Abstract
Our previous research showed that enolates formed from 1,3-dicarbonyl compounds, such as 2-acetylcyclopentanone (2-ACP), could provide protection in cell culture models from electrophile- or oxidative stress-induced toxicity. In the present study, we evaluated the protective abilities of 2-ACP in a mouse model of acetaminophen (APAP) hepatotoxicity. Results show that oral APAP overdose (500 mg/kg) was nearly 90% lethal within 72 hours and that the resulting hepatotoxicity was associated with substantial changes in plasma liver enzyme activities, histopathological indices, and markers of hepatocyte oxidative stress. 2-ACP administered intraperitoneally 20 minutes before APAP completely prevented lethality over a 7-day observation period. This effect was dose-dependent (0.80–2.40 mmol/kg) and was correlated with normalization of measured parameters. Nearly complete protection was afforded when 2-ACP was administered 20 minutes post-APAP, but not 60 minutes after intoxication. Although intraperitoneal administration of N-acetylcysteine (NAC) was not effective over a broad dose range (2.40–7.20 mmol/kg), temporal studies indicated that intraperitoneal NAC was hepatoprotective when injected 60 minutes after APAP intoxication. Because of a loss of function in stomach acid, oral administration of 2-ACP was associated with modest APAP protection. In contrast, NAC administered orally provided dose-dependent (0.80–2.40 mmol/kg) protection against APAP hepatotoxicity. In chemico studies and quantum mechanical calculations indicated that 2-ACP acted as a surrogate nucleophilic target for the reactive electrophilic APAP metabolite N-acetyl-p-benzoquinone imine. Our findings suggest that 2-ACP or a derivative might be useful in treating acquired toxicities associated with electrophilic drugs and metabolites or environmental toxicants.
Introduction
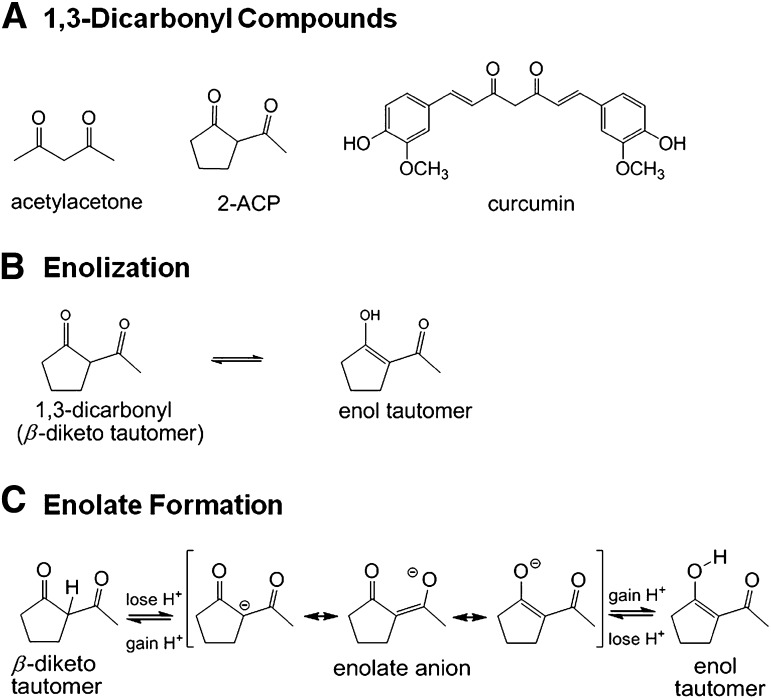
Recent studies (LoPachin et al., 2011) showed that enolates formed from 1,3-dicarbonyl compounds (Fig. 1A), such as acetylacetone (AcAc) and 2-acetylcyclopentanone (2-ACP), are cytoprotective in cell culture models from oxidative stress (H2O2) or electrophile (acrolein)-induced toxicity. The idea that these β-diketones might be cytoprotective stems from the recognition that the central heptadienone bridge of curcumin (Fig. 1A) is also a dicarbonyl compound and that, although the contributing mechanism is uncertain, the presence of this substructure is critically important for cytoprotection (Weber et al., 2006; Begum et al., 2008; see detailed discussion in LoPachin et al., 2011).
Fig. 1.
Line structures and ionization of 1,3-dicarbonyl compounds. (A) Line structures for acetylacetone, 2-acetylcyclopentanone (2-AC), and curcumin. (B) Schematic diagram illustrating the existence of 2-ACP as equilibrating keto-enol tautomers. (C) Schematic diagram showing that loss of a proton from either the central (“α”) carbon in the diketo tautomer of 2-ACP or the hydroxyl group of the enol isomer yields the same resonance-stabilized enolate anion.
1,3-Dicarbonyls exist as equilibrating tautomers (Fig. 1B) with the enol-containing isomers generally predominating (Balasubramanian, 2006; Payton et al., 2007). In ionizing solutions such as biologic buffers, loss of a proton from either the central (“α”) carbon of the diketo tautomer or the enol hydroxyl group of the isomer yields a resonance-stabilized nucleophilic enolate anion (Fig. 1C). Enolate sites on dicarbonyl compounds are Michael donors that can form covalent adducts with electrophiles (Awasthi et al., 1996; Loudon, 2002; Bug and Mayr, 2003). Indeed, recent studies of oxidative stress (LoPachin et al., 2011) showed that although free radical trapping was not involved, enolate-forming 1,3-dicarbonyl compounds provided cytoprotection by scavenging electrophilic α,β-unsaturated carbonyl derivatives (e.g., acrolein, 4-hydroxy-2-nonenal) that participate in the corresponding cell injury process.
The enolate of a 1,3-dicarbonyl compound can also function as a bidentate chelator of iron [Fe(III)], copper [Cu(II)], and other electrophilic metal ions (Jiao et al., 2006; Eames, 2009). In our studies of oxidative stress (LoPachin et al., 2011), we provided evidence that the metal chelating abilities of 2-ACP and AcAc were important components of 1,3-dicarbonyl cytoprotection. Metal ion chelation can be cytoprotective by limiting the participation of iron and copper in the metal-catalyzed Fenton reaction, which reduces generation of the highly toxic hydroxyl radical (Halliwell, 2006) and thereby blocks the oxidative stress cascade. Therefore, the 1,3-dicarbonyl compounds have at least two cytoprotective actions specifically linked to their ability to form enolate moieties, i.e., electrophile scavenging and metal ion chelation. These properties are the mechanistic basis of cytoprotection (LoPachin et al., 2011, 2012).
Our previous investigations identified 2-ACP as the most potent 1,3-dicarbonyl compound with respect to in vitro cytoprotection (LoPachin et al., 2011). However, the protective capacity of this type of compound has not been evaluated in an animal model. Therefore, in the present study, we determined the ability of 2-ACP to prevent hepatotoxicity in a mouse model of acetaminophen (Tylenol; APAP) poisoning. Experimental APAP intoxication in laboratory animals is a clinically relevant model that has been used to assess putative cytoprotectants (Jaeschke et al., 2011). Moreover, although some information gaps remain, the molecular mechanism of APAP hepatoxicity has been investigated extensively and is relatively well understood. Specifically, in overdose, excess acetaminophen is metabolized by the liver cytochrome P450 system to a highly reactive electrophile metabolite, N-acetyl-p-benzoquinone imine, which causes hepatocyte damage via glutathione (GSH) depletion, covalent modification of mitochondrial proteins, and oxidative stress (Jaeschke and Bajt, 2006; Hinson et al., 2010). The primary involvement of an electrophilic metabolite and development of secondary oxidative stress suggests that APAP intoxication in mice might be an appropriate model for testing the in vivo protective properties of 2-ACP.
Materials and Methods
Reagents.
All chemicals were of the highest grade commercially available and, unless otherwise stated, were purchased from Sigma-Aldrich (St. Louis, MO). Pierce bicinchoninic acid (BCA) protein assay kits and radioimmunoprecipitation assay (RIPA) buffer were purchased from Thermo Fisher Scientific Inc. (Rockford, IL). Protease inhibitor cocktail was purchased from Roche (Indianapolis, IN).
Animals and Treatments.
All aspects of animal use in this study were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Montefiore Medical Center Animal Care Committee. Three-month-old male C57BL/6N mice (mean weight 27 g) were purchased from Charles River Laboratory (Wilmington, MA). Mice were housed individually in polycarbonate boxes, and filtered drinking water and Purina Rodent Laboratory Chow (Purina Mills, Inc., St. Louis, MO) were available ad libitum. The animal room was maintained at approximately 22°C and 50% humidity with a 12-hour light/dark cycle. Before each experiment, mice were fasted overnight and treatments began at 8:00 AM the next morning. Food was returned 1 hour post-treatment. APAP and all experimental compounds were administered in phosphate-buffered polyethylene glycol. Preliminary studies demonstrated that this vehicle did not affect the experimental outcome. As a general protocol, groups of mice (n = 15) were pretreated by intraperitoneal injection (10 ml/kg) of either 2-ACP (2.4 mmol/kg) or equimolar N-acetylcysteine (NAC) followed 20 minutes later by oral (4 ml/kg) administration of APAP (500 mg/kg). Animals in a separate group were given intraperitoneal injections of vehicle followed 20 minutes later by oral administration of APAP. Control mice received an intraperitoneal/oral sequence of vehicle injections. Range-finding studies demonstrated that acute intraperitoneal or oral doses of 2-ACP up to 7.2 mmol/kg did not produce gross toxicity or lethality over a 7-day observation period (see also Ballantyne and Cawley, 2001). The doses of NAC used in this study were based on previous published experiments (Corcoran et al., 1985a; Saito et al., 2010). The general health and survival of mice in the different treatment groups were followed by a blinded observer for 7 days postintoxication. Kaplan-Meier survival curves were used to illustrate the cumulative percent daily lethality of mice in different experimental groups and were generated in Prism 6.0 (GraphPad Software, San Diego, CA). In separate studies, animals were divided according to the previously defined groups (n = 15–30/group) and were sacrificed at 2, 6, 24, 48, and 168 hours after APAP or vehicle administration. At the selected times, mice were killed in a carbon dioxide chamber and decapitated, and blood was collected. Livers were excised and weighed, and small tissue samples were fixed in 10% phosphate-buffered formalin. Livers were then frozen in liquid nitrogen for later biochemical analyses. In subsequent studies, we determined the dose- (2-ACP = 0.80–2.40 mmol/kg; NAC = 0.80–7.20 mmol/kg) and route- (intraperitoneal vs. oral) dependent characteristics of the hepatoprotection provided by 2-ACP and NAC in the APAP model. Finally, we determined that the temporal characteristics of hepatoprotection; i.e., hepatoprotectant, was administered at various times before (–20 minutes) or after (+20 or +60 minutes) APAP intoxication.
Hepatoxicity Parameters and Histopathological Analyses.
To assess APAP-induced hepatocyte damage, the temporal appearances of the liver-specific enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), in plasma were measured. In addition, we determined serum levels of lactate dehydrogenase (LDH) as a measure of general cell damage. Blood was collected in heparin-coated tubes (1.5-ml; BD Biosciences, Franklin Lakes, NJ), and serum samples were obtained by centrifugation (14,000g for 5 minutes). Samples were subsequently analyzed by an automated analyzer (Hitachi Modular Automated Clinical Chemistry Analyzer; Roche Diagnostics, Indianapolis, IN) and expressed as international units per liter of plasma.
As indices of hepatocyte oxidative stress, soluble thiols and the aldehyde byproducts of lipid peroxidation, 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA), were measured in liver homogenates at several time points (2–168 hours) after APAP intoxication or vehicle administration. For aldehyde measurements, frozen livers were pulverized, and samples (2-g) were added to 2.5 ml of RIPA buffer containing protease inhibitor cocktail and butylated hydroxytoluene (BHT; 5 mM). Tissue samples were homogenized in a Dounce tissue grinder (10 strokes), and the homogenate was centrifuged at 500g (4°C) for 15 minutes to remove cellular debris. The pellet was washed once in RIPA buffer (2.5 ml), and the supernatant (S2) was combined with the S1 supernatant. Aldehyde concentrations were determined in aliquots of liver homogenates (1 mg of protein) by the spectrophotometric method of Gerard-Monnier et al. (1998). In brief, an aliquot of sample (200 μl) was added to 650 μl of 1-methyl-2-phenylindole in an acetonitrile/methanol (3:1) mixture. The reaction was started by adding 150 μl of 12 N hydrochloric acid. Absorbance (586 nm) was measured after incubation of the reaction mixture at 45°C for 60 minutes. The final absorbance was used to determine MDA concentrations (extinction coefficient = 110,000 M−1 cm−1) based on a standard curve for 1,1,3,3-tetramethoxypropane (TMP; a source of MDA). To determine the respective HNE concentrations, parallel samples (200 μl) were added to the 1-methyl-2-phenylindole mixture, and the reaction was started by adding 150 μl of methanesulfonic acid (37%) containing 100 μM Fe(III). Absorbance (586 nm) was measured after incubation at 45°C for 60 minutes. The final absorbance is a linear function of both the HNE and MDA concentrations, and therefore, the HNE content can be derived by subtracting the previously determined MDA concentration from the combined unsaturated aldehyde content.
To measure sulfhydryl contents in liver supernatants, hepatocyte proteins were precipitated (1:1 v/v methanol/acetonitrile) and then removed by centrifugation at 13,000g (4°C) for 15 minutes. This procedure simplifies the analysate and preserves acid-labile N-acetyl-p-benzoquinone imine (NAPQI)-sulfhydryl adducts on proteins and small molecular weight thiol molecules (Gerard-Monnier et al., 1998; Hou et al., 2004). Methanol/acetonitrile-soluble sulfhydryl contents were measured by a colorimetric method using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) as previously modified by LoPachin et al. (2009). Data are expressed as micromoles per milligram protein ± S.E.M. In groups of control mice (n = 3/time point) that received an intraperitoneal/oral sequence of vehicle injections (see above), the thiol and unsaturated aldehyde parameters were measured on a temporal basis (2–168 hours). For each parameter, the respective control data among individual time points did not differ statistically and therefore were pooled.
For histological analyses, liver samples were excised from mice in all experimental groups at the 24-hour post-APAP time point. Samples were fixed in 10% buffered formalin solution, paraffin embedded, sectioned (5-μm), and stained with hematoxylin and eosin. Sections were examined by a blinded observer for evidence of inflammation, fatty changes, and hepatocellular necrosis, nuclear pleomorphism, and vacuolization.
In Chemico Studies.
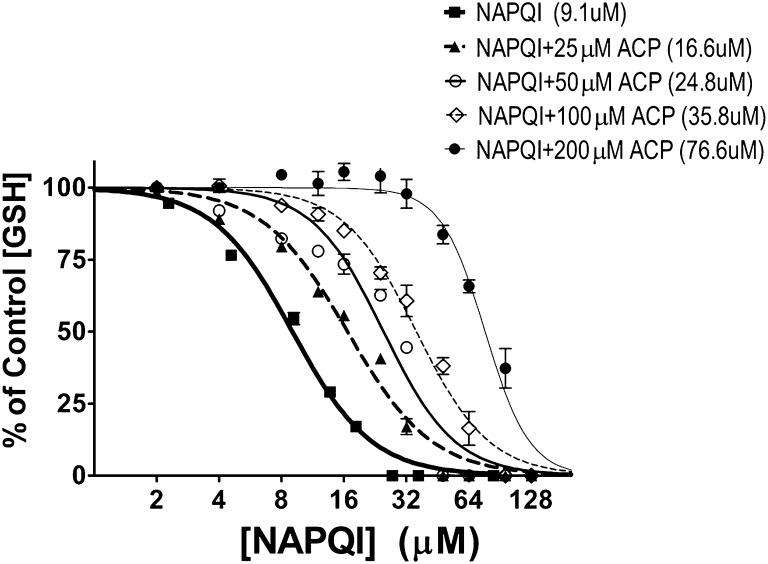
Initial in chemico studies determined the relative concentration-dependent abilities of selected quinone and α,β-unsaturated carbonyl derivatives to deplete GSH sulfhydryl groups (Table 1). Graded concentrations (2–128 μM; 8 μM–1.6M) of electrophile were incubated (15 minutes) in phosphate-buffered saline (pH 7.4, 25°C) with GSH (30 μM). At the end of the incubation period, remaining sulfhydryl content was measured spectrophotometrically via the 5,5′-dithiobis(2-nitrobenzoic acid) method of LoPachin et al. (2009). To assess the concentration-dependent ability of 2-ACP to prevent thiol loss (Fig. 10), graded NAPQI concentrations (2–128 μM) were incubated (15 minutes) in phosphate-buffered saline (pH 7.4, 25°C) with different concentrations of 2-ACP (25–200 μM). At the end of the preincubation period, GSH (30 μM) was added, and sulfhydryl content was measured after 15 minutes via the DTNB method of LoPachin et al. (2009). Thiol data were fitted by nonlinear regression analyses (r2 for all curves ≥ 0.90), and electrophile concentrations that produced 50% thiol loss (IC50 values) and their 95% confidence intervals were calculated by the sigmoidal dose-response equation (variable slope; GraphPad Prism).
TABLE 1.
Softness, electrophilicity, and IC50 values for quinone and α,β-unsaturated carbonyl derivatives
Softness (σ) and electrophilicity (ω) for the selected compounds were calculated as described under Materials and Methods. The respective IC50 values represent in vitro electrophile concentrations that produce 50% thiol loss and reflect the relative electrophilic potency of each chemical.
| Electrophile | σ | ω | IC50 |
|---|---|---|---|
| × 10–3 ev–1 | ev | μM | |
| NAPQI | 500 | 6.83 | 9.1 |
| pBQ | 524 | 7.78 | 11.6 |
| tBQ | 505 | 7.20 | 17.5 |
| HNE | 393 | 3.78 | ND |
| Acrolein | 379 | 3.57 | 52.5 |
| Acrylamide | 346 | 2.62 | 436,515 |
tBQ, 2-tert-butyl-1,4-benzoquinone
Fig. 10.
To assess the concentration dependency of 2-ACP thiol protection, graded NAPQI concentrations (2–128μM) were incubated (15 minutes) with different concentrations of 2-ACP (25–200 μM). At the end of the preincubation period, GSH (30 μM) was added, and sulfhydryl content was measured after 15 minutes by the DTNB method. Data are expressed as mean percent control ± S.E.M. (n = 3–4 experiments). Calculated IC50 values are provided in parentheses.
Calculations of Hard and Soft Acid Base Parameters.
The Lowest Unoccupied Molecular Orbital (LUMO) energy (ELUMO) and Highest Occupied Molecular Orbital (HOMO) energy (EHOMO) were determined using Spartan’08 (version 1.1.1) software (Wavefunction Inc., Irvine CA). For each structure, ground state equilibrium geometries were calculated with Density Functional B3LYP 6-31G* in water starting from 6-31G* geometries. Global (whole molecule) hardness (η) was calculated as η = (ELUMO – EHOMO)/2, and softness (σ) was calculated as the inverse of hardness (i.e., σ = 1/η). The electrophilicity index (ω) was calculated as ω = μ2/2η, where μ is chemical potential of the electrophile [μ = (ELUMO + EHOMO)/2]. An index of nucleophilicity (ω–; see LoPachin et al., 2012 for more detailed discussion) was calculated as ω− = ηA(μA – μB)2/2(ηA + ηB)2, where A = reacting nucleophile and B = NAPQI (μ = –5.235 ev, η = 2.005 ev).
Statistical Analyses.
All statistical analyses were conducted using Prism 6.0 (GraphPad Software) with significance set at the 0.05 level of probability. In studies evaluating the relative abilities of potential hepatoprotectants to modify APAP lethality, the Mantel-Cox log-rank test was used to compare survival rates among the experimental groups. For analysis of liver enzymes in serum and oxidative stress parameters, statistically significant differences between group mean data were determined by a Bonferroni test for multiple comparisons. IC50 values for electrophiles in the in chemico studies were compared statistically by Student’s t test.
Results
Effects of Intraperitoneal 2-ACP or NAC on Oral APAP-Induced Lethality.
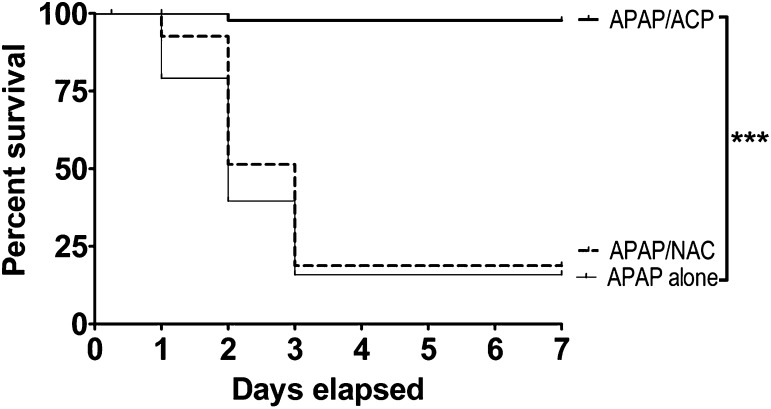
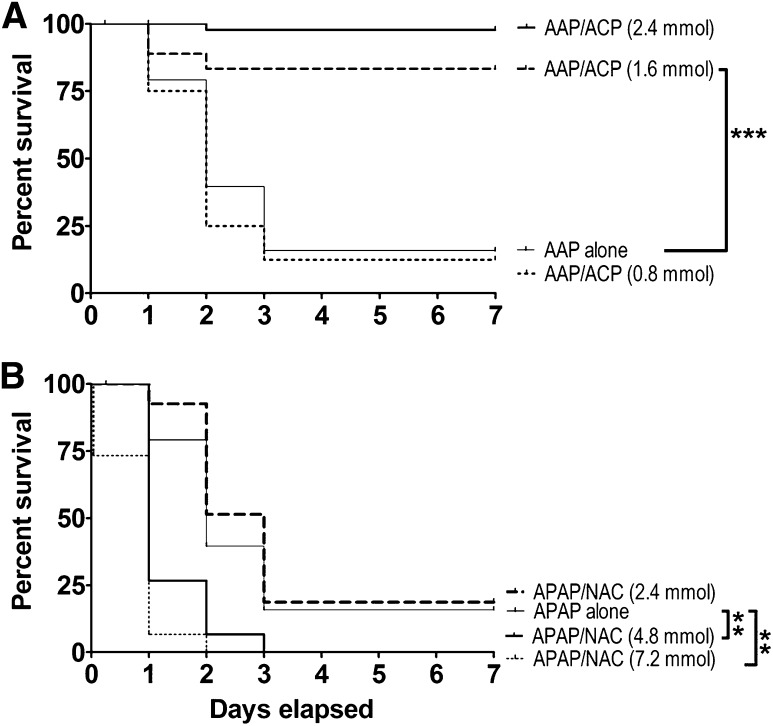
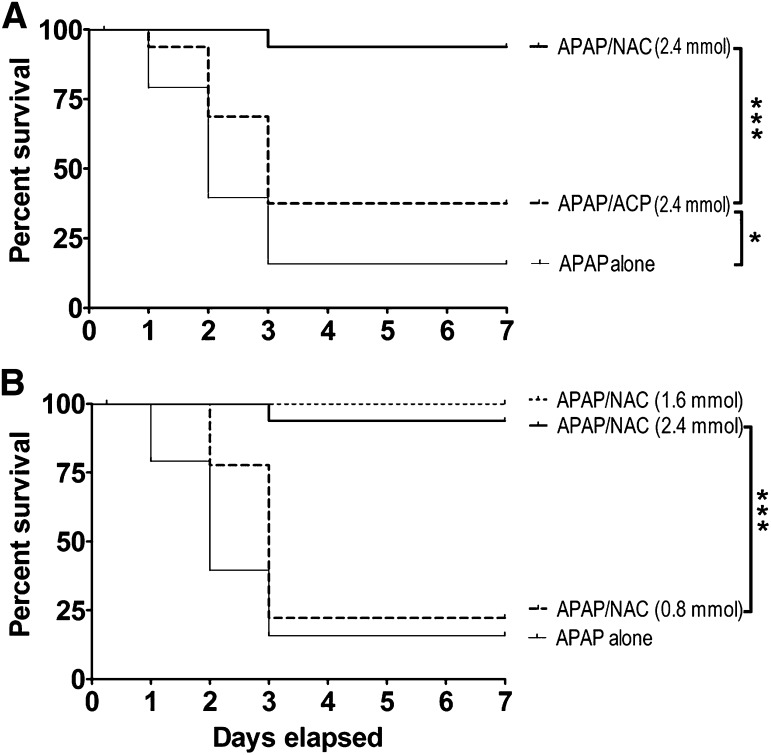
The Kaplan-Meier survival curves presented in Fig. 2 demonstrate that mice in the APAP only and the APAP/NAC treatment groups followed a similar cumulative rate of lethality. Specifically, in both groups approximately 10–20% of mice died within the first 24 hours (day 1) after acute oral APAP administration. In contrast, all mice in the intraperitoneal 2-ACP treatment group survived during this initial period. Over the next 24-hour period (day 2), substantial cumulative lethality was noted in both the APAP-intoxicated (62% lethality) and NAC/APAP (55% lethality) groups, whereas in the 2-ACP treatment group, lethality was limited to a single death (97%). By day 3, mice continued to die in both the APAP-intoxicated and NAC/APAP group (∼85% cumulative lethality). However, no additional deaths occurred in the 2-ACP treatment group. Figure 2 shows that, over the next 96 hours (days 4–7), the percent survival in all groups remained constant. Statistical analyses of the respective survival curves revealed that 2-ACP provided nearly complete protection against the hepatotoxic effects of oral APAP, whereas intraperitoneal administration of NAC failed to produce significant protection.
Fig. 2.
Effects of i.p. 2-ACP (2.40 mmol/kg) or equimolar NAC on oral APAP- (500 mg/kg) induced hepatotoxicity in mice (n = 15–30/group). Kaplan-Meier survival curves illustrate the cumulative percent daily lethality in the APAP alone, APAP/NAC and APAP/ACP groups. Joining line indicates statistically significant differences in treatment groups at ***P < 0.001 level of significance.
Effects of Protectants on APAP-Induced Changes in Hepatocyte Biomarkers.
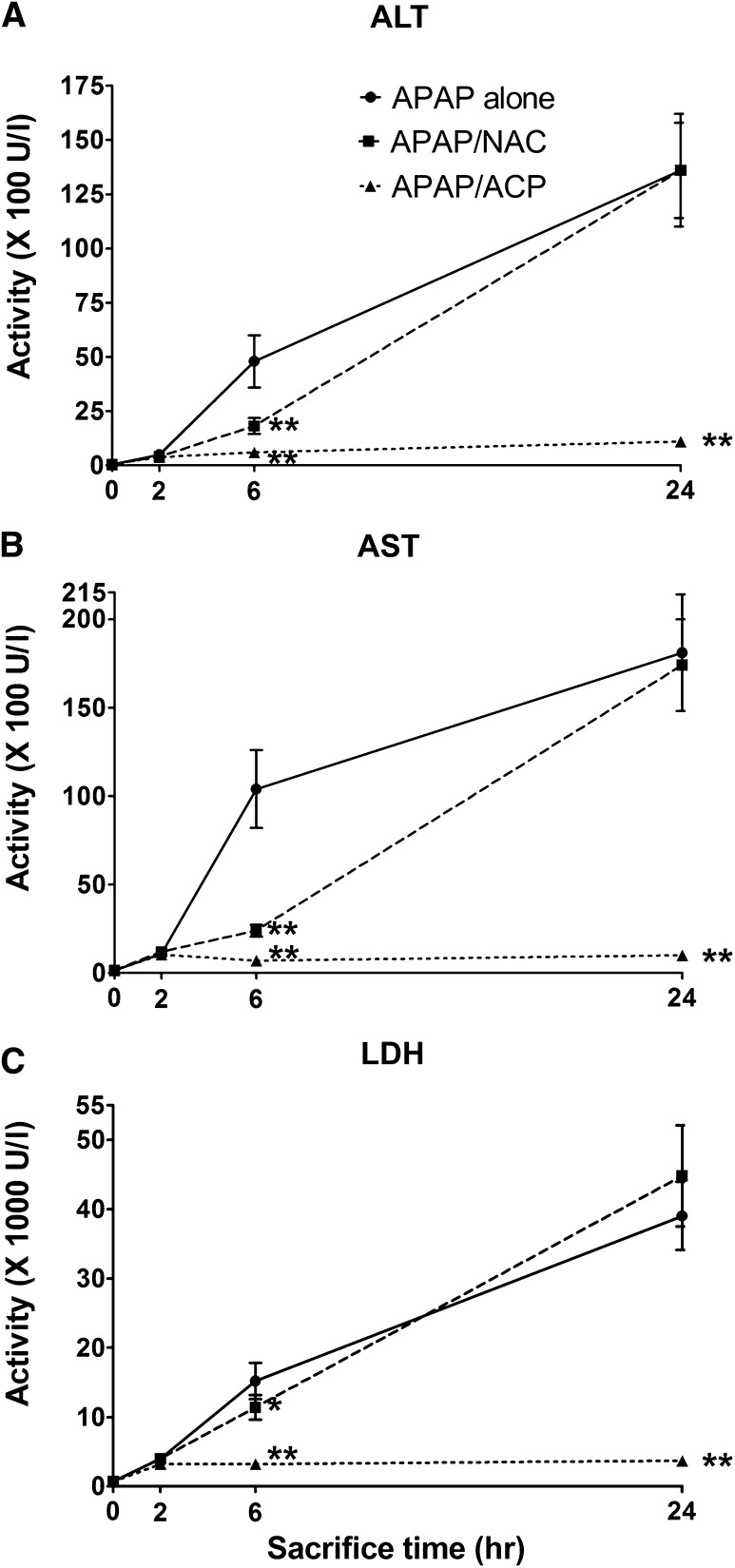
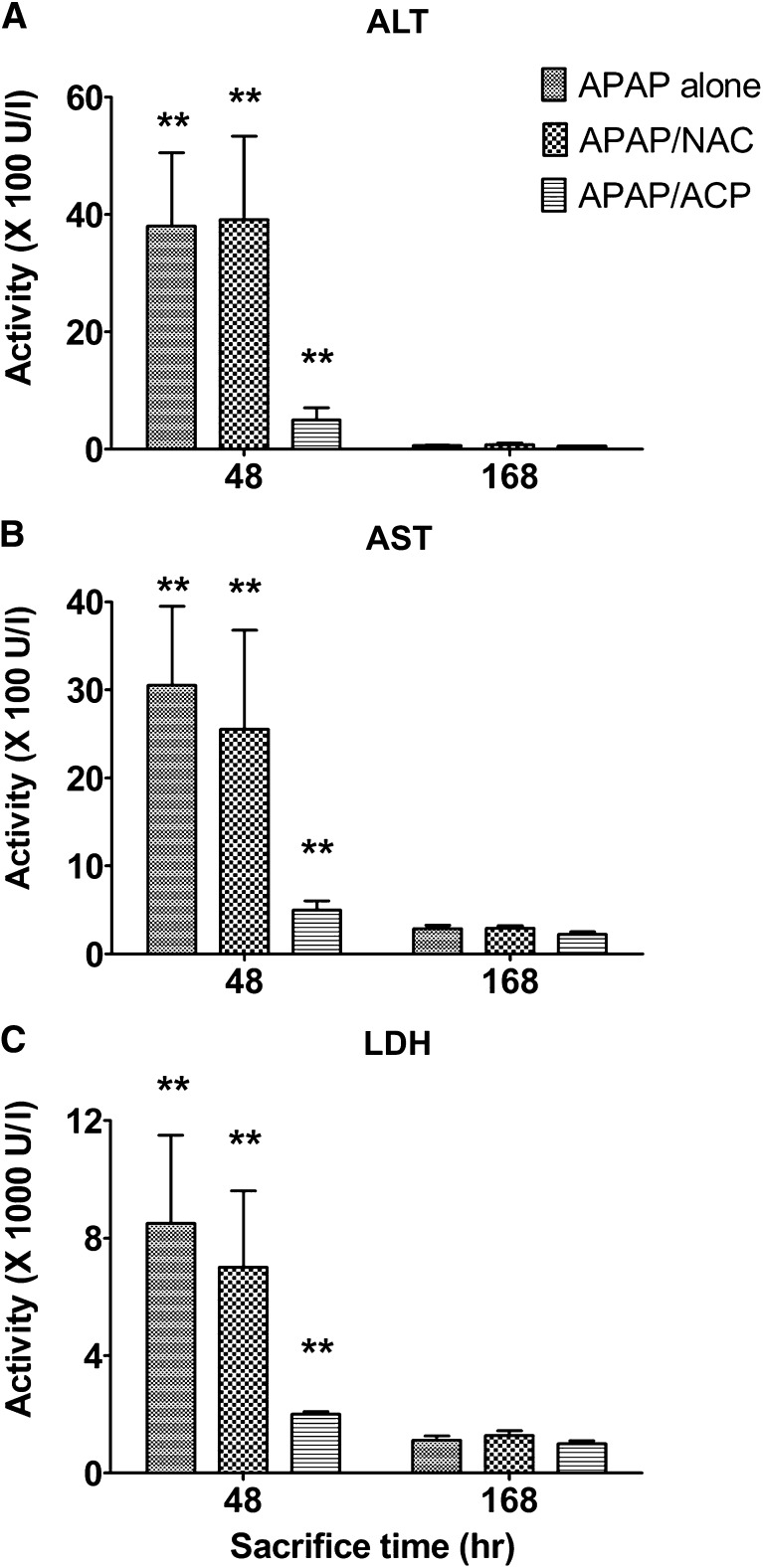
The lethality associated with APAP intoxication (Fig. 2) corresponded to the progressive derangement of both serum biomarkers (ALT, AST, and LDH) and indicators of oxidative stress (aldehyde accumulation and thiol loss). During the initial 2 hours post-APAP intoxication, serum biomarker levels rose slowly (Fig. 3, A–C). Thereafter, biomarker activity increased rapidly, and at the 24 time point, the mean peak levels achieved were remarkably elevated indicating significant hepatocyte damage (Agarwal et al., 2012; Jaeschke, 1990), e.g., mean (± S.E.M.) 24-hour AST levels were 18,100 ± 3300 IU/l, which represents more than a 110-fold increase relative to the pooled control, 160 ± 20 IU/l (Fig. 3A). At 48 hours (day 2), mean serum biomarker levels decreased by approximately 80% relative to 24-hour values in surviving mice of the APAP-only group (Fig. 4, A–C). The return to control values probably reflects pooled measurements of those animals that will survive combined with those that will succumb by day 3. At 168 hours, or the 7-day experimental period, mean ALT levels were not different from control, whereas AST and LDH levels were slightly elevated relative to the pooled control data (Fig. 4, A–C).
Fig. 3.
Effects of intraperitoneal 2-ACP (2.40 mmol/kg) or equimolar NAC on the time course (≤24 hours) for the plasma appearance of LDH (C) and the liver enzymes, ALT (A), and AST (B), in APAP-intoxicated mice (n = 15/group) of the different experimental groups. Data are expressed as mean activity ± S.E.M. and **P < 0.01 and *P < 0.05 indicate levels of significance relative to APAP-alone group.
Fig. 4.
Effects of intraperitoneal 2-ACP (2.40 mmol/kg) or equimolar NAC on the time course (48–168 hours) for the plasma appearance of ALT (A), AST (B), and LDH (C) and the liver enzymes in APAP-intoxicated mice (n = 15/group) of the different experimental groups. Data are expressed as mean activity ± S.E.M. and **P < 0.01 indicate levels of significance relative to the vehicle-treated control.
2-ACP injected intraperitoneally 20 minutes before oral APAP intoxication (–20 minutes) prevented the APAP-induced serum changes in hepatic biomarkers. For example, the 2-ACP pretreatment group exhibited an initial (2 hours), relatively small but significant increase in mean AST compared with control (i.e., 970 ± 130 vs. 160 ± 20 IU/l, respectively). At the experimental time points over 6–48 hours, AST serum levels remained constant at approximately 500–700 IU/l, whereas at the 168-hour time point, AST level decreased to 220 ± 25 (Figs. 3B and 4B). In contrast, although NAC pretreatment slowed the APAP-induced rise in serum biomarkers up to 6 hours post-APAP intoxication, the corresponding 24-hour data were not statistically different from that of the APAP-alone group (Fig. 3, A–C). The 48–168-hour serum biomarker data (Fig. 4, A–C) for the NAC/APAP group did not differ statistically from the data of the APAP-alone group.
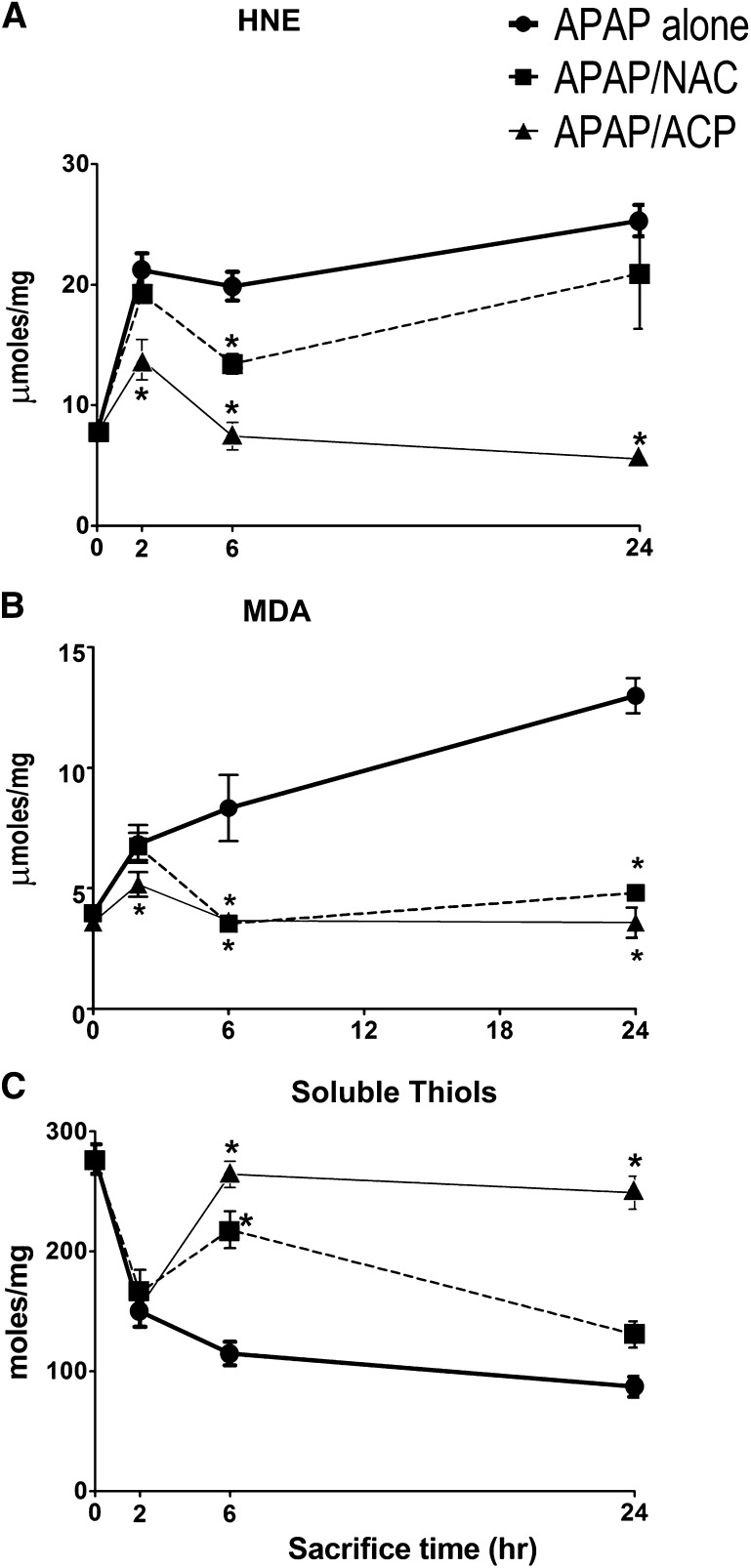
With respect to liver aldehyde concentrations in APAP-treated mice, HNE (Fig. 5A) and MDA (Fig. 5B) increased substantially at 2 hours and then continued to rise slowly over the next 22 hours. The hepatic thiol content of APAP-intoxicated mice exhibited a similar temporal pattern, i.e., early decreases at 2 hours followed by continuous slow thiol loss up to the 24-hour time point (Fig. 5C). Over the 48–168-hour experimental period, the liver thiol and aldehyde concentrations of surviving animals returned to control values (data not shown). Pretreatment (–20) with intraperitoneal 2-ACP (2.4 mmol/kg) substantially modified the APAP-induced changes in liver parameters. Thus, 2-ACP significantly reduced the initial (2-hour) increase in mean HNE (Fig. 5A) and MDA (Fig. 5B) concentrations and, by 6 hours post-APAP, aldehyde levels returned to control values. In contrast, NAC did not alter the initial (2-hour) rise in mean HNE (Fig. 5A) or MDA (Fig. 5B) but did significantly reduce the mean 6-hour data. At 24 hours post-APAP administration, mean liver HNE (Fig. 5A) and MDA (Fig. 5B) values in 2-ACP-pretreated animals remained similar to control, whereas in the NAC-treated group, aldehyde levels were increased (Fig. 5, A and B).
Fig. 5.
Effects of intraperitoneal 2-ACP (2.40 mmol/kg) and equimolar NAC on oral APAP- (500 mg/kg) induced changes in 4-hydroxy-2-nonenal (A), malondialdehyde (B), and soluble thiol (C) content in mouse liver homogenates (n = 10/group). Data are expressed as mean μmol/mg ± S.E.M. and *P < 0.05 indicates the level of significance relative to the APAP alone group.
With respect to APAP-induced thiol loss (Fig. 5C), 2-ACP pretreatment did not modify early (2-hour) thiol depletion. However, similar to the aldehydes, thiol levels were similar to control by the 6-hour time point. NAC pretreatment also did not alter the initial decline in soluble thiol levels and, despite returning to control values at 6 hours, by 24 hours significant thiol loss was noted (Fig. 5C). During the 48–168-hour experimental period, the liver thiol and aldehyde concentrations returned to control values in surviving animals of the 2-ACP and NAC pretreatment groups (data not shown).
Histopathology.
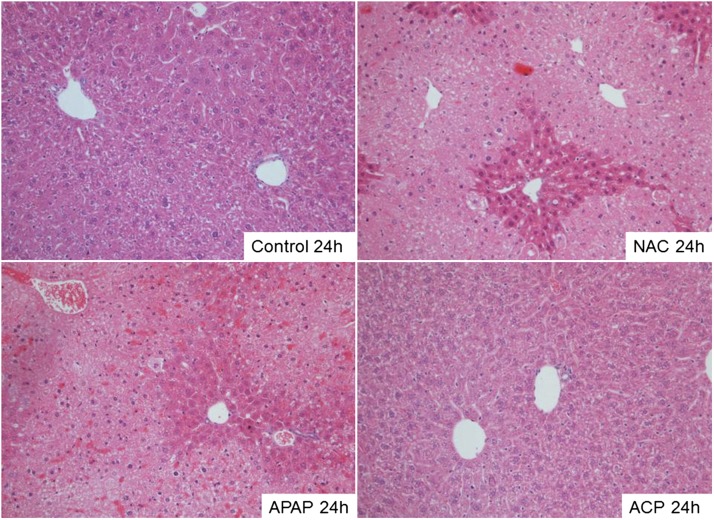
The liver histopathological changes noted in the different treatment groups corroborated the corresponding changes in the respective hepatic biomarkers. As expected, all sections from the control group exhibited unremarkable liver histology; i.e., necrosis and steatosis were not observed (Fig. 6A). Liver sections from the 24-hour APAP-only group demonstrated marked hepatocellular necrosis surrounding the central veins (Zone 3) and exhibited frequent bridging necrosis (Fig. 6B). In addition, a significant amount of microvesicular steatosis ranging from moderate to severe was observed. The morphologic pattern and degree of hepatocellular injury associated with APAP intoxication were indistinguishable from the level of tissue injury in the NAC-pretreated group (Fig. 6C). In contrast, the 2-ACP pretreatment group showed near-complete protection of hepatocytes (Fig. 6D).
Fig. 6.
Representative photomicrographs showing the effects of intraperitoneal 2-ACP (2.40 mmol/kg) and equimolar NAC on oral APAP- (500 mg/kg) induced histopathological changes in mouse liver. Tissue was excised at 24 hours post-APAP intoxication, fixed, and then stained with hematoxylin and eosin. Magnification, ×200.
Route, Dose, and Temporal Characteristics of 2-ACP Hepatoprotection.
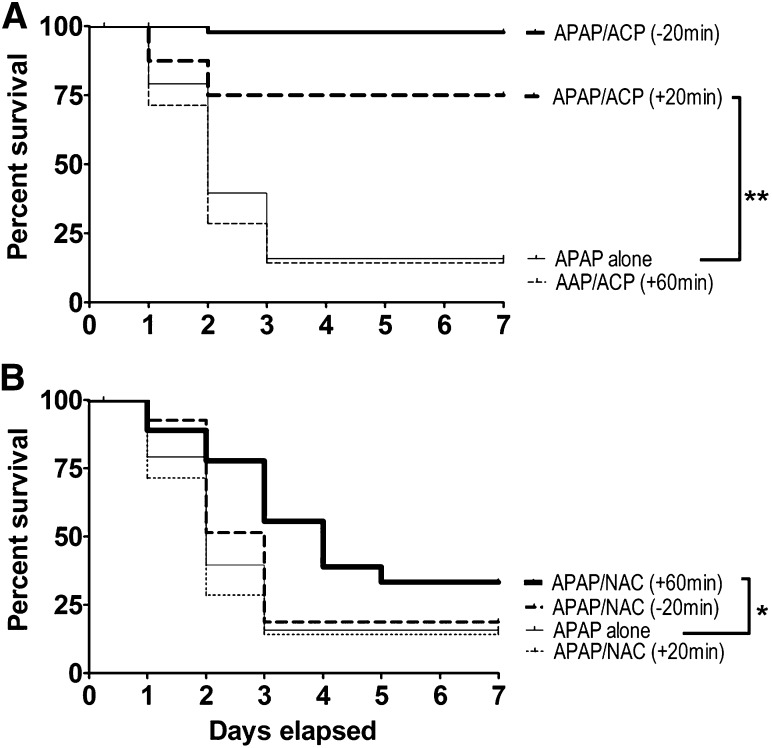
The findings presented in Fig. 2 indicated that intraperitoneal injection of 2-ACP (2.4 mmol/kg) given 20 minutes before (–20) an oral overdose of APAP (500 mg/kg) can completely prevent subsequent hepatotoxicity. In contrast, intraperitoneal pretreatment with an equimolar dose of NAC did not provide protection. Dose-dependent analyses (Fig. 7A) showed that, although intraperitoneal administration of 2-ACP at 0.8 mmol/kg was not protective, the 1.6 mmol/kg dose provided protection that was equivalent to that produced by the 2.4 mmol/kg dose. However, intraperitoneal NAC pretreatment over a broad dose range (4.8–7.2 mmol/kg) did not provide any level of hepatoprotection (Fig. 7B). In fact, the higher intraperitoneal NAC doses (4.80 and 7.20 mmol/kg) significantly accelerated the APAP lethality rate. To determine the temporal characteristics of hepatoprotection, 2-ACP or NAC was injected intraperitoneal at selected times after oral APAP intoxication. Results (Fig. 8A) show that 2-ACP (2.4 mmol/kg) administered intraperitoneally 20 minutes before (–20 minutes) or after (+20 minutes) APAP provided significant hepatoprotection, whereas equimolar administration after 60 minutes (+60 group) was completely ineffective. Relative to 2-ACP, intraperitoneal NAC administration exhibited the opposite temporal relationship of hepatoprotection (Fig. 8B); i.e., the +60 NAC group exhibited modest protection, whereas +20 administration did not prevent APAP hepatotoxicity.
Fig. 7.
(A) Dose dependency of intraperitoneal 2-ACP (0.80–2.40 mmol/kg) on oral APAP- (500 mg/kg) induced lethality. (B) Dose dependency of intraperitoneal NAC (2.40–7.20 mmol/kg) on oral APAP-induced lethality. Kaplan-Meier survival curves illustrate the cumulative percent daily lethality in the APAP-alone, APAP/NAC, and APAP/ACP groups (n = 15 mice/group). Joining line indicates statistically significant differences in treatment groups at ***P < 0.001 and **P < 0.01 levels of significance.
Fig. 8.
Temporal dependency of intraperitoneal (2.40 mmol/kg) 2-ACP (A) or equimolar NAC (B) on oral APAP- (500 mg/kg) induced lethality. Hepatoprotectants were administered 20 minutes before (–20), 20 minutes after (+20), or 60 minutes after (+60) APAP intoxication. Kaplan-Meier survival curves illustrate the cumulative percent daily lethality in the APAP-alone, APAP/NAC, and APAP/ACP groups (n = 15 mice/group). Joining line indicates statistically significant differences in treatment groups at **P < 0.01 and *P < 0.05 levels of significance.
To evaluate the route dependency of hepatoprotection, equimolar doses (2.4 mmol/kg) of 2-ACP or NAC were administered by oral gavage 20 minutes before (–20 groups) APAP overdose. Results (Fig. 9A) show that NAC pretreatment provided nearly complete protection against APAP hepatotoxicity, whereas 2-ACP was only modestly protective. This decreased effectiveness is probably related to the susceptibility of β-diketones to acid-catalyze reactions (e.g., aldol condensation) that can occur in the stomach. In support of this, we found that buffering the gavage solution (HEPES buffer, pH 9.0) or increasing the dose (4.80 mmol/kg) improved oral 2-ACP hepatoprotection (data not shown). Evaluation of dose dependency (Fig. 9B) revealed that the 2.4 and 1.6 mmol/kg NAC doses produced equivalent hepatoprotection, whereas the 0.8 mmol/kg dose was ineffective (Fig. 9B).
Fig. 9.
(A) Effects of oral 2-ACP (2.40 mmol/kg) or equimolar NAC on oral APAP- (500 mg/kg) induced lethality. (B) Dose dependency of oral NAC (0.80–2.40 mmol/kg) on oral APAP- (500 mg/kg) induced lethality. Kaplan-Meier survival curves illustrate the cumulative percent daily lethality in the APAP-alone, APAP/NAC, and APAP/ACP groups (n = 15 mice/group). Joining line indicates statistically significant differences in treatment groups at ***P < 0.001 and *P < 0.05 levels of significance.
Mechanism of 2-ACP Hepatoprotection.
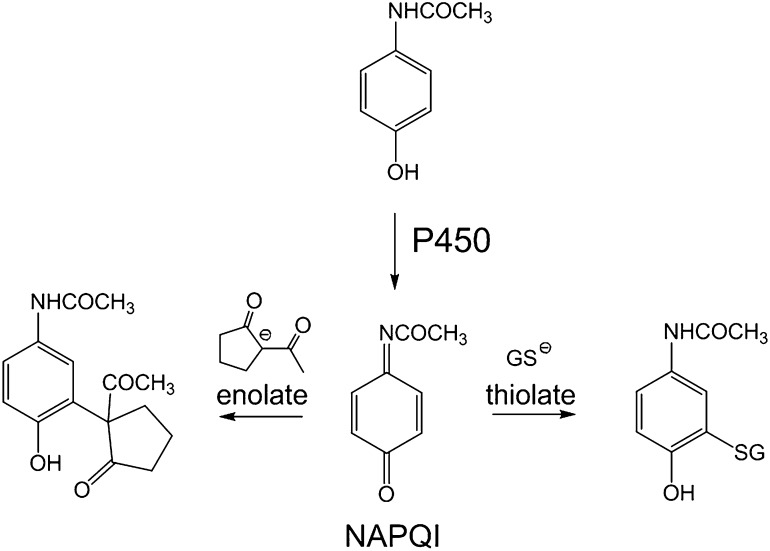
The active APAP metabolite NAPQI is an electrophile; therefore, we propose that 2-ACP provides hepatoprotection by acting as a surrogate nucleophile target for this metabolite (Fig. 11). Electrophilic toxicants, however, do not react arbitrarily with nucleophiles, and instead, these interactions are selective as predicted by the Hard and Soft, Acids and Bases (HSAB) theory. Thus, based on relative polarizability (electron mobility), electrophiles and nucleophiles are classified as being either soft (polarizable) or hard (nonpolarizable). In accordance with HSAB principles, toxic electrophiles will react preferentially with nucleophilic biologic targets of comparable softness or hardness. The designation of “hard” or “soft” is quantifiable based on corresponding inherent electronic characteristics that can be computed from the energies of the respective frontier molecular orbitals, i.e., the Highest Occupied Molecular Orbital (EHOMO) and the Lowest Unoccupied Molecular Orbital (ELUMO). These energies have been used to develop parameters that define the electrophilicity (ω) and nucleophilicity (ω–) of chemical species (reviewed in LoPachin et al., 2012; LoPachin and Gavin, 2012). Table 1 shows that NAPQI and related quinones are, in general, exceptionally soft and highly electrophilic chemicals (larger σ and ω values), whereas acrolein and other selected toxicologically relevant α,β-unsaturated carbonyl derivatives exhibit a significantly lower range of softness and electrophilicity (see also LoPachin et al., 2007a,b, 2009). As a soft electrophile, NAPQI will rapidly form covalent adducts with soft nucleophilic sulfhydryl groups (e.g., see Dietze et al., 1997; Gibson et al., 1996; Hoffmann et al., 1985; Madsen et al., 2007). This reaction is reflected in the correspondence of the respective IC50 values for GSH loss among chemicals with varying electrophilicity (Table 1). The relative nucleophilicity (ω–) of 2-ACP and other putative cytoprotectants can also be calculated. Data presented in Table 2 show that, as a carbon-based nucleophile (ω– value), the enolate of 2-ACP is comparable to the respective nucleophilic thiolate forms of NAC and GSH (for detailed discussions see LoPachin et al., 2012; LoPachin and Gavin, 2012). However, because of a lower pKa value, the 2-ACP enolate will be present in significantly higher concentrations than either thiolate at physiologic conditions. To determine whether the enolate is capable of cytoprotection through electrophile scavenging, the adduct reaction between NAPQI and a nucleophilic target (GSH) was studied experimentally. Results (Fig. 10) show that NAPQI produced a graded decrease in GSH-derived sulfhydryl groups (IC50 = 9.1 μM) and that the addition of 2-ACP prevented this sulfhydryl loss in a concentration-dependent manner; i.e., 2-ACP progressively increased the NAPQI IC50 (Fig. 10). Thus, the relative cytoprotective ability of 2-ACP is determined by the aqueous ionization efficiency (low pKa value) and the inherent nucleophilic strength (high ω–) of the resulting enolate (see Discussion).
Fig. 11.
Schematic diagram showing cytochrome P450 (P450) metabolism of acetaminophen to the electrophilic metabolite, NAPQI, and the subsequent adduct-forming reactions with the enolate of 2-ACP or the GSH thiolate.
TABLE 2.
HSAB and ionization parameters for thiolate and enolate nucleophiles
Softness (σ) and nucleophilicity (ω−; NAPQI as the reacting electrophile) for the selected compounds were calculated as described under Materials and Methods.
| Nucleophile | σ | ω– | pKa | % Anion (pH = 7.4) |
|---|---|---|---|---|
| × 10–3 ev–1 | × 10–3 ev | |||
| 2-ACP (enolate) | 418 | 485 | 7.8 | 28.5 |
| GSH (thiolate) | 427 | 548 | 8.6 | 5.9 |
| NAC (thiolate) | 367 | 667 | 9.5 | 0.8 |
Discussion
We have proposed that β-diketones such as 2-ACP provide cytoprotection through their ability to form nucleophilic enolates. This supposition was based on the recognition that enol moieties of certain phytopolyphenols (e.g., curcumin) can ionize to form nucleophilic enolates and that these carbanions are important structural components of their well-documented cytoprotective properties. Our initial research demonstrated that 1,3-dicarbonyl enolates were cytoprotective in several in vitro models of electrophile toxicity (LoPachin et al., 2011). In the present study, we have expanded our evaluation of cytoprotection into an animal model of APAP hepatotoxicity. Results show that an oral APAP overdose (500 mg/kg) administered to mice was nearly 90% lethal within 72 hours (Fig. 2). However, we found that 2-ACP (2.40 mmol/kg) given intraperitoneally 20 minutes before intoxication completely prevented APAP lethality over a 7-day experimental period. Whereas 2-ACP hepatoprotection was dose-dependent (Fig. 7A), pretreatment with intraperitoneal NAC over a broad dose range (4.80–7.20 mmol/kg) was not protective and instead accelerated the rate of APAP lethality (Fig. 7B). Measurements of several biochemical indices of hepatocyte death (Figs. 3 and 4) and oxidative stress (Fig. 5) suggested that 2-ACP, but not NAC, prevented APAP-induced liver cell death. Histopathological analyses (Fig. 6) confirmed hepatocyte preservation and showed that intraperitoneal pretreatment with 2-ACP prevented the centrilobular necrosis that characterizes APAP hepatotoxicity, whereas intraperitoneal NAC did not stop this damage. Studies designed to determine the temporal dependency of hepatoprotection showed that 2-ACP (2.40 mmol/kg i.p.) afforded complete protection when administered 20 minutes before (–20) or after (+20) APAP intoxication, whereas the +60 time point was ineffective (Fig. 8A). NAC (2.40 mmol/kg i.p.) was not protective at either 20-minute (+/–) time points, although when administered 60 minutes (+60) after APAP, NAC partially improved the survival of intoxicated mice (Fig. 8B; also see Corcoran et al., 1985a). Although pharmacokinetic differences might be involved, the corresponding temporal nature of hepatoprotection could indicate that 2-ACP and NAC block different stages of APAP-induced hepatotoxicity.
In contrast to the intraperitoneal route, oral gavage administration of 2-ACP 20 minutes before oral APAP intoxication was modestly protective (Fig. 9A), whereas equimolar oral NAC provided complete protection against APAP-induced lethality (Fig. 9B). Similar to intraperitoneal 2-ACP (Fig. 7A), oral NAC hepatoprotection was dose-dependent over the same narrow dose range (Fig. 9B). This dosing characteristic has been reported previously for NAC analogs (Roberts et al., 1998) and suggests a threshold concentration for protection. The route dependency (intraperitoneal vs. oral) of NAC protection is well recognized (Corcoran et al., 1985a; Shalansky et al., 2005; Dickey et al., 2008) and could be a product of pharmacokinetic and/or toxicodynamic attributes. Although both the intraperitoneal or oral routes lead to the hepatic portal system, the relatively rapid absorption and elimination kinetics of the intraperitoneal route (Gibaldi and Perrier, 1974) might render the resulting liver NAC concentration insufficient to provide hepatoprotection or temporally disconnected from the later developing target stage of APAP toxicity.
Our initial research demonstrated that 1,3-dicarbonyl compounds could provide protection in several cell culture models of electrophile (acrolein)- and oxidative stress (H2O2)-induced cytotoxicity (LoPachin et al., 2011). In the acrolein model, the rank order of cytoprotection was directly related to the differential rates of individual acrolein-dicarbonyl adduct reactions. These second-order rates are determined by the enolate nucleophilicity (which affects the rate constant of the reaction) and the pKa of the parent dicarbonyl compound (which affects the concentration of the reacting nucleophile). In the H2O2 model, we found that metal chelation was an important component of 1,3-dicarbonyl cytoprotection. Thus, compounds (e.g., 1,3-cyclopentanedione) with rigid structures that preclude bidentate metal ion coordination were ineffective in the H2O2 model, whereas those compounds with flexible structures and metal chelating abilities (e.g., 2-ACP, AcAc) provided substantial cytoprotection. These data indicate that the hepatoprotective mechanism of the 1,3-dicarbonyls could involve: 1) scavenging of toxic electrophiles, which prevents protein inactivation and changes in cellular redox status, and 2) metal ion chelation, which reduces cellular free radical loads by inhibiting the metal-catalyzed Fenton reaction. These cytoprotective mechanisms, considered within the framework of APAP pathophysiology, suggest that 2-ACP could act at multiple sites within the hepatotoxicity pathway. Specifically, the hepatotoxic mechanism appears to involve rapid biotransformation of APAP to NAPQI, which is followed closely by GSH depletion and protein inactivation via sulfhydryl adduct formation (e.g., see Hoffman et al., 1985; Gibson et al., 1996; Dietze et al., 1997). Many researchers consider these to be originating effects that occur within 1–2 hours of NAPQI generation and accordingly this “initial phase” leads to a NAPQI-independent cascade of irreversible hepatocyte injury. This later developing “toxic phase” is characterized by mitochondrial damage, DNA fragmentation, and the generation of reactive oxygen/nitrogen species (Bajt et al., 2003, 2011; James et al., 2003; Reid et al., 2005; Agarwal et al., 2011).
Based on the preceding discussion, we hypothesize that 2-ACP acts as a surrogate nucleophilic target for NAPQI (Fig. 11) and thereby prevents APAP hepatotoxicity by blocking development of the initial phase. Whereas hepatoprotection might involve inhibition of APAP metabolic activation, we found early (2-hour) transient changes in the liver aldehyde and thiol contents of 2-ACP-pretreated APAP-intoxicated mice (Fig. 5) that were consistent with NAPQI-mediated toxicity (Jaeschke et al., 2011, 2012). This suggests ongoing APAP bioactivation with generation of NAPQI, the toxic effects of which are subsequently prevented by 2-ACP scavenging. Our HSAB calculations indicate that NAPQI is a very soft, highly reactive electrophile (Table 1). As a soft electrophile, NAPQI will react preferentially with soft nucleophiles, which in biologic systems are sulfhydryl thiolate groups on specific cysteine residues of cellular proteins (reviewed in LoPachin and Barber, 2006; LoPachin et al., 2008, 2012; LoPachin and Gavin, 2012). With respect to hepatoprotection, the 2-ACP enolate is a soft, relatively strong carbanion nucleophile with a pKa close to physiologic pH (Table 2). Accordingly, our in chemico studies (Fig. 10) demonstrated that 2-ACP could adduct NAPQI and thereby prevent GSH loss. Although the thiolate of GSH is also a strong nucleophile, because of the relatively high (8.6) pKa, the majority (94%) of this sulfhydryl species will be present in the nonreactive thiol state (see also LoPachin et al., 2007a,b, 2009). That 2-ACP can scavenge NAPQI and thereby prevent the initiating stage of hepatotoxicity is suggested by the narrow temporal window of in vivo hepatoprotection, i.e., protection at ±20 minutes, but not +60 minutes (Fig. 8A). This is consistent with the observation that 2-ACP cannot trap free radicals (LoPachin et al., 2011) and therefore would not directly affect the cellular burden of reactive oxygen/nitrogen species during the later developing toxic phase. Nonetheless, iron-catalyzed free radical formation during this second stage (e.g., see Kon et al., 2010) might be suppressed by the ability of 2-ACP to chelate metal ions, which would contribute to overall hepatoprotection.
In contrast to 2-ACP, NAC is a poor target for NAPQI; i.e., although a significant sulfhydryl nucleophile (Table 2), the high pKa of NAC (9.6) means that at physiologic pH very little (<1%) of the corresponding cysteine sulfhydryl groups will be in the reactive anionic state. Indeed, previous research has suggested that NAC does not scavenge NAPQI and therefore cannot prevent the initial phase. Rather, NAC hepatoprotection might involve an alternative mechanism that impacts the toxic phase, e.g., free radical trapping, increased GSH synthesis, or enhanced mitochondrial energy production (Lauterburg et al., 1983; Corcoran et al., 1985b; reviewed in De Flora et al., 2001; Zwingmann and Bilodeau, 2006; Saito et al., 2010). Our observation that i.p. NAC provided protection only at 60 minutes after APAP is consistent with such a delayed hepatoprotective mechanism (Fig. 8B; see also Corcoran et al., 1985a).
The present study has shown that 2-ACP can provide significant protection in an animal model of APAP poisoning. The anionic enolate of this 1,3-dicarbonyl is a soft nucleophile that can form 1,4-Michael adducts with soft electrophiles of different chemical classes, e.g., quinones, α,β-unsaturated carbonyl/aldehyde derivatives. Our HSAB calculations and in chemico studies indicate that 2-ACP can scavenge NAPQI and thereby prevent the initial phase of APAP hepatotoxicity. Although unlikely to be a singular treatment of APAP poisoning in humans, a potentially effective approach might be a combination of 2-ACP and NAC, since this mixture would impact both phases of toxicity. In this regard, it is noteworthy that the 1,3-dicarbonyl compounds are chemically stable, relatively water-soluble compounds that are rapidly absorbed and have large volumes of distribution (Ballantyne and Cawley, 2001; LoPachin et al., 2011). Furthermore, the acute animal toxicity of these chemicals is low (LD50 > 800 mg/kg) and longitudinal dosing studies indicate a low incidence of systemic toxicity (e.g., 400–600 mg/kg per day for 60 days; Ballantyne and Cawley, 2001; R. LoPachin and B. Geohagen, unpublished data). The mechanism of cytoprotection suggests that the 1,3-dicarbonyls might be useful in the treatment of other drugs that produce toxicity via electrophilic intermediates, e.g., diclofenac, cyclophosphamide, valproic acid (Erve, 2006). In addition, enolate cytoprotection could stem the toxicity of drugs that are significant electrophiles, e.g., cisplatin, clopidogrel (Dedon and Borch, 1987; Singh et al., 2011). This suggestion is feasible, since electrophile-mediated toxicity can often be prevented without affecting the therapeutic (e.g., antineoplastic) efficacy of these drugs (Borch et al., 1980; Neuwelt et al., 2004). Finally, many environmental chemicals are electrophiles (e.g., acrolein, acrylamide, methylmercury) that produce toxicity as a consequence of acute or chronic exposure. The 1,3-dicarbonyl compounds might therefore be useful in treating the acquired toxicities that occur as a result of environmental exposure to these toxicants (LoPachin et al., 2012; LoPachin and Gavin, 2012).
Acknowledgments
The authors thank Dr. Allan W. Wolkoff, Division of Gastroenterology and Liver Diseases, Albert Einstein College of Medicine, for helpful comments and guidance. The authors thank Irene Ostrovsky (Chemistry Laboratory, Department of Pathology), Tewolde Yimer, and their staff—Anie George, Faina Kremerman, Don Kutsyk, Kseniya Vayner, and Aleksandr Yakubov—for the analysis of mouse plasma biochemistry.
Abbreviations
- 2-ACP
2-acetylcyclopentanone
- AcAc
acetylacetone
- ALT
aspartate aminotransferase
- APAP
acetaminophen
- AST
alanine aminotransferase
- DTNB
5,5′-dithiobis-2-nitrobenzoic acid
- ev
electronvolt
- GSH
glutathione
- HNE
4-hydroxy-2-nonenal
- HSAB
hard and soft acid base
- LDH
lactate dehydrogenase
- MDA
malondialdehyde
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p-benzoquinone imine
- pBQ
benzoquinone
- RIPA
radioimmunoprecipitation assay
Authorship Contributions
Participated in research design: Zhang, Gavin, LoPachin.
Conducted experiments: Zhang, Geohagen, Liu, Downey.
Performed data analysis: Zhang, Gavin, Geohagen, Liu, Downey.
Wrote or contributed to the writing of the manuscript: Gavin, Downey, LoPachin.
Footnotes
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grant R01 ES003830-25].
References
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. (2011) Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther 337:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, MacMillan-Crow LA, Hinson JA. (2012) Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther 340:134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Srivatava SK, Piper JT, Singhal SS, Chaubey M, Awasthi YC. (1996) Curcumin protects against 4-hydroxy-2-trans-nonenal-induced cataract formation in rat lenses. Am J Clin Nutr 64:761–766 [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Farhood A, Jaeschke H. (2003) Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther 307:67–73 [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. (2011) Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci 122:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian K. (2006) Molecular orbital basis for yellow curry spice curcumin’s prevention of Alzheimer’s disease. J Agric Food Chem 54:3512–3520 [DOI] [PubMed] [Google Scholar]
- Ballantyne B, Cawley TJ. (2001) 2,4-Pentanedione. J Appl Toxicol 21:165–171 [DOI] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, et al. (2008) Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 326:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch RF, Katz JC, Lieder PH, Pleasants ME. (1980) Effect of diethyldithiocarbamate rescue on tumor response to cis-platinum in a rat model. Proc Natl Acad Sci USA 77:5441–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bug T, Mayr H. (2003) Nucleophilic reactivities of carbanions in water: the unique behavior of the malodinitrile anion. J Am Chem Soc 125:12980–12986 [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Racz WJ, Smith CV, Mitchell JR. (1985a) Effects of N-acetylcysteine on acetaminophen covalent binding and hepatic necrosis in mice. J Pharmacol Exp Ther 232:864–872 [PubMed] [Google Scholar]
- Corcoran GB, Todd EL, Racz WJ, Hughes H, Smith CV, Mitchell JR. (1985b) Effects of N-acetylcysteine on the disposition and metabolism of acetaminophen in mice. J Pharmacol Exp Ther 232:857–863 [PubMed] [Google Scholar]
- Dedon PC, Borch RF. (1987) Characterization of the reactions of platinum antitumor agents with biologic and nonbiologic sulfur-containing nucleophiles. Biochem Pharmacol 36:1955–1964 [DOI] [PubMed] [Google Scholar]
- Dickey DT, Muldoon LL, Doolittle ND, Peterson DR, Kraemer DF, Neuwelt EA. (2008) Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother Pharmacol 62:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Flora S, Izzotti A, D’Agostini F, Balansky RM. (2001) Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis 22:999–1013 [DOI] [PubMed] [Google Scholar]
- Dietze EC, Schäfer A, Omichinski JG, Nelson SD. (1997) Inactivation of glyceraldehyde-3-phosphate dehydrogenase by a reactive metabolite of acetaminophen and mass spectral characterization of an arylated active site peptide. Chem Res Toxicol 10:1097–1103 [DOI] [PubMed] [Google Scholar]
- Eames J. (2009) Acid-base properties of enols and enolates, in The Chemistry of Metal Enolates (Zablicky J. ed) pp 411–460, John Wiley & Sons, West Sussex, England [Google Scholar]
- Erve JCL. (2006) Chemical toxicology: reactive intermediates and their role in pharmacology and toxicology. Expert Opin Drug Metab Toxicol 2:923–946 [DOI] [PubMed] [Google Scholar]
- Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudière J. (1998) Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol 11:1176–1183 [DOI] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D. (1974) Route of administration and drug disposition. Drug Metab Rev 3:185–199 [DOI] [PubMed] [Google Scholar]
- Gibson JD, Pumford NR, Samokyszyn VM, Hinson JA. (1996) Mechanism of acetaminophen-induced hepatotoxicity: covalent binding versus oxidative stress. Chem Res Toxicol 9:580–585 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658 [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. (2010) Mechanisms of acetaminophen-induced liver necrosis. Handbook Exp Pharmacol 196:369–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann KJ, Streeter AJ, Axworthy DB, Baillie TA. (1985) Identification of the major covalent adduct formed in vitro and in vivo between acetaminophen and mouse liver proteins. Mol Pharmacol 27:566–573 [PubMed] [Google Scholar]
- Hou W, Watters JW, McLeod HL. (2004) Simple and rapid docetaxel assay in plasma by protein precipitation and high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 804:263–267 [DOI] [PubMed] [Google Scholar]
- Jaeschke H. (1990) Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther 255:935–941 [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. (2006) Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89:31–41 [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. (2011) Current issues with acetaminophen hepatotoxicity—a clinically relevant model to test the efficacy of natural products. Life Sci 88:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, McGill MR. (2012) Caveats of using acetaminophen hepatotoxicity models for natural product testing. Toxicol Lett 215:40–41 [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. (2003) Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci 75:458–467 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wilkinson J, 4th, Christine Pietsch E, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. (2006) Iron chelation in the biological activity of curcumin. Free Radic Biol Med 40:1152–1160 [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ. (2010) Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci 117:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterburg BH, Corcoran GB, Mitchell JR. (1983) Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest 71:980–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Barber DS. (2006) Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol Sci 94:240–255 [DOI] [PubMed] [Google Scholar]
- Lopachin RM, Barber DS, Geohagen BC, Gavin T, He D, Das S. (2007a) Structure-toxicity analysis of type-2 alkenes: in vitro neurotoxicity. Toxicol Sci 95:136–146 [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, Geohagen BC, Das S. (2007b) Neurotoxic mechanisms of electrophilic type-2 alkenes: soft soft interactions described by quantum mechanical parameters. Toxicol Sci 98:561–570 [DOI] [PubMed] [Google Scholar]
- LoPachin RM, Barber DS, Gavin T. (2008) Molecular mechanisms of the conjugated α,β-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci 104:235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopachin RM, Geohagen BC, Gavin T. (2009) Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal. Toxicol Sci 107:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, Geohagen BC, Zhang L, Casper D, Lekhraj R, Barber DS. (2011) β-Dicarbonyl enolates: a new class of neuroprotectants. J Neurochem 116:132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopachin RM, Gavin T, Decaprio AP, Barber DS. (2012) Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant—target interactions. Chem Res Toxicol 25:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, Gavin T. (2012) Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environ Health Perspect 120:1650–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon GM (2002) Chemistry of enolate ions, enols and α,β-unsaturated carbonyl compounds, in Organic Chemistry, 4th ed, Oxford University Press, New York, pp 997–1068. [Google Scholar]
- Madsen KG, Olsen J, Skonberg C, Hansen SH, Jurva U. (2007) Development and evaluation of an electrochemical method for studying reactive phase-I metabolites: correlation to in vitro drug metabolism. Chem Res Toxicol 20:821–831 [DOI] [PubMed] [Google Scholar]
- Neuwelt DA, Pagel MA, Kraemer DF, Peterson DR, and Muldoon LL (2004) Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther 309:594–599. [DOI] [PubMed]
- Payton F, Sandusky P, Alworth WL. (2007) NMR study of the solution structure of curcumin. J Nat Prod 70:143–146 [DOI] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. (2005) Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther 312:509–516 [DOI] [PubMed] [Google Scholar]
- Roberts JC, Phaneuf HL, Szakacs JG, Zera RT, Lamb JG, Franklin MR. (1998) Differential chemoprotection against acetaminophen-induced hepatotoxicity by latentiated L-cysteines. Chem Res Toxicol 11:1274–1282 [DOI] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. (2010) Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51:246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalansky SJ, Pate GE, Levin A, Webb JG. (2005) N-acetylcysteine for prevention of radiocontrast induced nephrotoxicity: the importance of dose and route of administration. Heart 91:997–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Petter RC, Baillie TA, Whitty A. (2011) The resurgence of covalent drugs. Nat Rev Drug Discov 10:307–317 [DOI] [PubMed] [Google Scholar]
- Weber WM, Hunsaker LA, Gonzales AM, Heynekamp JJ, Orlando RA, Deck LM, Vander Jagt DL. (2006) TPA-induced up-regulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem Pharmacol 72:928–940 [DOI] [PubMed] [Google Scholar]
- Zwingmann C, Bilodeau M. (2006) Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology 43:454–463 [DOI] [PubMed] [Google Scholar]