Abstract
Because of persistent social problems caused by methamphetamine (METH), new therapeutic strategies need to be developed. Thus, we investigated the response of central nervous system neurotensin (NT) systems to METH self-administration (SA) and their interaction with basal ganglia dopamine (DA) pathways. Neurotensin is a peptide associated with inhibitory feedback pathways to nigrostriatal DA projections. We observed that NT levels decreased in rats during extinction of METH SA when lever pressing resulted in intravenous infusions of saline rather than METH. Thus, 6 h after the first session of extinction, NT levels were 53, 42, and 49% of corresponding controls in the anterior dorsal striatum, posterior dorsal striatum, and globus pallidus, respectively. NT levels were also significantly reduced in corresponding yoked rats in the anterior dorsal striatum (64% of control), but not the other structures examined. The reductions in NT levels in the anterior dorsal striatum particularly correlated with the lever pressing during the first session of extinction (r =s; 0.745). These, and previously reported findings, suggest that the extinction-related reductions in NT levels were mediated by activation of D2 receptors. Finally, administration of the neurotensin receptor 1 (NTR1) agonist [PD149163 [Lys(CH2NH)Lys-Pro,Trp-tert-Leu-Leu-Oet]; 0.25 or 0.5 mg/kg] diminished lever pressing during the first extinction session, whereas the NTR1 antagonist [SR48692 [2-[(1-(7-chloro-4-quinolinyl)-5-(2,6-imethoxyphenyl)pyrazol-3-yl)carbonylamino]tricyclo(3.3.1.1.(3.7))decan-2-carboxylic acid]; 0.3 mg/kg per administration] attenuated the reduction of lever pressing during the second to fourth days of extinction. In summary, these findings support the hypothesis that some of the endogenous basal ganglia NT systems contribute to the elimination of contingent behavior during the early stages of the METH SA extinction process.
Introduction
Methamphetamine (METH) abuse continues to create major individual and social problems; it is highly addictive, difficult to treat, and often associated with psychosis, violence, and criminal behavior (Baldessarini, 1996; Meredith et al., 2005). Because there currently are no approved medications for METH dependence, developing new approaches for treating METH addiction is important (National Institute on Drug Abuse. Methamphetamine: Abuse and Addiction (2006); www.drugabuse.gov/publications/research-reports/methamphetamine-abuse-addiction/what-treatments-are-effective-methamphetamphine-abusers). Thus, to this end, we and others have investigated the response of the excitatory tridecapeptide neurotensin (NT) and its associated central nervous system (CNS) pathways to METH exposure and dopamine (DA) systems.
NT in dorsal striatum is associated with the two major efferent projections referred to as the direct (striatonigral) and indirect (striatopallidal) feedback loops (Castel et al., 1993, 1994a,b) and the overall net impact of increasing NT release is to inhibit drug-induced DA activation and related effects (Ervin et al., 1981; Wagstaff et al., 1994), possibly by stimulating GABA release that in turn diminishes corresponding DA release (Ferraro et al., 2007; Torregrossa and Kalivas, 2008).
Until recently, our understanding of how NT systems contribute to METH effects has been based on animal models that employed noncontingent administration of METH, and examined CNS NT tissue levels (Letter et al., 1987; Merchant et al., 1989a,b), synthesis (Adams et al., 2001), and release (Wagstaff et al., 1996a,b; Frankel et al., 2005). These findings suggest that relatively low doses of METH (approximately 0.5 mg/kg s.c.), through a D2-receptor mechanism (Merchant et al., 1989b), in the basal ganglia 1) release NT (Wagstaff et al., 1996a,b; Frankel et al., 2005), 2) reduce the striatal NT tissue level (Wagstaff et al., 1996a), and 3) limit METH-induced DA-mediated activities (Wagstaff et al., 1994). In contrast, high doses/concentrations (5–10 mg/kg s.c.) of METH through D1 mechanisms 1) reduce release of NT in basal ganglia (Hanson et al., 1992; Wagstaff et al., 1996a), and 2) increase NT tissue levels by increasing NT synthesis and accumulation (Letter et al., 1987; Castel et al., 1994a,b; Adams et al., 2001).
Although these noncontingent studies implicate NT systems in the pharmacology of METH, their relevance to the role of NT in METH dependence per se is unclear. Thus, using lever pressing as an operant behavior, we examined the response of NT systems to METH self-administration (SA). It was observed in these studies that self-administration of METH (SAM), through a D1 mechanism, elevated NT levels in basal ganglia-related regions by approximately 200–300% in a manner similar to that reported in response to noncontingent administration of high doses of METH (Frankel et al., 2011; Hanson et al., 2012). Due to these findings that NT systems are altered by contingent METH SA in rats, we examined in the current studies the possibility that these same NT systems may also be influenced when rats trained to lever press for METH SA no longer were receiving drug, but saline instead (which we operationally called extinction). Reported herein is the observation that NT systems of basal ganglia structures of rats undergoing extinction to METH SA, possibly through D2 mechanisms, appeared to respond opposite to that caused by METH SA itself, manifesting a reduction in NT levels in some basal ganglia structures. These and other findings suggested that basal ganglia NT systems likely contributed to the associated elimination of the operant behavior.
Materials and Methods
Animals.
Male Sprague-Dawley rats (300–350 g; Charles River Laboratories, Raleigh, NC) were allowed to acclimate for at least 1 week prior to experimentation. For most studies, the rats to be used for METH SA were initially group-housed during food training (Fuchs et al., 2005) and then housed individually after jugular catheter implantation.
Drugs and Chemicals.
Methamphetamine hydrochloride was furnished by the National Institutes of Health (NIH) National Institute on Drug Abuse (Bethesda, MD), and infusion quantities were calculated as the free base. D1 (SCH23390, R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrohydro-1H-3-benzazepine hydrochloride; Research Biochemicals Inc., Natick, MA) or D2 (eticlopride, S-(−)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride) antagonists were obtained commercially. A NTR1 agonist [PD149163 [a gift from the National Institute of Mental Health, NIH]; a stable NT fragment (NT[8-13]) that stimulates CNS NTR1 receptors when delivered systemically; 0.25 or 0.5 mg/kg s.c.; Feifel et al., 2008] or antagonist [SR48692 [2-[(1-(7-chloro-4-quinolinyl)-5-(2,6-imethoxyphenyl)pyrazol-3-yl)carbonylamino]tricyclo(3.3.1.1.(3.7))decan-2-carboxylic acid; purchased from Tocris Bioscience]; 0.3 mg/kg; Wagstaff et al., 1994; Antonelli et al., 2007] was used to study the role of NT receptors in METH SA.
Food Training.
Because we have observed in our treatment paradigm that food training predicted an animal’s ability to acquire drug SA, all rats used in the METH SA studies were required to pass food training as described previously (Fuchs et al., 2005; See et al., 2007; Frankel et al., 2011; Hanson et al., 2012). In brief, rats were restricted to approximately 85% of their free-feeding food quantity and then placed in Coulbourn operant chambers connected to a PC computer running Graphic State software (Coulbourn Instruments, Whitehall, PA). Each chamber was equipped with two retractable levers, a food-pellet dispenser between the levers, and a house light on the wall opposite the levers. One lever was the “active” lever resulting in the delivery of a food pellet, whereas the other lever had no programmed consequences. Training consisted of a schedule of food reinforcement (FR) (45-mg Rodent Grain food pellets; Bio-Serv Delivering Solutions, Frenchtown, NJ). FR1 comprised only the stimulus-appropriate response (drug-lever) causing release of a food pellet. If, during each overnight food-training session, a rat received 50 pellets, the FR was increased to 2. If the rat obtained another 50 food pellets, the FR increased to 3 for the balance of the session. Rats remained in the food-training phase for either 4 days or until they achieved two consecutive sessions on the FR3 schedule after which they received a jugular vein catheter implant (approximately 90% of the rats successfully completed food training) in preparation for METH SA/extinction studies.
Catheter Implantation.
For most of the studies after food training, rats were anesthetized with Equithesin (i.p.) and indwelling catheters consisting of a screw-type connecter, silastic tubing (10 cm i.d., 0.64 mm o.d., 1.19 mm), ProLite (Atrium Medical Corporation, Hudson, NH) polypropylene monofilament mesh, and cranioplastic cement were implanted beneath the skin of the back (at the shoulder-blades). The outlet of the catheter ran subcutaneously around the underside of the animal with the end inserted into the right jugular vein. The catheter was secured to the surrounding tissue with sutures. A 0.1-ml antibiotic solution containing cefazolin (10.0 mg/ml) dissolved in heparinized saline (70 U/ml; Sigma-Aldrich, St Louis, MO) was flushed through the catheter for 3 days after surgery to extend catheter patency. Thereafter, catheters were flushed with 0.1 ml of heparinized saline before and after each SA session to prevent clotting. Stylets were inserted into the catheters when rats were not connected to infusion pumps. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee and adhered to the National Academy of Sciences Guide for the Care and Use of Laboratory Animals.
METH Self-Administration Training.
Operant training was based on procedures as previously described (Fuchs et al., 2005; See et al., 2007; Frankel et al., 2011; Hanson et al., 2012). All SA sessions were conducted during the light cycle; however, animals were exposed to a 14-/10-h light/dark environment while in their home cages.
For all experiments except that shown in Fig. 4, each SAM rat underwent 4-h sessions in Coulbourn operant chambers for 7 consecutive days and were exposed to the presentation of an identical right and left lever. One of the levers was selected (it did not matter which) as active such that appropriate pressing resulted in a primary stimulus of METH infusion (0.06 mg/infusion i.v.) followed by lever retraction for a 20-s time-out period until subsequent presentation of the levers. The SAM rats selectively pressed the active lever >90% of the time. Data collection and reinforcer delivery were controlled by a PC computer using Graphic State Notation (Coulbourn Instruments). For studies represented in Figs. 2, 3, and 5–7, prior to initiation of SA training, each SAM rat was randomly paired with one or two groups of yoked animals. The yoked rats were prepared and treated identically to the SAM animals except that neither lever in the operant chamber had programmed consequences. Furthermore, these yoked animals received either METH (0.06 mg/infusion; YM) or saline (equal volume; YS) at times and quantities determined by the lever-pressing behavior of the linked SAM rats. After initial food-training, YS rats that were then exposed to only inactive levers after the placement of jugular catheters averaged approximately 19 lever presses on what had been the active lever for food training, during their first session. In these rats, the lever-pressing behavior dropped off to an average of 4–5 presses/session by the fourth through seventh daily sessions.
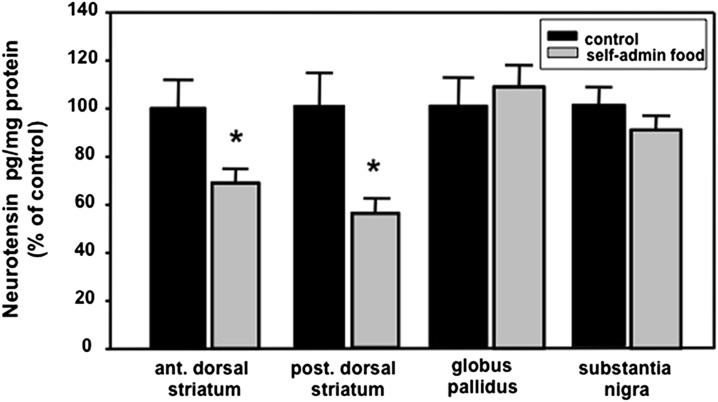
Fig. 4.
Effects of extinction for self-administration of food pellets on NT levels in basal ganglia structures. The two groups compared in these studies included the following: 1) rats that self-administered food pellets (self-admin food) followed by a session of extinction during which lever pressing had no effect, and 2) control rats that were exposed to only inactive levers and received no food pellets. The total food intake of the control rats was adjusted to equal that of the self-administration rats as explained in Materials and Methods. All rats were killed 6 h after the final session as described in Materials and Methods. In control groups the NT levels (pg/mg protein) in the anterior and posterior dorsal striatum, globus pallidus, and substantia nigra were 126, 150, 540, and 589, respectively. *P < 0.05 versus corresponding controls. n = 9–10.
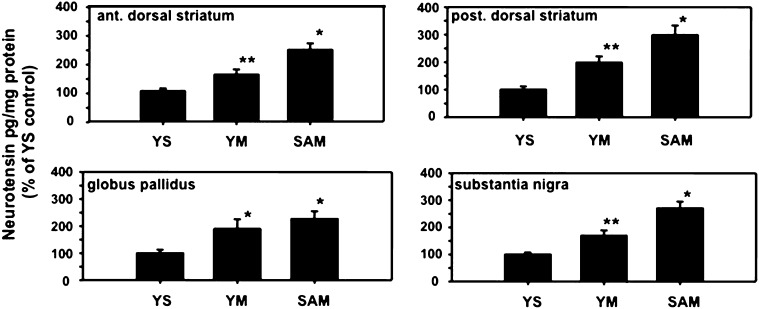
Fig. 2.
Effects of METH self-administration on NT levels in basal ganglia structures. The three groups compared in these studies included rats that self-administered METH (SAM) for seven 4-h daily sessions, rats yoked to the SAM animals that received METH in an identical fashion regardless of lever pressing patterns (YM), and rats yoked to the SAM animals but received saline infusions instead of METH, regardless of lever pressing (YS). All rats were killed 6 h after the final session as described in Materials and Methods. The levels of NT in the anterior and posterior of the dorsal striatum, globus pallidus, and substantia nigra were determined. *P < 0.05 versus other corresponding groups, but not each other (i.e., globus pallidus); **P < 0.05 versus other corresponding groups. n = 9–10. NT levels (pg/mg protein) in control YS animals were 106, 82, 362, and 664 in anterior dorsal striatum, posterior dorsal striatum, globus pallidus, and substantia nigra, respectively.
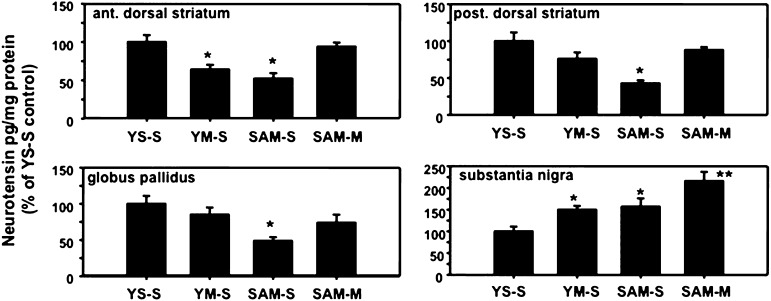
Fig. 3.
Effects of extinction on NT levels in basal ganglia structures. The four groups compared in these studies included the following: 1) rats that self-administered METH followed by a session of extinction during which lever pressing caused saline, rather than METH, infusions (SAM-S); 2) rats with only inactive levers and received METH during self-administration and the saline infusions during extinction yoked to the lever pressing of the SAM-S rats (YM-S); 3) rats with only inactive levers and received saline infusions when the SAM-S rats lever pressed (YS-S); and 4) rats treated like SAM-S animals except appropriate lever pressing always resulted in METH infusions even during the extinction session (SAM-M). All rats were killed 6 h after the final session as described in Materials and Methods and levels of NT were determined in anterior and posterior dorsal striatum, globus pallidus, and substantia nigra. *P < 0.05 versus all other corresponding groups but not from each other; **P < 0.05 versus all other corresponding groups. n = 9–10. NT levels (pg/mg protein) in YS-S controls were 177, 105, 595, and 648 in anterior dorsal striatum, posterior dorsal striatum, globus pallidus, and substantia nigra, respectively.
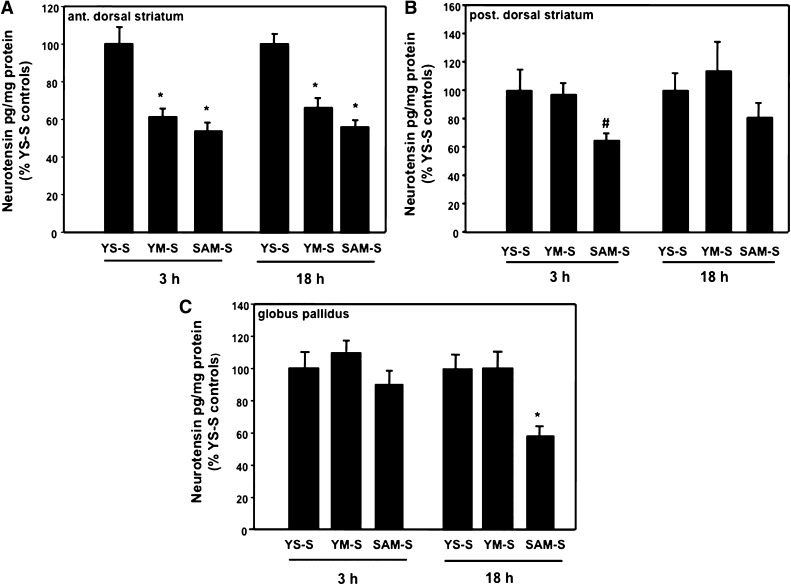
Fig. 5.
Temporal effects of extinction on NT levels in basal ganglia structures. The SAM-S, YM-S, and YS-S groups were treated as described for Fig. 3 except rats were killed either 3 or 18 h after the first extinction session. NT levels were determined in the anterior dorsal striatum (A), posterior dorsal striatum (B), and globus pallidus (C). *P < 0.05 versus all other corresponding groups but not from each other (i.e., ant. dorsal striatum); #P = 0.06 versus all other corresponding groups. n = 10. NT levels (pg/mg protein) in YS-S controls were 175–194, 145–161, and 425–492 for anterior dorsal striatum, posterior dorsal striatum, and globus pallidus, respectively.
Fig. 7.
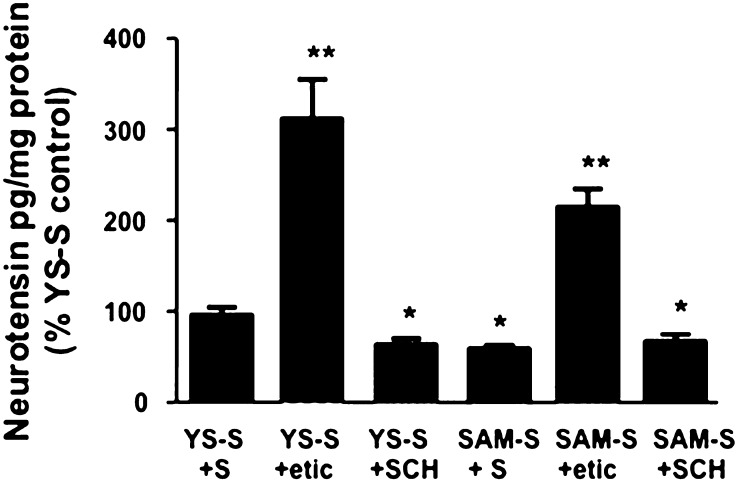
Role of DA receptors in mediating changes in neurotensin levels of the anterior dorsal striatum after the first session of extinction. The rats were all treated like the SAM-S or YS-S groups described in Fig. 3 except they were pretreated 15 min prior to the first extinction session with either saline (YS-S+S or SAM-S+S), eticlopride (YS-S+etic or SAM-S+etic), or SCH23390 (YS-S+SCH or SAM-S+SCH). *P < 0.05 versus other groups but not from each other; **P < 0.05 versus other groups but not from each other. n = 25 (YS-S+S), n = 7 (YS-S+etic), n = 33 (SAM-S+S), n = 12 (SAM-S+etic), n = 8 (YS-S+SCH), and n = 15 (SAM-S+SCH). Mean NT levels in anterior dorsal striata of YS-S+S controls = 133 pg/mg protein.
After each SA session, all rats were returned to their home cages and given access to 15–20 g of Purina rat chow (Purina, St. Louis, MO). The METH SA protocol within each session consisted of lever pressing at an FR1 for 3 presses, which was followed by, and increased to, FR2 for the next 6 presses, followed by an increase to FR3 for 12 presses, and finally to an FR5 for the balance of the session. Rats typically reached the FR5 level each day by the end of the fourth through seventh sessions. For these studies, SAM rats were considered to have reached criterion if they maintained at least relatively steady lever pressing during days 5–7 (with at least 0.6 mg/kg per day METH infusions per day and self-administered a total of at least 3 mg of i.v. METH during days 1–7; this was typical of 80% of our SAM animals). The pattern of METH SA was relatively stable during days 5–7 as previously described (Frankel et al., 2011; Hanson et al., 2012). Six hours after the final SA session (Fig. 2), or 3, 6, or 18 h after the first extinction (see Figs. 3, 5–7) sessions, the rats were killed, and brains were removed and frozen on dry ice for dissection and NT analysis.
Extinction.
To evaluate the impact of extinction (i.e.,operationally defined as when METH infusion was no longer available to SAM rats for active lever pressing) on NT responses, SAM rats were housed individually in their home cages for 48 h after the seventh session of METH SA (see Figs. 1, 3, 5–8) to allow METH to be cleared from the animals and basal ganglia NT systems to recover from METH exposure. During extinction sessions in Figs. 1–3 and 5–8, one group of SAM rats was allowed to lever press but only received saline (SAM-S). For comparison, YM and YS groups (Figs. 3 and 5) after a 48-h abstinence were returned to their operant chambers and passively received saline infusions linked to the SAM-S rats lever-pressing (YM-S and YS-S, respectively). In one study, a SAM group received METH infusions in response to appropriate lever pressing during the extinction session for the SAM-S and YM-S groups (SAM-M) (Fig. 3) to better establish the effect of extinction on the NT system.
Fig. 1.
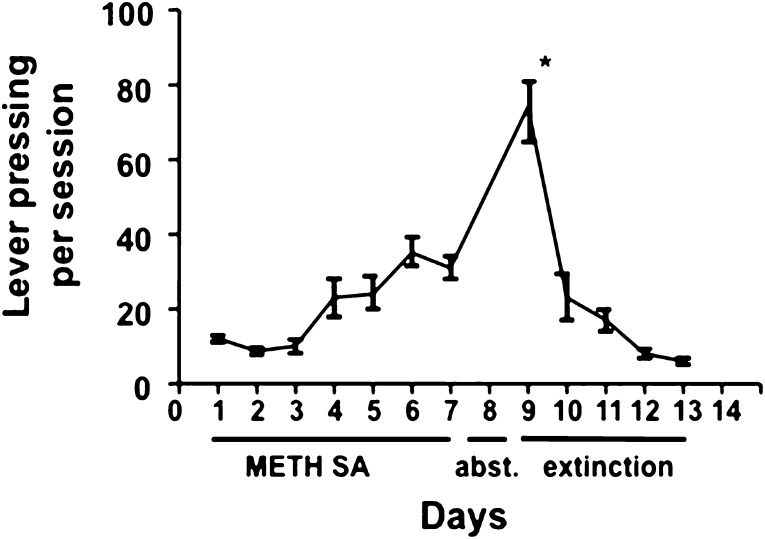
Lever pressing responses by rats during METH self-administration and extinction. Rats trained to lever press as described in Materials and Methods participated in seven 4-h daily sessions when appropriate lever pressing resulted in intravenous infusions of METH (0.06 mg/injection). Days 1–7 are marked as METH self-administration (SA). After day 7, rats were housed individually in their home cages for 48 h indicated as abstinence (abst.) in the figure because they were not given access to METH. Rats were returned to the operant chambers for 4-h sessions on days 9–13 during which lever pressing resulted in saline, not METH, infusions. This period is labeled extinction in the figure. *P < 0.05 versus all other lever pressing values. n = 14–15.
Fig. 8.
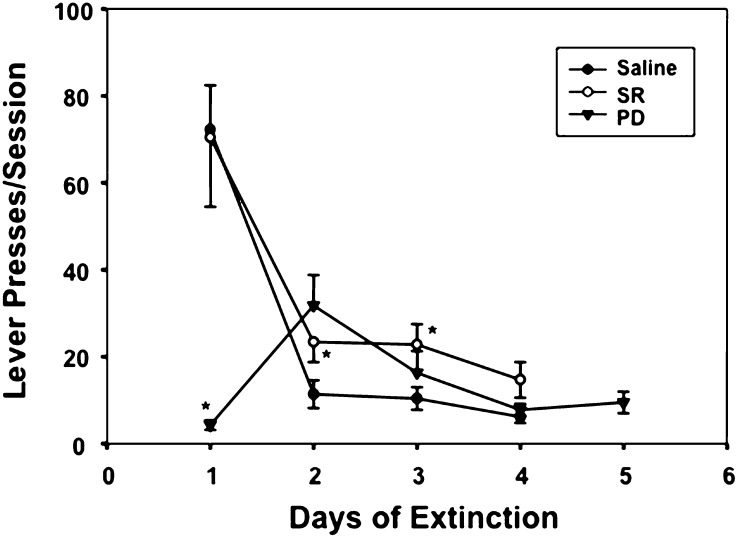
Effect of pretreatment with the NTR1 agonist PD149163 (PD; 0.5 mg/kg), antagonist SR48692 (SR; 0.3 mg/kg), or saline on lever-pressing behavior in SAM rats during 4–5 daily extinction sessions. The PD compound was administered 15 min prior to extinction session 1 but not 2–5. The SR compound was administered 15 min prior to 1–4 extinction sessions. *P < 0.05 versus corresponding saline groups. n = 23 (saline), n = 9 (SR), and n = 9 (PD).
Also for comparison, the effects of extinction on NT levels were measured in rats trained to lever press for food pellets in a manner similar to that described above for METH SA (i.e., rats lever pressed for food pellets during 4-h daily sessions for a total of 7 days) (Fig. 4). A control group was also placed in the operant chambers and exposed to inactive levers. After 48 h of abstinence in their home cages, these animals were returned to their respective operant chambers, except lever pressing no longer resulted in the release of a food pellet for any of the rats. These animals were killed 6 h after this first extinction session and compared with the control rats. Total food consumption was matched and adjusted between the two groups with the total weight consumed in food pellets in the chamber plus the free-standing food in the home cages being comparable for all rats
Drug Treatments.
In the experiments to study the DA receptor mechanisms underlying the NT responses to extinction, SAM or YS rats were pretreated by intraperitoneal injections of saline or 0.5 mg/kg dose of either 0.5 mg/kg SCH23390 (D1 antagonist) or eticlopride (D2 antagonist) (Merchant et al., 1989a,b; Castel et al., 1994b; Wagstaff et al., 1996a,b). The drugs were administered 15 min prior to the first extinction session, and rats were killed 6 h later (see Fig. 6).
Fig. 6.
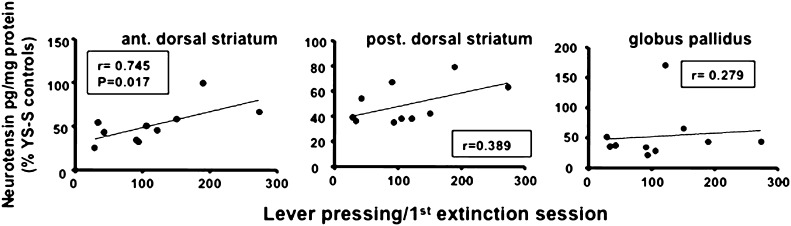
Correlation between neurotensin tissue levels and lever pressing during the first day of extinction. All rats were treated as described in Fig. 3 for SAM-S animals and killed 6 h after the first extinction session. Neurotensin levels were determined for each rat in anterior and posterior dorsal striatum and globus pallidus and correlated with the respective total of lever pressing during the first extinction period. The Spearman correlation coefficients and the only significant P value are expressed for relevant groups in the corresponding figures. n = 10.
To determine the role of NTR1 receptors in the extinction of METH SA, the agonist or antagonist was delivered prior to the first (PD149163) or the first through fourth (SR48692) sessions of extinction in SAM animals. As previously reported, the 0.5 mg/kg dose of PD149163 alone did not impair motor functions (Frankel et al., 2011) nor did it have stimulant or SA properties like METH (Hanson et al., 2012). In addition, in the operant chambers, the behaviors of the rats receiving the lower 0.25 mg/kg dose of PD149163 were indistinguishable from rats receiving saline consistent with previous reports that 1) intracerebroventricular pretreatment with NT blocks stimulant (including amphetamine)–induced locomotion (Norman et al., 2008), 2) NT receptor stimulation blocks stimulant-induced DA release (Liang et al., 2008), and 3) NT receptor knockouts have hyperactivity (Liang et al., 2010).
Analysis of Neuropeptide Levels in Tissue.
Levels of NT were determined by a specific and sensitive RIA previously described (Frankel et al., 2011; Hanson et al., 2012). The anterior and posterior dorsal striatum, globus pallidus, and substantia nigra were dissected (Paxinos and Watson, 1982) and frozen at −80°C until assayed. The tissue levels of NT for each control group are indicated in the figure legends. To facilitate comparisons, data were normalized by dividing with respective control mean values and expressed as a percentage.
Statistical Analysis.
The data presented are the ± S.E.M. All data were analyzed using the SAS 9.1 program (SAS Institute, Inc. Cary, NC) or GraphPad Prism (version 5.01; GraphPad Software, Inc., La Jolla, CA). For analysis of NT levels, three or more groups were compared by one-way analysis of variance followed by a Newman–Keuls post hoc test or t test. Two groups were assessed using the t test. Correlations were conducted using Spearman’s correlation coefficient. Pressing behavior was analyzed using a repeated-measures analysis of variance with a Huynh-Feldt-Lecoutre correction if the sphericity assumption was violated. All results were considered significant when P < 0.05.
Results
Using the METH SA model, we observed that lever pressing went from approximately 12 (day 1) to 32 (day 7) presses per 4-h session (Fig. 1) similar to our previous report (Frankel et al., 2011). The rats were left in their home cages without access to METH (referred to as abstinence) for 48 h to allow the METH and its effect to be cleared and then rats were returned to the operant chambers for a routine 4-h session. Importantly, we included a 48-h abstinence period prior to the extinction session because we previously found that it requires approximately 48 h for NT levels to return to normal after exposure to noncontingent stimulants like METH (Hanson et al., 1989). This recovery pattern was confirmed in separate SA studies by the observation that control (YS) rats receiving saline and SAM animals that were left in their home cage for approximately 48 h, rather than re-exposed to an operant chamber and associated levers during an extinction session, had similar dorsal striatal NT tissue levels (i.e., the SAM effect had recovered by 48 h) (data not shown). Thus, we concluded that the direct pharmacological effect of METH SA wears off in time for the extinction sessions and YS rats receiving saline during extinction (YS-S) were appropriate controls for these studies. In the YS rats, lever pressing activity during the first session of extinction was greater than during the later stages of METH SA (e.g., days 6 and 7) [F(11,143) =s; 25.30, P < 0.05, Fig. 1], even though there was no drug injection associated with the operant behavior. However, lever pressing dramatically reduced on the second day of extinction (10th day).
The response of basal ganglia NT systems to METH SA was determined by measuring NT levels 6 h after the seventh SA session in the anterior and posterior dorsal striatum (sites of the origin of NT striatal efferent neurons), as well as in the substantia nigra and globus pallidus (sites of terminals associated with striatal direct and indirect feedback projections, respectively; Castel et al., 1993,1994b) (Fig. 2). NT levels in all of these basal ganglia structures were significantly elevated in SAM rats [anterior dorsal striatum: F(2,25) =s; 20.26, P < 0.05; posterior dorsal striatum: F(2,25) =s; 12.68, P < 0.05; globus pallidus: F(2,26) =s; 5.01, P < 0.05; substantia nigra: F(2,26) =s; 22.96, P < 0.05]. Although the NT levels in these same regions were also increased in the associated YM rats, the elevations in NT levels were significantly greater in the corresponding SAM animals in each area except the globus pallidus.
To better appreciate the significance of the NT changes caused by METH SA, we evaluated the response by NT systems 3–18 h after the first session of extinction in these same basal ganglia structures. In contrast to the effects of METH SA, 6 h after the first extinction session (Fig. 3), NT levels were significantly reduced in anterior and posterior dorsal striatum and the globus pallidus in SAM rats receiving saline after lever pressing (SAM-S) [anterior dorsal striatum: F(3,32) =s; 17.49, P < 0.05; posterior dorsal striatum: F(3,33) =s; 8.29, P < 0.05; globus pallidus: F(3,34) =s; 4.64, P < 0.05]. NT levels in both dorsal striatal regions of YM rats were also reduced, but this effect tended to be less than that observed in corresponding SAM animals, particularly in the posterior dorsal striatum. Extinction appeared to have no significant effect on pallidal NT levels in the YM animals. In contrast, nigral NT levels similarly increased in both YM and SAM rats [F(3,34) =s; 7.11, P < 0.05]. For comparison, we also examined NT levels in these same regions in SAM rats that received METH (SAM-M) instead of saline (SAM-S) associated with lever pressing during the first extinction session. The NT levels were not significantly altered in the dorsal striatal regions or the globus pallidus but were elevated in the substantia nigra of these animals.
Relative to these findings, we examined whether these NT decreases were a direct consequence of the drug itself or reward seeking per se. This was done by determining the effect of nonpharmacological behavior extinction on NT levels in the basal ganglia of rats trained for 7 days to lever press for food pellets during 4-h daily sessions. By use of an extinction paradigm similar to that used with METH SA for Figs. 1 and 3 (see Materials and Methods), we observed that extinction of lever pressing for food pellets caused similar patterns of reduction in both anterior and posterior [t(20) =s; 2.28, P < 0.05 and t(26) =s; 4.19, P < 0.05, respectively] dorsal striatal NT levels 6 h after the first extinction session but not in the globus pallidus or substantia nigra (Fig. 4).
To assess the temporal features of this unique NT response to extinction in SAM rats, we measured NT levels in the anterior and posterior dorsal striatum as well as in the globus pallidus 3 and 18 h after the first extinction session. Reductions in NT levels were observed in the anterior dorsal striatum both 3 h [F(2,27) =s; 13.68, P < 0.05] and 18 h [F(2,27) =s; 19.46, P < 0.05] after the first day extinction session in both SAM-S and YM-S animals (Fig. 5A). NT levels in the posterior dorsal striatum revealed a trend toward significant decrease after 3 h [F(2,27) =s; 3.08, P =s; 0.06], but not 18 h [F(2,25) =s; 2.57, N.S.] in SAM-S rats with no changes after either time in YM-S animals (Fig. 5B). In contrast, the only NT change in the globus pallidus was a significant reduction in SAM-S animals 18 h after treatment [3 h: F(2, 26) =s; 2.54, N.S.; 18 h: F(2,25) =s; 6.74, P < 0.05; Fig. 5C].
Based on the assumption that when activated, the basal ganglia NT feedback pathways reduce the behavior of lever pressing (Hanson et al., 2012) and that reduced NT tissue levels soon after treatment are associated with increased release, turnover, and depletion of NT (Wagstaff et al., 1996a), we evaluated whether the reduction in NT levels in individual animals correlated with their lever pressing behavior during the first session of extinction. We observed a significant correlation between the anterior dorsal striatum NT levels and lever pressing of SAM-S rats during extinction such that lower NT tissue levels (likely reflecting greater release and turnover and enhanced inhibitory feedback activity) related to reduced lever-pressing behavior (r =s; 0.745, P < 0.05). Although it did not reach significance, a similar relationship between reduced NT levels and extinction lever pressing tended to exist also in the posterior dorsal striatum, whereas there was no evidence of such a relationship in the globus pallidus (Fig. 6).
We tested the possibility that decreases in NT levels of this dorsal striatal region were mediated by METH-induced activation of the DA receptors. The extinction-related reduction in NT levels appeared to be still expressed in SAM rats that were pretreatment with the D1 antagonist, SCH23390; however, it should be noted that SCH23390 treatment alone (YS-S+SCH) also reduced the NT tissue content in this brain region [F(5,94) =s; 48.68, P < 0.05]. In contrast, antagonism of D2 receptors with eticlopride pretreatment prevented the extinction-induced reduction of NT in the anterior dorsal striatum of SAM-S+etic compared with YS-S+S controls, but eticlopride by itself (YS-S+etic) also increased the NT in this structure.
As mentioned above, our findings suggest that during the first session of extinction in SAM rats, NT in the dorsal striatum, particularly the anterior region, is released through a D2 mechanism resulting in a NT-mediated feedback inhibitory response that suppresses the lever-pressing operant response for METH. To further test this hypothesis, SAM rats were treated with either the NTR1 receptor agonist (PD149163) or antagonist (SR48692) and the effects of these compounds on the operant behavior during the first session of extinction were measured (Fig. 8). The general pattern of lever-pressing extinction displayed in Fig. 1 was confirmed with elevated pressing activity the first day of extinction followed by reduction of the operant behavior during the second through fourth sessions (see saline group in Fig. 8). When pretreated with the 0.5 mg/kg PD149163 prior to the first extinction session, the operant behavior was dramatically reduced [day × drug interaction: F(3,114) =s; 9.05, P < 0.05] with some rebound when the NTR1 agonist pretreatment was discontinued for the second session followed by a tapering of effect for days 3–5. In a separate experiment, we administered the PD149163 compound on both days 1 and 2 of extinction and found identical effects on lever pressing both days (data not shown). To determine the potency of the PD149163 compound to block extinction-related lever pressing, a lower dose of PD149163 (0.25 mg/kg) was administered prior to the first extinction session. Despite the fact that this lower dose had no observable effects on rats in the chambers, lever pressing decreased from 65 ± 12 presses in saline-treated animals to 13 ± 4 in PD149163-treated rats [F(1,26) =s; 4.19, P < 0.05]. In contrast, pretreatment to the first through fourth sessions with the NTR1 antagonist (SR48692 compound) significantly reduced the effects of extinction on lever pressing on extinction days 2 and 3.
Because of the complex nature of the results for these experiments, a summary of findings is presented in Table 1.
TABLE 1.
Summary of principal findings
| METH SA increases NT levels in basal ganglia structures with the effect generally being greater in the SAM versus YM animals. |
| In contrast to METH SA, extinction of lever pressing for METH correlates with a decrease in most basal ganglia structures (except substantia nigra) with the best correlation in the anterior, closely followed by the posterior, dorsal striatum. The extinction-induced decreases in NT levels were generally greatest in the SAM versus YM rats. |
| Similar to METH SA, extinction of lever pressing for food SA also correlated with NT decreases in both anterior and posterior dorsal striatum, but not globus pallidus or the substantia nigra. |
| Results suggest that activation of D2 receptors contribute to the basal ganglia NT response to extinction of METH SA, while previous studies demonstrated that activation of D1 receptors contribute to NT responses to METH SA itself (Frankel et al., 2011). |
| Activation of NTR1 receptors appears to contribute to the reduction in lever pressing associated with extinction of METH SA. |
Discussion
In this study, we examined the role of basal ganglia NT systems in extinction to METH SA. Because of the D2 receptor link with striatopallidal NT projections (Gerfen et al., 1990), it is likely that D2-mediated changes in the dorsal striatal NT system are principally associated with this indirect feedback pathway (Castel et al., 1994a,b). For example, D2-mediated reductions in NT levels in the dorsal striatum probably correspond with elevated NT release (Wagstaff et al., 1996a,b), suggesting that reduced NT stores under these conditions is the result of increased release, turnover, and depletion. Predictably, D2 antagonists have the opposite effects on NT, implying a rise in accumulation with increased synthesis of NT enhancing its buildup (Letter et al., 1987; Merchant et al., 1994). In contrast, D1 receptor stimulation increases NT levels in basal ganglia structures that correspond with elevated NT precursor mRNA, suggesting that raised NT content relates to accumulation of NT from increased synthesis. This effect is similar to that caused by D2 antagonists, although probably associated with a different cell population (i.e., striatonigral versus striatopallidal pathways, respectively) (Castel et al., 1993, 1994b).
Similarly, noncontingent administrations of low and high doses of METH have contrasting effects on NT systems. For example, a low dose of METH (0.5 mg/kg) reduces NT tissue levels and increases NT release in the dorsal striatum apparently by activation of striatopallidal-related D2 receptors (Castel et al., 1994b; Wagstaff et al., 1996a). In contrast, like activation of D1 receptors, a high dose of METH (10 mg/kg) increases both NT striatal-related tissue levels and associated mRNA expression, an effect blocked by a D1, but not a D2, antagonist and likely linked with the direct striatonigral feedback pathway (Castel et al., 1994a).
These differential roles of D1 and D2 receptors in the dose-dependent effects of noncontingent METH on NT responses may reflect the fact that a low dose of METH elevates extracellular DA levels approximately 50–100% (Wagstaff et al., 1994; Pereira et al., 2006), only enough to preferentially activate D2 high-affinity receptors (Schiffer et al., 2009). Consequently, activation of these high-affinity D2 receptors would preferentially enhance the striatopallidal NT system and its inhibitory feedback influence on nigrostriatal DA systems associated with a noncontingent, low-dose METH administration. In contrast, a high dose of METH elevates extracellular striatal DA levels 10- to 50-fold (Kuczenski et al., 1997), thereby activating the lower-affinity D1 receptor (Dearry et al., 1990) linked to the direct feedback striatonigral pathway, elevating associated NT levels by increasing NT synthesis in striatal cell bodies (Castel et al., 1994b).
To determine the relevance of these noncontingent METH effects to drug dependence, we examined the role of NT systems in METH SA models and focused on the dorsal striatum-related pathways due to their role in emotional elements such as anticipation and habit formation (Koob and Volkow, 2010). We observed that NT levels increased in basal ganglia-related structures after METH SA in both the SAM and YM groups (Fig. 2). Because NT levels were significantly higher in the SAM versus YM rats in most of these regions, it is likely that elements associated with the operant training (in SAM rats) embellished this DA response to the pharmacological action of the drug (i.e., the response seen in the YM rats) (Frankel et al., 2011; Hanson et al., 2012). It is also probable that this NT response to METH SA was D1 mediated and associated with the striatonigral pathway, as previously reported (Frankel et al., 2011).
In addition, to appreciate the role of NT in the operant behavior associated with METH SA, we previously administered a NTR1 agonist (PD149163) prior to a METH SA session. The NT agonist dramatically, but reversibly, reduced lever pressing but did not suppress the METH-related operant response by impairing motor functions. In contrast, the administration of the NTR1 antagonist, SR48692, had no significant effect on METH SA (Frankel et al., 2011; Hanson et al., 2012).
The present experiments examined the impact of extinction on basal ganglia NT responses. After the first extinction session, NT levels in SAM-S rats decreased in anterior and posterior dorsal striatum and the globus pallidus, whereas NT levels significantly decreased only in the anterior dorsal striatum of the linked YM-S rats (see Fig. 3). If the decreases in striatal NT levels were associated with extinction of operant behavior linked to METH infusions, these findings suggest that this process was not only occurring in the SAM animals, but also, to a lesser degree in some regions, in the YM rats. Thus, it is possible that factors in addition to those associated with lever pressing, such as placement of rats into the operant chambers, are also associated with exposure to METH and cause some extinction processes when METH infusions in either the SAM or the YM groups are terminated. For comparison, we also examined NT responses in the same basal ganglia structures during extinction of lever pressing associated with SA for food pellets (Fig. 4) and observed a similar reduction in NT levels in both anterior and posterior dorsal striatum, but no changes in globus pallidus or substantia nigra. These findings suggest that the extinction-related decreases in NT levels in the dorsal striatum of SAM rats are to some extent related to the extinction process itself (i.e., it was caused by extinction from both METH and food SA). However, a lack of response by pallidal and nigral NT systems to food extinction suggests that NT in striatal efferent projection terminals (Castel et al., 1994b) do not contribute to the process of extinction itself.
SAM rats that received METH infusions when they lever pressed during the first extinction session (SAM-M; i.e., they did not experience extinction per se) in Fig. 3, did not manifest a significant decline of NT levels in basal ganglia structures. A lack of NT response in these brain regions of SAM-M rats suggests that an association exists between the process of operant behavior elimination when METH SA is stopped and the reduction in NT levels in the basal ganglia structures reported in Fig. 3. NT levels were not elevated in most of the basal ganglia regions of the SM-M rats as observed in Fig. 2 for the SAM animals 6 h after the seventh day of METH SA possibly because the first “extinction” session was deliberately 48 h after the last METH SA session (day 7; see Fig. 2), to allow the NT effect to return to control levels (see the “Extinction” under Materials and Methods, and discussion of the Results for details). The fact that METH SA for the SAM-M rats was not sufficient to significantly elevate NT levels 6 h after a single 4-h session suggests that the METH-induced increases in NT levels are likely dependent on a process that requires more time to express as elevated NT tissue levels, such as increased mRNA expression and enhanced NT synthesis (Adams et al., 2001).
As described above, previous reports support a role for both the activation of D2 receptor and NT release in the extinction process in that they demonstrated that low doses (approximately 0.5 mg/kg, which causes an approximately 150% increase in DA release), but not high doses (10 mg/kg, which causes an approximately 10- to 50-fold increase in DA release), of METH and a D2 agonist administered noncontingently cause both a release of NT in the dorsal striatum and a corresponding reduction in NT levels in this structure (Wagstaff et al., 1996a,b). Thus, these reports suggest that extinction-related decreases in NT tissue levels reflect increases in release and turnover, and participate in the process of extinction of METH SA through a D2 mechanism (Merchant et al., 1989a,b; Castel et al., 1994b). This is supported by the present findings that decreases in NT levels correlate with the reduction of lever presses in extinction (Fig. 6), and that pretreatment with the NTR1 agonist (PD149163) or antagonist (SR48692) reduced lever pressing or attenuates the extinction-related decreases in lever pressing, respectively (Figs. 7 and 8). Despite the fact that several of the findings reported herein are consistent with the hypothesis of a role for a D2-mediated mechanism, this conclusion is confounded by the findings in Fig. 7. Thus, the observation that both eticlopride and SCH23390 had significant effects of their own on NT levels in basal ganglia structures makes it difficult to interpret the impact of pretreatment with D2 and D1 antagonists on the NT responses to METH SA. Thus, additional studies employing strategies such as microdialysis and expression of NT-related mRNA will be necessary to establish confidently what are likely to be complex mechanisms and how release of endogenous NT contributes to the process of operant behavior extinction associated with reward seeking.
In summary (Table 1), the present studies suggest that in rats the effect of extinction on basal ganglia NT systems is opposite that caused by METH SA, in that extinction through D2 mechanisms appears to release NT and lower NT levels in most of the associated structures, which in turn diminishes the operant behavior associated with infusions of METH (i.e., lever pressing).
Acknowledgments
The authors appreciate the gift of the methamphetamine from the National Institute on Drug Abuse and the PD149163 compound from the National Institute of Mental Health.
Abbreviations
- CNS
central nervous system
- DA
dopamine
- METH
methamphetamine
- NT
neurotensin
- NTR1
neurotensin receptor 1
- PD149163
Lys(CH2NH)Lys-Pro,Trp-tert-Leu-Leu-Oet
- SA
self-administration
- SAM
self-administration of methamphetamine
- SCH23390
R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrohydro-1H-3-benzazepine hydrochloride
- SR48692
2-[(1-(7-chloro-4-quinolinyl)-5-(2,6-imethoxyphenyl)pyrazol-3-yl)carbonylamino]tricyclo(3.3.1.1.(3.7))decan-2-carboxylic acid
- YM
yoked methamphetamine
- YS
yoked saline
Authorship Contributions
Participated in research design: Hanson, Hoonakker, McFadden, Frankel, Alburges.
Conducted experiments: Hoonakker, Robson, McFadden.
Performed data analysis: Hanson, Hoonakker, McFadden.
Wrote or contributed to the writing of the manuscript: Hanson, McFadden, Alburges.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA031883, DA000378, DA013367].
References
- Adams DH, Hanson GR, Keefe KA. (2001) Differential effects of cocaine and methamphetamine on neurotensin/neuromedin N and preprotachykinin messenger RNA expression in unique regions of the striatum. Neuroscience 102:843–851 [DOI] [PubMed] [Google Scholar]
- Antonelli T, Tomasini MC, Fuxe K, Agnati LF, Tanganelli S, Ferraro L. (2007) Receptor-receptor interactions as studied with microdialysis. Focus on NTR/D2 interactions in the basal ganglia. J Neural Transm 114:105–113 [DOI] [PubMed] [Google Scholar]
- Baldessarini R (1996) Drugs and the treatment of psychiatric disorders, in Pharmacological Basis of Therapeutics (Hardman J and Limbird L eds) pp 399–459, McGraw Hill Publishers, New York. [Google Scholar]
- Castel MN, Morino P, Frey P, Terenius L, Hökfelt T. (1993) Immunohistochemical evidence for a neurotensin striatonigral pathway in the rat brain. Neuroscience 55:833–847 [DOI] [PubMed] [Google Scholar]
- Castel MN, Morino P, Dagerlind A, Hökfelt T. (1994a) Up-regulation of neurotensin mRNA in the rat striatum after acute methamphetamine treatment. Eur J Neurosci 6:646–656 [DOI] [PubMed] [Google Scholar]
- Castel MN, Morino P, Nylander I, Terenius L, Hökfelt T. (1994b) Differential dopaminergic regulation of the neurotensin striatonigral and striatopallidal pathways in the rat. Eur J Pharmacol 262:1–10 [DOI] [PubMed] [Google Scholar]
- Dearry A, Gingrich JA, Falardeau P, Fremeau RT, Jr, Bates MD, Caron MG. (1990) Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature 347:72–76 [DOI] [PubMed] [Google Scholar]
- Ervin GN, Birkemo LS, Nemeroff CB, Prange AJ., Jr (1981) Neurotensin blocks certain amphetamine-induced behaviours. Nature 291:73–76 [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Murray RJ, Tina Tran DN, Rullan MA, Shilling PD. (2008) The reversal of amphetamine-induced locomotor activation by a selective neurotensin-1 receptor agonist does not exhibit tolerance. Psychopharmacology (Berl) 200:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Fuxe K, Agnati LF, Mazza R, Tanganelli S, Antonelli T. (2007) Mesolimbic dopamine and cortico-accumbens glutamate afferents as major targets for the regulation of the ventral striato-pallidal GABA pathways by neurotensin peptides. Brain Res Brain Res Rev 55:144–154 [DOI] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. (2011) Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther 336:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Hanson GR, Bush L, Keefe KA, Alburges ME. (2005) Differential neurotensin responses to low and high doses of methamphetamine in the terminal regions of striatal efferents. Eur J Pharmacol 522:47–54 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432 [DOI] [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Alburges ME, McFadden LM, Robson CM, Frankel PS. (2012) Response of limbic neurotensin systems to methamphetamine self-administration. Neuroscience 203:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Singh N, Merchant K, Johnson M, Bush L, Gibb JW. (1992) Responses of limbic and extrapyramidal NT systems to stimulants of abuse. Ann N Y Acad Sci 668:165–172 [DOI] [PubMed] [Google Scholar]
- Hanson GR, Smiley P, Johnson M, Letter A, Bush L, Gibb JW. (1989) Response by the neurotensin systems of the basal ganglia to cocaine treatment. Eur J Pharmacol 160:23–30 [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Melega WP, Cho AK, Segal DS. (1997) Extracellular dopamine and amphetamine after systemic amphetamine administration: comparison to the behavioral response. J Pharmacol Exp Ther 282:591–596 [PubMed] [Google Scholar]
- Letter AA, Merchant KM, Gibb JW, Hanson GR. (1987) Effect of methamphetamine on neurotensin concentrations in rat brain regions. J Pharmacol Exp Ther 241:443–447 [PubMed] [Google Scholar]
- Liang Y, Boules M, Shaw AM, Williams K, Fredrickson P, Richelson E. (2008) Effect of a novel neurotensin analog, NT69L, on nicotine-induced alterations in monoamine levels in rat brain. Brain Res 1231:6–15 [DOI] [PubMed] [Google Scholar]
- Liang Y, Boules M, Li Z, Williams K, Miura T, Oliveros A, Richelson E. (2010) Hyperactivity of the dopaminergic system in NTS1 and NTS2 null mice. Neuropharmacology 58:1199–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant KM, Hanson GR, Dorsa DM. (1994) Induction of neurotensin and c-fos mRNA in distinct subregions of rat neostriatum after acute methamphetamine: comparison with acute haloperidol effects. J Pharmacol Exp Ther 269:806–812 [PubMed] [Google Scholar]
- Merchant KM, Bush LG, Gibb JW, Hanson GR. (1989a) Dopamine D2 receptors exert tonic regulation over discrete neurotensin systems of the rat brain. Brain Res 500:21–29 [DOI] [PubMed] [Google Scholar]
- Merchant KM, Gibb JW, Hanson GR. (1989b) Role of dopamine D-1 and D-2 receptors in the regulation of neurotensin systems of the neostriatum and the nucleus accumbens. Eur J Pharmacol 160:409–412 [DOI] [PubMed] [Google Scholar]
- Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. (2005) Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry 13:141–154 [DOI] [PubMed] [Google Scholar]
- Norman C, Beckett SR, Spicer CH, Ashton D, Langlois X, Bennett GW. (2008) Effects of chronic infusion of neurotensin and a neurotensin NT1 selective analogue PD149163 on amphetamine-induced hyperlocomotion. J Psychopharmacol 22:300–307 [DOI] [PubMed] [Google Scholar]
- Paxinos P, Watson C. (1982) The Rat Brain in Stereotaxic Coordinates, Academic Press, New York [Google Scholar]
- Pereira FC, Lourenço E, Milhazes N, Morgadinho T, Ribeiro CF, Ali SF, Macedo TR. (2006) Methamphetamine, morphine, and their combination: acute changes in striatal dopaminergic transmission evaluated by microdialysis in awake rats. Ann N Y Acad Sci 1074:160–173 [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Liebling CN, Reiszel C, Hooker JM, Brodie JD, Dewey SL. (2009) Cue-induced dopamine release predicts cocaine preference: positron emission tomography studies in freely moving rodents. J Neurosci 29:6176–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. (2007) The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 194:321–331 [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW. (2008) Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement. J Pharmacol Exp Ther 325:556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff JD, Gibb JW, Hanson GR. (1996a) Microdialysis assessment of methamphetamine-induced changes in extracellular neurotensin in the striatum and nucleus accumbens. J Pharmacol Exp Ther 278:547–554 [PubMed] [Google Scholar]
- Wagstaff JD, Gibb JW, Hanson GR. (1996b) Dopamine D2-receptors regulate neurotensin release from nucleus accumbens and striatum as measured by in vivo microdialysis. Brain Res 721:196–203 [DOI] [PubMed] [Google Scholar]
- Wagstaff JD, Bush LG, Gibb JW, Hanson GR. (1994) Endogenous neurotensin antagonizes methamphetamine-enhanced dopaminergic activity. Brain Res 665:237–244 [DOI] [PubMed] [Google Scholar]