF-α, tumor necrosis factor-α).
cQuality score is a modified version of the STARD criteria and is fully detailed in Table 1, Score ≥9 is ‘good’ quality, 7 and 8 are ‘fair’ quality and ≤6 is ‘poor’ quality.
dNumber of deceased donors (urine studies).
Additionally, six studies examined 18 different urine biomarkers in 275 deceased donors, all published after 1998. Quality assessment revealed that one urine biomarker study was ‘good’ quality, two were ‘fair’ and the remaining three were ‘poor’ quality.
Three key outcomes analyzed in perfusate biomarker studies were DGF, primary non-function (PNF) and graft function (GF). DGF and GF were also the two main outcomes examined in the urine biomarker studies with one study evaluating allograft rejection. Typically, DGF was characterized by the temporary need for post-transplant dialysis and PNF by the continued/permanent need for post-transplant dialysis. GF was assessed by serum creatinine concentration or creatinine clearance.
Perfusate biomarker performance
Lactate dehydrogenase
Lactate dehydrogenase (LDH), a non-specific marker of cellular injury, was evaluated in the largest number of studies and was shown to be significantly associated with DGF, PNF and GF (Table 3). Of the 12 studies with perfusate LDH, 7 found it to be significantly associated with allograft outcomes. The only two perfusate studies of ‘good’ quality found pre-transplant perfusate LDH to be significantly higher in kidneys that developed DGF after transplant compared with those that did not [46, 47]. In the largest (306 transplanted kidneys) and most recent (2010) study, Moers et al. [47] found the AUC for predicting DGF with perfusate LDH to be 0.60.
Table 3.
Studies of specific biomarkers from deceased-donor kidney perfusate and their associations with allograft outcomes
| Outcomesa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DGF |
PNF |
GF |
||||||||
| Perfusate biomarkerb | Studies | Kidney Txp'sc | Significant association | Studies | Significant association | Studies | Significant association | Studies | Significant association | |
| Traditional markers | Flow | 12 | 1680 | 4 | 8 | 4 | 2 | 0 | 3 | 0 |
| Resistance | 6 | 1318 | 3 | 5 | 3 | 2 | 0 | 1 | 0 | |
| Perfusion pressure | 5 | 365 | 2 | 3 | 2 | 1 | 0 | 0 | 0 | |
| Chemical markers | LDH | 12 | 1320 | 7 | 9 | 6 | 3 | 1 | 3 | 1 |
| pH | 7 | 948 | 1 | 5 | 1 | 1 | 0 | 2 | 0 | |
| GST | 7 | 777 | 5 | 6 | 4 | 3 | 2 | 1 | 0 | |
| Lactate | 7 | 599 | 2 | 5 | 2 | 0 | 0 | 3 | 0 | |
| Electrolytes | 5 | 872 | 1 | 3 | 1 | 1 | 0 | 1 | 0 | |
| AST | 3 | 458 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | |
| pO2/pCO2 | 3 | 162 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | |
| Ala-AP | 2 | 380 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | |
| HFABP | 2 | 380 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | |
| Osmolarity | 2 | 136 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | |
| NAG | 1 | 306 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | |
| Redox-active Fe | 1 | 231 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| Glucose | 1 | 52 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| MDA | 1 | 50 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| TAS | 1 | 50 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Acid phosphatase | 1 | 34 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
aOutcomes [DGF (as defined by each study, typically temporary post-transplant dialysis); GF (as defined by each study, typically recipient serum creatinine concentration or creatinine clearance); PNF (as defined by each study, typically continued/permanent post-transplant dialysis)].
bBiomarker abbreviations (Ala-AP, alanine aminopeptidase; Fe, iron).
cTxp's, transplants.
pH
Lower perfusate pH may suggest stressed or even abnormal cellular function in the allograft. Of the seven studies that investigated perfusate pH, only one, published in 1973 and of ‘poor’ quality, reported a significant association with any outcome (DGF) [23]. None were ‘good’ quality, four were ‘fair’ and three were ‘poor’ quality.
Glutathione-S-transferase
Glutathione-S-transferase (GST) is a proposed biomarker of renal tubule injury. Of the seven studies with perfusate GST, five reported significant associations with allograft outcomes. Similar to the LDH results, both perfusate studies of ‘good’ quality found GST to be significantly elevated in DGF kidneys [46, 47], with an AUC of 0.67 for predicting DGF as determined by Moers et al. [47].
Lactate
Lactate is a non-specific marker of ischemic cellular injury. Significant associations between perfusate lactate levels and DGF were found in two of five studies that analyzed their relationship. The most recent study of perfusate lactate reported a sensitivity of 60.6% and specificity of 68.6% for detecting DGF [38]. This study was of ‘fair’ quality, while the other study reporting a significant association was of ‘poor’ quality.
Electrolytes
A total of five published studies have examined four different electrolytes (sodium, potassium, chloride and calcium) in perfusate solution. Only one of the five studies reported a significant association between electrolyte concentrations and outcome (DGF) [48]. In this ‘fair’ quality study, perfusate calcium concentration was significantly higher (by 40.7%) for DGF. No other study reported calcium concentrations in relation to allograft outcomes, and two articles did not specify which electrolytes were assessed [24, 25].
Aspartate transaminase
Aspartate transaminase (AST), a biomarker that may be expressed from injured renal parenchymal cells, is also identified in the literature as serum glutamic oxaloacetic transaminase or aspartate aminotransferase. Two of the three studies that reported perfusate AST levels noted significant associations with DGF [44, 47]. Moers et al. [47] reported an AUC of 0.61.
Gas pressures (pO2 and pCO2)
Perfusate gas pressures are determined by gas exchange between cells and the external environment. Measurement of perfusate pO2 and pCO2 was undertaken in three studies, with only one (published in 1973 and of ‘poor’ quality) that described a significant association with DGF [23].
Alanine aminopeptidase
Alanine aminopeptidase, a potential marker of renal tubular injury, was not associated with kidney allograft outcomes in either of the two studies that measured this biomarker. Moers et al. [47] noted a non-significant AUC of 0.57 for predicting DGF.
Heart-type fatty acid-binding protein
Heart-type fatty acid-binding protein (HFABP) is a marker of ischemic cellular injury and was assessed in two perfusate studies. Moers et al. [47] noted a significant AUC of 0.64 for predicting DGF. The second study was of ‘fair’ quality. The authors did not report DGF as an outcome and noted no association between HFABP and GF [26].
Osmolarity
While perfusate osmolarity has been considered as a possible injury biomarker, neither of the two studies that described this measurement noted a significant relationship with subsequent GF.
Single study biomarkers
Six additional perfusate biomarkers were assessed in single investigations without replication in other studies. These included N-acetyl-β-D-glucosaminidase (NAG, ischemic tubular injury marker), redox-active iron (ischemia–reperfusion injury marker), malondialdehyde (MDA, oxidative stress marker) and total antioxidant status (TAS, oxidative stress marker). All were found to be significantly associated with DGF in their respective studies [27, 46, 47]. Redox-active iron was also noted to be significantly associated with PNF [46]. While these perfusate biomarker evaluations have not been replicated in additional studies, those that examined NAG and redox-active iron were the two perfusate studies of ‘good’ quality, [46, 47] and the study with MDA and TAS was of ‘fair’ quality [27]. The two other perfusate biomarkers that have not been reported in more than one study are glucose and acid phosphatase (non-specific markers of cellular dysfunction). The studies that measured these markers were published in the 1970s, were of ‘poor’ quality and showed no significant associations with GF [25, 39].
Donor urine biomarker performance
Single study biomarkers
All 18 urine biomarkers examined were assessed by single studies without repeat examination in additional studies (Table 4). Neutrophil gelatinase-associated lipocalin (NGAL), a marker of distal tubular injury, was evaluated in the largest, most recent and highest quality (good) of the urine biomarker studies by Hollmen et al. They reported prolonged DGF (lasting ≥14 days) occurred more frequently (23 versus 11%, P = 0.03) and eventual 1-year allograft survival was lower (90 versus 97%, P = 0.05) in recipients of transplants from donors with urine NGAL concentrations above versus below the mean (18 ng/mL) [51].
Table 4.
Studies of specific deceased-donor urine biomarkers and their associations with allograft outcomes
| Outcomesa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| DGF |
GF |
Rejection |
|||||||
| Urine biomarkerb | Studies | Deceased donors | Significant association | Studies | Significant association | Studies | Significant association | Studies | Significant association |
| NGAL | 1 | 95 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| GST | 1 | 61 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Acetate | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Alanine | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Citrate | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Dimethylamine | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Dimethylglycine | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lactate | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Succinate | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Trimethylamine | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| TMAO | 1 | 48 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| TEAC | 1 | 29 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| IsoP | 1 | 29 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| TNF-α | 1 | 22 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| IL-2 | 1 | 22 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| IL-6 | 1 | 22 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| IL-8 | 1 | 22 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| KIM-1 | 1 | 20 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
aOutcomes, [DGF (as defined by each study, typically recipient dialysis within the immediate post-operative period); GF (for KIM-1, GF is outcome measured by recipient serum creatinine. For GST, GF is measured by a standardized recipient GST over creatinine rubric.); rejection is outcome where recipient had a kidney graft rejection episode (including corticoid-treated rejection episodes and rejection episodes resistant to high-dose steroids)].
bBiomarker abbreviations (IsoP, 8-iso-prostaglandin F2α; TMAO, trimethylamine-N-oxide; TNF-α, tumor necrosis factor-α).
GST and lactate are the two perfusate biomarkers that have also been measured in donor urine in the context of graft outcomes. In a study of ‘poor’ quality, Sarvary et al. [35] found urine GST to be significantly associated with the recovery of allograft function as defined by a comparison to the tubular enzymuria of GST in healthy controls.
In a separate study of ‘fair’ quality, lactate, along with eight other biomarkers (acetate, alanine, citrate, dimethylamine, dimethylglycine, succinate, trimethylamine and trimethylamine-N-oxide), was measured in donor urine using proton nuclear magnetic resonance spectroscopy (H1-NMR) [50]. No associations were reported with DGF.
In a ‘poor’ quality study that evaluated both Trolox equivalent antioxidant capacity (TEAC, a marker of oxidative function) and 8-iso-prostaglandin F2α (IsoP, a marker of oxidant stress), only TEAC was found to be significantly lower in donor urine for kidneys that were ultimately discarded or developed DGF compared with donor urine for kidneys that were transplanted and had good early function [36].
Tumor necrosis factor-α, interleukin (IL)-2, IL-6 and IL-8, all markers of inflammation, were reported to lack significant association with allograft rejection by the same ‘poor’ quality study [37].
Donor urine concentrations of kidney injury molecule-1 (KIM-1, a marker of tubular injury) were significantly associated with, and an independent predictor of, GF at 14 days and 1 year [49].
Discussion
While descriptive reviews have briefly addressed the topic of biomarkers in the context of deceased-donor kidney perfusion, this is the first systematic review of the published literature on the use of machine perfusate and deceased-donor urine biomarkers for the evaluation of kidney allograft outcomes [19, 52]. We have identified a broad range of donor biomarker studies, reviewed the reported potential of those biomarkers for evaluating deceased-donor kidney quality and revealed the shortcomings of the current literature on this subject.
For a biomarker to be adopted in clinical practice, it must typically undergo several phases of development (from exploratory studies to large prospective validation studies in various clinical settings) and be shown to be reliable/reproducible in subsequent investigations [53]. In addition, a biomarker may be associated with certain diagnoses or outcomes, but unless it demonstrates significant improvement (e.g. better accuracy, lower cost and/or easier to use) over traditional assessment tools, it may not be clinically useful.
Several perfusate biomarkers have been shown to be significantly associated with allograft outcomes. Of the biomarkers examined in relation to DGF in multiple studies, LDH, GST and AST were the three that demonstrated significant associations in the majority of their respective studies (6/9, 4/6 and 2/2 studies, respectively). GST was the only perfusate biomarker found to be significantly associated with PNF in a majority of its studies (2/3). No marker was found to be significantly associated with GF in a majority of its studies. Although LDH is a general marker of tissue breakdown, measuring LDH in kidney perfusate may allow for its ability to assess tissue damage in the kidney specifically. Both LDH and GST (a kidney tubule-specific marker) showed significant associations with DGF in the two recent perfusate studies of good quality. Thus, these two markers display initial promise as predictors of allograft outcomes, but they must be validated in additional studies before implementation. In addition to confirming findings related to LDH and GST, future studies of kidney perfusate should also investigate more modern and specific kidney injury biomarker candidates (NGAL, KIM-1, IL-18, etc.) that have shown promise in other clinical settings [9]. Contemporary kidney biomarkers such as NGAL and KIM-1 have also been localized to specific regions of the kidney, potentially enabling these biomarkers to be linked to different forms of injury. We conclude that perfusate biomarker measurements could provide transplant clinicians with an additional, novel, non-invasive tool to monitor kidney quality at multiple time points pre-transplantation (after procurement and before transplantation). Perfusate information may also specifically aid in the pump management of kidneys with respect to adjusting flow and resistance.
With respect to donor urine biomarkers, NGAL, GST, TEAC and KIM-1 were the only ones shown to have significant associations with allograft outcomes. Unfortunately, every urine biomarker identified in this review was examined by only one study, limiting the strength of any conclusions by this review. Furthermore, the outcomes assessed in these studies were all different from one another, making it difficult to draw comparisons about the strengths or weaknesses of specific urine biomarkers in this population. Though limited to a small number of studies, the recent literature on deceased-donor urine biomarkers reflects the growing interest in urine biomarkers of kidney injury in general. In contrast to perfusate studies, modern urine biomarkers like NGAL and KIM-1 have been biomarker candidates evaluated in the initial studies of deceased-donor urine. Given their promise in other kidney injury settings, future studies would do well to focus on these urine markers as well as similar novel modern urine markers being developed in the rapidly progressing field of kidney injury. Compared to perfusate markers, urine biomarkers can provide an assessment of kidney quality prior to procurement and may be able to help with initial allocation decisions. Urine biomarkers may therefore prove to be more valuable than perfusate biomarkers in a transplant outcome prediction model because urine biomarkers may be evaluated at the earliest time point and could therefore more easily affect organ allocation. Notwithstanding the allocation process, perfusate biomarkers may supplement prediction models by allowing clinicians the ability to monitor kidney injury during the preservation process.
One major difference between kidney injury in non-donors and kidney injury in deceased donors is the process of brain death which the latter undergo. Brain death has been shown to lead to a cascade of negative neurohormonal changes through diverse organ systems, including brain death-induced renal injury [54]. Deceased-donor biomarkers may be well suited to capture these changes. A separate issue to consider is that some candidate injury biomarkers may be elevated in the setting of chronic kidney injury rather than AKI (or even in both settings). With reference to evaluating donor kidney quality, a biomarker that reflected either acute injury or chronic disease would serve a valuable purpose in a predictive model with a primary goal of predicting recipient outcomes. However, if the biomarker is also to be used to direct therapies to the allograft, a biomarker may need to reflect a precise type of injury. As an example, it is plausible that a biomarker that was specific for tubular injury might identify allografts that benefit from pumping, whereas pumping is unlikely to benefit kidneys with biomarker elevations due to chronic disease.
Although the search strategy for this review did not specifically focus on parameters of pump performance—such as resistance—these parameters were evaluated in a number of included studies (Table 3). It is important to note that some studies have reported that perfusion flow and resistance predict allograft outcomes, particularly in kidneys thought to be at high risk for complications because of donor characteristics [55, 56]. Our review found that flow, resistance and pressure were all significantly associated with DGF in at least half of the respective studies that reported these findings (4/8, 3/5 and 2/3 studies, respectively). Compared to these pumping parameters, the successful perfusate biomarkers listed above display similar promise for assessing kidney quality during organ transport.
The adjusted STARD quality score index classified only 2 of the 23 perfusate studies as ‘good’ quality, suggesting potential areas for improvement. Statistical analysis was one such area. Twenty perfusate studies did not report estimates of diagnostic accuracy or measures of statistical uncertainty (Table 1). Most studies employed t-tests or chi-squared tests to determine whether biomarker levels were significantly different between groups. This statistical approach is not sufficient to assess a biomarker's performance in classifying an injury or predicting risks in patients. Instead, the strength of association between a biomarker and outcome is better described by odds ratio or relative risk analysis. For validated clinical use, a biomarker should accurately differentiate between patients who experience the given outcome and those who do not, which is better represented by sensitivity, specificity and related diagnostic criteria. The receiver operating characteristic curve is a plot of sensitivity versus one minus specificity and the AUC can provide a meaningful summary of a test's discriminatory ability. Additionally, future statistical analysis should include multivariable adjustment in order to determine whether a biomarker is a true independent predictor of GF.
Aside from the statistical deficiencies, studies may have been biased in three ways. In 20 of the 23 perfusate studies, ‘review bias’ was possible as the examiners who evaluated the experimental tests and graft outcomes were not stated to be blinded. Selection bias was also possible in 12 perfusate studies because donor enrollment methods were not clearly stated (i.e. random, consecutive or convenience sampling), which may indicate that the enrolled donors were not representative of the entire pool of donors. Publication bias was an additional concern as studies with negative findings may have been less likely to have been published.
The studies exhibited methodological heterogeneity as well. Nine studies did not report the technical specifications of their materials and methods. Definitions for allograft outcomes were varied or unclear. While DGF was most often defined as temporary post-transplant dialysis, there was substantial variation in the length of time considered as the post-transplant period and the number of dialysis sessions required to make this diagnosis—a significant problem also highlighted elsewhere [57].
Moving forward, more studies are needed that examine biomarkers with respect to short- and long-term outcomes, including allograft function, allograft failure and associated costs. Only seven studies of longer term outcomes were identified by this systematic review—primarily focusing on 1-year recipient serum creatinine levels [24, 26–28, 40, 47, 48]. Particularly among lower quality kidneys (DCD and ECD), biomarkers could add significant value in assessment of kidney injury and prediction of short- to medium-term recipient outcomes. Predicting long-term allograft outcomes (i.e. survival beyond 2 years) may be more difficult due to the impact of many later hard-to-predict events like non-compliance, patient death and rejection. Additional studies that investigate modern kidney injury biomarkers (e.g. KIM-1, NGAL, IL-18 and cystatin C) are also needed given the growing body of evidence for these markers. Ideally, more studies should be multicenter and evaluate both urine and perfusate biomarkers for more meaningful and generalizable results. A single biomarker has yet to be proven accurate and reliable for predicting allograft outcomes; however, future high-quality studies that incorporate the research aspects identified here have the opportunity to advance the use of non-invasive biomarkers in kidney transplantation. Most likely, individual biomarkers will not become the single tool to predict kidney graft outcomes. Instead, individual biomarkers or panels of markers that supplement current clinical measures of deceased-donor kidney quality may be validated, which could lead to clinical trials aimed at improving organ allocation and allograft outcomes.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Acknowledgements
We thank Mark Gentry, library liaison at the Yale Medical Library, for his assistance in developing the search strategy for this review.
Transparency declarations. None declared.
References
- 1.Tuttle-Newhall JE, Krishnan SM, Levy MF, et al. Organ donation and utilization in the United States: 1998–2007. Am J Transplant. 2009;9:879–893. doi: 10.1111/j.1600-6143.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 2.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–125. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 3.Yarlagadda SG, Coca SG, Formica RN, Jr, et al. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 4.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. J Urol. 1996;155:1831–1840. doi: 10.1016/s0022-5347(01)66023-3. [DOI] [PubMed] [Google Scholar]
- 5.Sung RS, Christensen LL, Leichtman AB, et al. Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant. 2008;8:783–792. doi: 10.1111/j.1600-6143.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD—fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. 2009;4:1827–1831. doi: 10.2215/CJN.02270409. [DOI] [PubMed] [Google Scholar]
- 7.Jochmans I, Pirenne J. Graft quality assessment in kidney transplantation: not an exact science yet! Curr Opin Organ Transplant. 2011;16:174–179. doi: 10.1097/MOT.0b013e3283446b31. [DOI] [PubMed] [Google Scholar]
- 8.Louvar DW, Li N, Snyder J, et al. “Nature versus nurture” study of deceased-donor pairs in kidney transplantation. J Am Soc Nephrol. 2009;20:1351–1358. doi: 10.1681/ASN.2008070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, Yalavarthy R, Concato J, et al. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 10.Sarwal MM, Benjamin J, Butte AJ, et al. Transplantomics and biomarkers in organ transplantation: a report from the first international conference. Transplantation. 2011;91:379–382. doi: 10.1097/TP.0b013e3182105fb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anglicheau D, Loupy A, Lefaucheur C, et al. A simple clinico-histopathological composite scoring system is highly predictive of graft outcomes in marginal donors. Am J Transplant. 2008;8:2325–2334. doi: 10.1111/j.1600-6143.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- 12.Nyberg SL, Matas AJ, Kremers WK, et al. Improved scoring system to assess adult donors for cadaver renal transplantation. Am J Transplant. 2003;3:715–721. doi: 10.1034/j.1600-6143.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 13.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 14.Schold JD, Kaplan B, Baliga RS, et al. The broad spectrum of quality in deceased donor kidneys. Am J Transplant. 2005;5(4 Pt 1):757–765. doi: 10.1111/j.1600-6143.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasiske BL, Israni AK, Snyder JJ, et al. A simple tool to predict outcomes after kidney transplant. Am J Kidney Dis. 2010;56:947–960. doi: 10.1053/j.ajkd.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, et al. The Maryland aggregate pathology index: a deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant. 2008;8:2316–2324. doi: 10.1111/j.1600-6143.2008.02370.x. [DOI] [PubMed] [Google Scholar]
- 17.Cockfield SM, Moore RB, Todd G, et al. The prognostic utility of deceased donor implantation biopsy in determining function and graft survival after kidney transplantation. Transplantation. 2010;89:559–566. doi: 10.1097/TP.0b013e3181ca7e9b. [DOI] [PubMed] [Google Scholar]
- 18.Jochmans I, Moers C, Smits JM, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am J Transplant. 2011;11:2214–2220. doi: 10.1111/j.1600-6143.2011.03685.x. [DOI] [PubMed] [Google Scholar]
- 19.Bond M, Pitt M, Akoh J, et al. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess. 2009;13:iii–iv. doi: 10.3310/hta13380. xi–xiv, 1–156. [DOI] [PubMed] [Google Scholar]
- 20.Wight J, Chilcott J, Holmes M, et al. The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors. Health Technol Assess. 2003;7:1–94. doi: 10.3310/hta7250. [DOI] [PubMed] [Google Scholar]
- 21.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138:W1–W12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 22.Smidt N, Overbeke J, de Vet H, et al. Endorsement of the STARD Statement by biomedical journals: survey of instructions for authors. Clin Chem. 2007;53:1983–1985. doi: 10.1373/clinchem.2007.090167. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RW, Anderson M, Taylor RM, et al. Significance of perfusate lactic acidosis in cadaveric renal transplantation. Br Med J. 1973;1:391–395. doi: 10.1136/bmj.1.5850.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sy G, Jr, Toledo-Pereyra LH, Dienst SG, et al. Are there any important predicting factors of renal function during hypothermic pulsatile perfusion for transplantation? Am Surg. 1980;46:340–343. [PubMed] [Google Scholar]
- 25.Sterling WA, Pierce JC, Hume DM, et al. Renal preservation by hypothermic storage plus pulsatile perfusion. Surg Gynecol Obstet. 1972;135:589–592. [PubMed] [Google Scholar]
- 26.Gok MA, Pelzers M, Glatz JFC, et al. Do tissue damage biomarkers used to assess machine-perfused NHBD kidneys predict long-term renal function post-transplant? Clin Chim Acta. 2003;338:33–43. doi: 10.1016/j.cccn.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Kosieradzki M, Kuczynska J, Piwowarska J, et al. Prognostic significance of free radicals: mediated injury occurring in the kidney donor. Transplantation. 2003;75:1221–1227. doi: 10.1097/01.TP.0000065282.46425.87. [DOI] [PubMed] [Google Scholar]
- 28.Newman CP, Shenton BK. Re-evaluation of viability testing of cadaveric kidneys for transplantation. Br J Urol. 1981;53:95–98. doi: 10.1111/j.1464-410x.1981.tb03143.x. [DOI] [PubMed] [Google Scholar]
- 29.Daemen JW, Oomen AP, Janssen MA, et al. Glutathione S-transferase as predictor of functional outcome in transplantation of machine-preserved non-heart-beating donor kidneys. Transplantation. 1997;63:89–93. doi: 10.1097/00007890-199701150-00017. [DOI] [PubMed] [Google Scholar]
- 30.Cho SI, Zalneraitis B, Ohmi N, et al. Prediction of cadaver kidney function by ligandin analysis. J Surg Res. 1981;30:361–364. doi: 10.1016/0022-4804(81)90172-4. [DOI] [PubMed] [Google Scholar]
- 31.Feinfeld DA, Levine RD, Levine SD, et al. Ligandin in perfusates from transplanted kidneys: a test for tubular necrosis. Nephron. 1978;21:38–41. doi: 10.1159/000181369. [DOI] [PubMed] [Google Scholar]
- 32.Horpacsy G, Schroder K, Zinsmeyer J, et al. Clinical experiences in the Gambro-preservation unit: analysis of 105 human cadaver kidneys. Acta Med Pol. 1978;19:59–65. [PubMed] [Google Scholar]
- 33.Burleson RL, Jones DB, Yenikomshian AM, et al. Clinical renal preservation by cryoperfusion with an albumin perfusate: renal perfusion with albumin. Arch Surg. 1978;113:688–692. doi: 10.1001/archsurg.1978.01370180030003. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RW, Taylor RM, Swinney J, et al. Perfusate lactic acidosis, an essential measurement for evaluating human cadaver kidneys. Surg Forum. 1974;25:266–267. [PubMed] [Google Scholar]
- 35.Sarvary E, Nemes B, Jaray J, et al. Prediction of early renal graft function by the measurement of donor urinary glutathione S-transferases. Transplantation. 2000;69:1397–1402. doi: 10.1097/00007890-200004150-00032. [DOI] [PubMed] [Google Scholar]
- 36.Shoskes DA, Webster R, Shahed A. Oxidant stress in cadaveric and living kidney donors as markers of renal injury: utility of total antioxidant capacity and isoprostane levels in urine. Transplant Proc. 2000;32:804–805. doi: 10.1016/s0041-1345(00)00991-x. [DOI] [PubMed] [Google Scholar]
- 37.Zivna H, Zivny P, Navratil P, et al. The role of cytokines and antioxidant status in graft quality prediction. Transplant Proc. 1999;31:2094. doi: 10.1016/s0041-1345(99)00272-9. [DOI] [PubMed] [Google Scholar]
- 38.Kosieradzki M, Danielewicz R, Kwiatkowski A, et al. Early function of kidneys stored by continuous hypothermic pulsatile perfusion can be predicted using a new “viability index”. Transplant Proc. 2002;34:541–543. doi: 10.1016/s0041-1345(01)02838-x. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson TP, Hendry WF, Ward JP, et al. Continuous perfusion of human cadaveric kidneys for transplantation. Br J Urol. 1975;47:37–44. doi: 10.1111/j.1464-410x.1975.tb03915.x. [DOI] [PubMed] [Google Scholar]
- 40.Kiser WS, Magnusson MO, Hewitt CB, et al. Experience with preservation of shipped-in cadaver kidneys. Transplant Proc. 1972;4:607–609. [PubMed] [Google Scholar]
- 41.Asher J, Wilson C, Gok M, et al. Factors predicting duration of delayed graft function in non-heart-beating donor kidney transplantation. Transplant Proc. 2005;37:348–349. doi: 10.1016/j.transproceed.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 42.Kootstra G, Kievit JK, Heineman E. The non heart-beating donor. Br Med Bull. 1997;53:844–853. doi: 10.1093/oxfordjournals.bmb.a011652. [DOI] [PubMed] [Google Scholar]
- 43.Danielewicz R, Kwiatkowski A, Polak W, et al. An assessment of ischemic injury of the kidney for transplantation during machine pulsatile preservation. Transplant Proc. 1997;29:3580–3581. doi: 10.1016/s0041-1345(97)01032-4. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CB, Sicard GA, Haid SD, et al. Cadaver kidney characteristics and immediate renal allograft function. Proc Clin Dial Transplant Forum. 1977;7:7–10. [PubMed] [Google Scholar]
- 45.Modgill VK, Wiggins PA, Rosenberg IL, et al. An evaluation of viability tests of human cadaveric kidneys. Br J Surg. 1977;64:548–553. doi: 10.1002/bjs.1800640806. [DOI] [PubMed] [Google Scholar]
- 46.de Vries B, Snoeijs MGJ, von Bonsdorff L, et al. Redox-active iron released during machine perfusion predicts viability of ischemically injured deceased donor kidneys. Am J Transplant. 2006;6:2686–2693. doi: 10.1111/j.1600-6143.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 47.Moers C, Varnav OC, van Heurn E, et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation. 2010;90:966–973. doi: 10.1097/TP.0b013e3181f5c40c. [DOI] [PubMed] [Google Scholar]
- 48.Polyak MMR, Arrington BOM, Stubenbord WT, et al. The influence of pulsatile preservation on renal transplantation in the 1990s. Transplantation. 2000;69:249. doi: 10.1097/00007890-200001270-00010. [DOI] [PubMed] [Google Scholar]
- 49.Nijboer WN, Schuurs TA, Damman J, et al. Kidney injury molecule-1 is an early noninvasive indicator for donor brain death-induced injury prior to kidney transplantation. Am J Transplant. 2009;9:1752–1759. doi: 10.1111/j.1600-6143.2009.02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robert R, Guilhot J, Pinsard M, et al. A pair analysis of the delayed graft function in kidney recipient: the critical role of the donor. J Crit Care. 2010;25:582–590. doi: 10.1016/j.jcrc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Hollmen ME, Kyllonen LE, Inkinen KA, et al. Deceased donor neutrophil gelatinase-associated lipocalin and delayed graft function after kidney transplantation: a prospective study. Crit Care. 2011;15:R121. doi: 10.1186/cc10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brook NR, Waller JR, Nicholson ML. Nonheart-beating kidney donation: current practice and future developments. Kidney Int. 2003;63:1516–1529. doi: 10.1046/j.1523-1755.2003.00854.x. [DOI] [PubMed] [Google Scholar]
- 53.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 54.Westendorp WH, Leuvenink HG, Ploeg RJ. Brain death induced renal injury. Curr Opin Organ Transplant. 2011;16:151–156. doi: 10.1097/MOT.0b013e328344a5dc. [DOI] [PubMed] [Google Scholar]
- 55.Talbot D, Shenton BK, Buckley PE, et al. Experiences learned in the successful establishment of a nonheart beating donor program for renal transplantation. J Urol. 2003;170(4 Pt 1):1088–1092. doi: 10.1097/01.ju.0000086774.12582.0f. [DOI] [PubMed] [Google Scholar]
- 56.Kozaki K, Sakurai E, Kubota K, et al. Prediction of kidney nonfunction after transplantation with machine perfusion preservation. Transplant Proc. 2000;32:275–276. doi: 10.1016/s0041-1345(99)00955-0. [DOI] [PubMed] [Google Scholar]
- 57.Yarlagadda SG, Coca SG, Garg AX, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23:2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.