Abstract
The D1 dopamine receptor (D1R) has been proposed to form a hetero-oligomer with the D2 dopamine receptor (D2R), which in turn results in a complex that couples to phospholipase C–mediated intracellular calcium release. We have sought to elucidate the pharmacology and mechanism of action of this putative signaling pathway. Dopamine dose-response curves assaying intracellular calcium mobilization in cells heterologously expressing the D1 and D2 subtypes, either alone or in combination, and using subtype selective ligands revealed that concurrent stimulation is required for coupling. Surprisingly, characterization of a putative D1-D2 heteromer-selective ligand, 6-chloro-2,3,4,5-tetrahydro-3-methyl-1-(3-methylphenyl)-1H-3-benzazepine-7,8-diol (SKF83959), found no stimulation of calcium release, but it did find a broad range of cross-reactivity with other G protein–coupled receptors. In contrast, SKF83959 appeared to be an antagonist of calcium mobilization. Overexpression of Gqα with the D1 and D2 dopamine receptors enhanced the dopamine-stimulated calcium response. However, this was also observed in cells expressing Gqα with only the D1R. Inactivation of Gi or Gs with pertussis or cholera toxin, respectively, largely, but not entirely, reduced the calcium response in D1R and D2R cotransfected cells. Moreover, sequestration of Gβγ subunits through overexpression of G protein receptor kinase 2 mutants either completely or largely eliminated dopamine-stimulated calcium mobilization. Our data suggest that the mechanism of D1R/D2R–mediated calcium signaling involves more than receptor-mediated Gq protein activation, may largely involve downstream signaling pathways, and may not be completely heteromer-specific. In addition, SKF83959 may not exhibit selective activation of D1-D2 heteromers, and its significant cross-reactivity to other receptors warrants careful interpretation of its use in vivo.
Introduction
Dopamine is a neurotransmitter that functions in the central nervous system to regulate neural processes that include motor control, cognition, and memory. Dysregulation of the dopamine (DA) system is associated with neurologic disorders such as Parkinson disease, schizophrenia, addiction, and attention deficit hyperactivity disorder. Five DA receptor (DAR) genes exist in mammals, each of which encodes a DAR subtype (D1R–D5R); these genes are grouped by structure and function into the D1-like (D1R and D5R) and D2-like (D2R, D3R, and D4R) DAR families. The D1-like receptors couple to the Gs/olf proteins to activate adenylyl cyclase–mediated formation of cAMP, whereas the D2-like receptors couple to the Gi/o proteins to inhibit adenylyl cyclase (Sibley and Monsma, 1992; Missale et al., 1998). Several studies, however, have proposed DAR-mediated signaling pathways that do not involve activation of either Gi/o or Gs/olf proteins.
The first evidence for alternate signaling pathways came from multiple studies reporting “D1-like” receptor stimulation of intracellular calcium mobilization, which was suggested to be a result of Gq-mediated activation of phospholipase C (PLC) (Mahan et al., 1990; Undie and Friedman, 1990; Wang et al., 1995; Pacheco and Jope, 1997). Subsequently, it was shown that in vitro cell cultures coexpressing the D1R and D2R could couple to intracellular calcium mobilization through the Gq-PLC-diacylglycerol pathway (Lee et al., 2004; Rashid et al., 2007a). This calcium response required both coexpression and coactivation of both receptor subtypes. This led to the proposal of a “noncanonical” mechanism for DAR-mediated signaling wherein the D1R forms a heteromeric complex with the D2R and induces PLC-mediated intracellular calcium mobilization (Lee et al., 2004; Rashid et al., 2007b; Hasbi et al., 2011). The precise mechanism for this type of signaling and its prevalence in vivo, however, remain unclear.
In vivo, there is evidence both for (Surmeier et al., 1992, 1996; Lester et al., 1993; Ariano et al., 1997; Aizman et al., 2000; Lee et al., 2004) and against (Gerfen et al., 1990; Le Moine et al., 1991; Hersch et al., 1995; Le Moine and Bloch, 1995; Bertran-Gonzalez et al., 2008) the existence of neural cells coexpressing both D1R and D2R. Interestingly, some neurons that appear to coexpress D1R and D2R have neuronal projections that express only D1R or only D2R (Lee et al., 2004). This finding, along with the different methods of detection and visualization, may partially explain the incongruent reports of D1R and D2R colocalization. However, several recent studies using confocal FRET techniques argue for direct demonstration of the existence of D1-D2 heteromers in 10–20% of the cell bodies and presynaptic terminals of medium spiny neurons within the nucleus accumbens (Hasbi et al., 2009; Perreault et al., 2011, 2012a), and the two DARs have been shown to cointernalize after selective activation of either receptor (O’Dowd et al., 2005; So et al., 2005).
Interestingly, several agonists of the benzazepine family seem to exhibit differential effects on the D1R monomer compared with the proposed D1-D2 heteromer (Rashid et al., 2007b). One such compound, 6-chloro-2,3,4,5-tetrahydro-1-(3-methylphenyl)-3-(2-propenyl)-1H-3-benzazepine-7,8-diol (SKF83822), has been proposed to selectively activate D1R-mediated cAMP production but have no effect on calcium mobilization (Rashid et al., 2007a,b). In contrast, another benzazepine, 6-chloro-2,3,4,5-tetrahydro-3-methyl-1-(3-methylphenyl)-1H-3-benzazepine-7,8-diol (SKF83959), has been proposed to selectively activate the heteromer-mediated calcium release and have no effect on cAMP production (Rashid et al., 2007a,b; Hasbi et al., 2011). More recent studies have used this finding to interpret the results of systemic SKF83959 injections in mice, which resulted in increased Ca2+/calmodulin-dependent protein kinase IIα phosphorylation and increased brain-derived neurotrophic factor expression in striatal neurons (Hasbi et al., 2009; Ng et al., 2010). It was also shown that expression of glutamate decarboxylase-67 and the vesicular glutamate transporters 1 and 2 in striatal neurons, when injected into rats, was altered by SKF83959 (Perreault et al., 2012b), which, again, was interpreted to be due to selective D1-D2 heteromer activation.
In the current study, we further investigated the biology and pharmacology of the proposed D1-D2 heteromer and the mechanism of calcium mobilization in heterologous expression systems. Although we found that coactivation of both D1R and D2R protomers is required for calcium mobilization to occur, there appear to be multiple mechanisms besides Gq activation through which this pathway is elicited. We also studied the functional characteristics of SKF83959 to determine its viability as a heteromer-selective in vivo ligand and found that it was significantly less selective than previously appreciated. In fact, we were not able to provide evidence for selective activation of the D1-D2 heteromer. These results indicate that D1Rs and D2Rs can synergize to induce calcium mobilization, although the mechanisms of activation are multiple and complex and there is not, as yet, a selective pharmacology.
Materials and Methods
Human embryonic kidney 293-tsa201 (HEK293T) cells were a gift from Dr. Vanitha Ramakrishnan. A D1R expressing stable cell line was purchased from Codex Biosolutions, Inc. (Gaithersburg, MD). [3H]N-methyl-(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH23390) (80.5 Ci/mmol) and [3H]N-methylspiperone (85.5 Ci/mmol) were obtained from PerkinElmer Life Sciences (Waltham, MA). Cell culture media and reagents were purchased from MediaTech/Cellgro (Manassas, VA). Cell culture flasks and materials and all assay plates were purchased from Greiner Bio-One (Monroe, NC). SKF83959 and SKF83822 were purchased from Tocris Bioscience/RD Systems (Minneapolis, MN). All other compounds and buffer components were purchased from Sigma-Aldrich (St. Louis, MO) except where indicated.
Cell Culture and Transfection.
HEK293T cells and D1R CODEX cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with a final concentration of 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 10 μg/ml gentamicin. Cells were incubated at 37°C, 5% CO2, and 90% humidity. They were passaged and plated mechanically using calcium-free Earle’s balanced salt solution and pelleted by centrifugation at 1000g for 10 minutes. For transfection studies, HEK293T cells were seeded in 150-mm plates at 10 × 106 cells per plate. After 24 hours, cells were transfected according to the manufacturer’s recommendations using Clontech’s CalPhos transfection kit (Clontech Laboratories, Inc., Mountain View, CA). The DAR plasmid constructs were FLAG-tagged rat D1R, D2SR (D2 short splice variant), or D2LR (D2 long splice variant) in the pCD-SRα vector (Takebe et al., 1988; Monsma et al., 1990; Zhang et al., 1994) and D4R in pcDNA3.1(+) vector (Schetz and Sibley, 2001). Additional experiments were done using the Gq protein in the pcDNA3.1(+) vector (Missouri S&T cDNA Resource Center, Rolla, MO) and various functionally dominant negative G protein receptor kinase 2 (GRK2) mutants: GRK2 C-terminus 495–689 in pcDNA3(+), GRK2 K220R in pcDNA3(+), and empty pcDNA3.1(+) (Koch et al., 1994; Freedman et al., 1995). For all transfections, 5 μg of each DNA construct was used to transfect cells, with the exception of D1R, in which 10 μg was used.
Radioligand Binding Assays.
Forty-eight hours after transfection, cells were dissociated from plates using calcium-free Earle’s balanced salt solution, and intact cells were collected by centrifugation at 900g for 10 minutes. Cells were resuspended and lysed using 5 mM Tris-HCl and 5 mM MgCl2 at pH 7.4 at 4°C. Cell lysate was pelleted by centrifugation at 20,000g for 30 minutes and resuspended in 5 mM Tris-HCl at pH 7.4; 100 μl of cell lysate (containing 8 μg of protein for D2R assays or 10 μg of protein for D1R assays) was incubated for 90 minutes at room temperature with various concentrations of [3H]N-methyl-SCH23390 (D1R binding) or [3H]N-methylspiperone (D2R binding) in a final reaction volume of 250 μl. Nonspecific binding was determined in the presence of 4 μM (+)-butaclamol. Bound ligand was separated from the unbound by filtration through a PerkinElmer Unifilter-96 GF/C 96-well microplate using the PerkinElmer Unifilter-96 Harvester, washing three times, 1 ml per well in ice-cold assay buffer. After drying, 50 μl of liquid scintillation cocktail (MicroScint PS; PerkinElmer) was added to each well, plates were sealed, and the plates were analyzed on a PerkinElmer Topcount NXT. For competition binding assays, a fixed concentration of 0.5 nM [3H]N-methyl-SCH23390 was incubated with various concentrations of SKF83959, and the remainder of the assay was performed as described already herein. Ki values were calculated from observed IC50 values using the Cheng-Prusoff equation and a Kd value of 0.5 nM for SCH23390, as determined in independent saturation isotherms (unpublished data). Expression of the D4R was determined in an identical assay format as that for the D2R.
Competition Radioligand Binding Screen.
A primary, single-point radioligand competition binding assay was performed to assay for radioligand binding inhibition by SKF83959 (10 μM). Forty-three G protein–coupled receptors (GPCRs) and neurotransmitter-related proteins were screened in the primary assay using radioligands with known binding properties. The percentage of inhibition was calculated by subtracting the percentage of specific binding in the presence of the test compound from the percentage of specific binding in the absence of the test compound (n = 4). Receptors whose corresponding radioligands had greater than 50% inhibition at 10 μM SKF83959 underwent secondary radioligand competition binding assays to generate full competition curves. Ki determinations and receptor binding profiles were provided by the National Institute of Mental Health (NIMH) Psychoactive Drug Screening Program (PDSP), Contract HHSN-271-2008-00025-C. The NIMH PDSP is directed by Dr. Bryan L. Roth (University of North Carolina, Chapel Hill, NC) and by Project Officer Jamie Driscol (NIMH, Bethesda, MD). For experimental details, including radioligands used and associated Kd values for each individual receptor, please refer to the PDSP website: http://pdsp.med.unc.edu/.
Calcium Mobilization Assays.
HEK293T cells were transiently transfected as described; 24 hours after transfection, cells were plated in 384-well, optical, clear-bottom, black-walled plates (20 μl/well, 30,000 cells/well; Greiner Bio-One). Forty-eight hours after transfection, cells were incubated for 60 minutes at room temperature in the dark with Fluo-8 NW calcium dye and an extracellular signal quencher to block any signal from extracellular calcium (Screen Quest Fluo-8 NW Calcium Assay Kit; AAT Bioquest, Inc., Sunnyvale, CA), as recommended by the manufacturer. The plates were then treated with various concentrations of antagonist or agonists (diluted in the presence of 0.2 mM sodium metabisulfite) as indicated in the Results and figure legends. For agonist reads, plates were read kinetically in real-time (every 0.6 second) by recording a baseline read for 14 seconds before the addition of an agonist compound and then continually measured for 2 minutes after agonist addition. For antagonist reads, plates were read kinetically in real-time (every 0.6 second) by recording a baseline reading for 20 seconds before the addition of that antagonist. Then, 3 minutes later, agonist compound was added, and the plates were read for an additional 3 minutes. All compound additions were done in unison using the 384-tip onboard robotics on a Functional Drug Screening System (FDSS) μCell (Hamamatsu, Bridgewater, NJ), and plates were continuously read using the FDSS μCell from the bottom throughout the assay with an excitation wavelength of 480 nm and an emission wavelength of 540 nm. Data were recorded and quantified as maximum minus minimum (max−min) relative fluorescence units within the assay window using FDSS software. Data are expressed as a percentage of the control max−min relative fluorescence units for given studies as indicated in the figure legends. In these experiments, D1R and D2R receptor expression levels typically varied between 1 and 3 pmol/mg protein. We found that coexpressing both receptors sometimes affected their expression compared with expressing them alone (unpublished data). However, this did not affect the calcium mobilization response, which, although not studied in detail, appeared to require simply a minimum level of dual receptor expression.
Statistical Analysis.
Data are expressed as a percentage of control values for individual experiments. Nonlinear regression of all data was conducted on GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA). Results are expressed as mean ± S.E.M.
See Supplemental Materials and Methods section for additional procedures.
Results
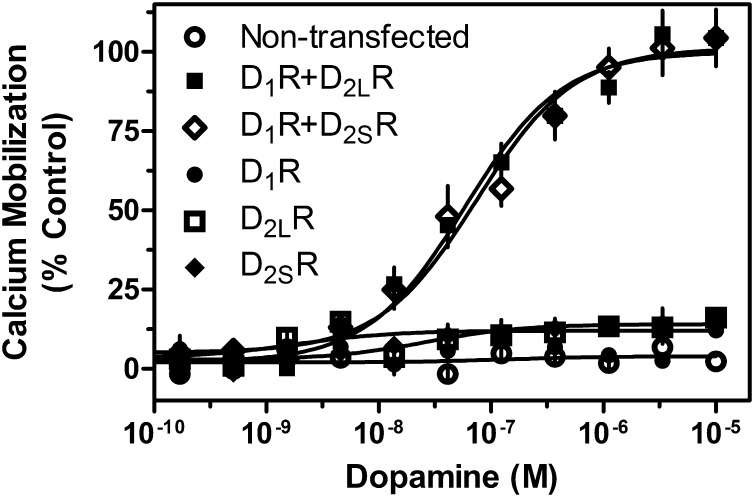
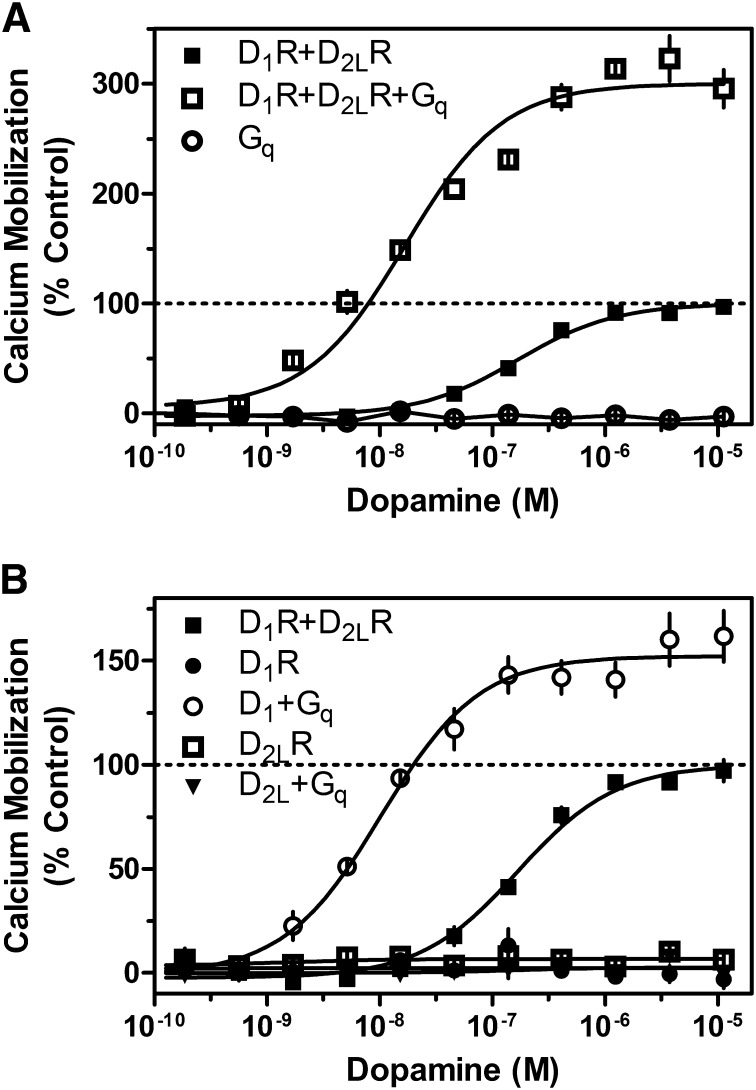
Previous studies have suggested that the D1-D2 receptor complex may signal as a heteromer and have implicated SKF83959 as a compound that may selectively activate this signaling complex (Lee et al., 2004; So et al., 2005; Rashid et al., 2007a,b). However, these findings have not been corroborated, and the mechanisms by which the D1-D2 receptor complex signals remain unclear. To investigate the apparent ability of D1-D2 receptor oligomerization to alter the G protein coupling of component receptors, we first transiently expressed the D1R either alone or concurrently with either the short (D2SR) or long (D2LR) isoforms of the D2R and measured intracellular calcium mobilization via kinetic fluorescence imaging. Preliminary coimmunoprecipitation experiments revealed that D1-D2 hetero-oligomers were indeed capable of forming under these expression conditions (Supplemental Fig. 1). When cells were transfected with the D1R and D2LR or the D1R with D2SR, a clear dose-dependent activation of calcium mobilization was observed in response to DA (Fig. 1). Importantly, we observed no difference in coupling efficacy or agonist potency between the short and long isoforms of the D2R. However, when cells were transfected with any of the subtypes alone, the receptors failed to couple to calcium mobilization (Fig. 1). These data suggest that expression and activation of both the D1R and D2R are essential for coupling to calcium mobilization and signaling.
Fig. 1.
Agonist-induced calcium mobilization in DA receptor–transfected cells. HEK293T cells were transiently transfected with D1R, D2LR, D2SR, D1R + D2LR, or D1R + D2SR, as indicated and described in Materials and Methods. Twenty-four hours later, cells were plated in 384-well plates and assayed the following day for calcium mobilization after stimulation by DA (D1R + D2LR EC50 = 73.8 nM, D1R + D2SR, EC50 = 58.2 nM). Data are representative of three independent experiments done with the same assay conditions on different days. Data are expressed as percentage of control, normalized to the maximum signal seen via DA stimulation of D1R + D2LR transfected cells. Error bars indicate S.E.M. from multiple wells within the representative experiment.
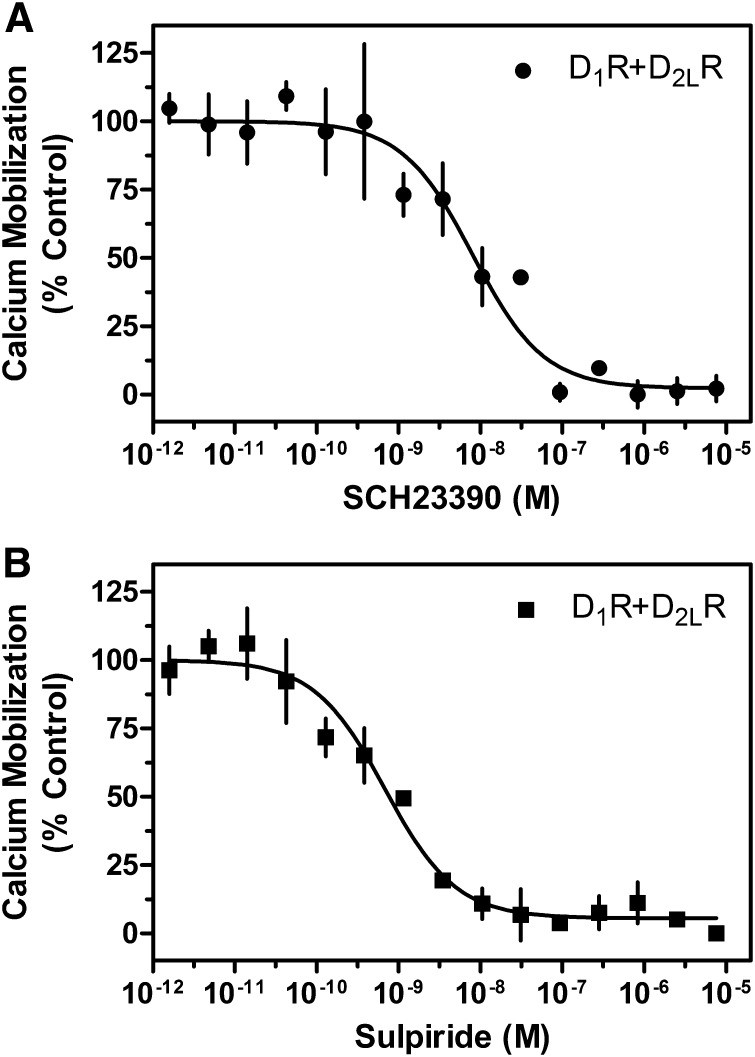
To investigate further that the activation of both receptor subtypes is required to stimulate calcium mobilization, we used receptor subtype–selective antagonists. Concentration response inhibition curves for the D1R-selective (SCH23390) and the D2R-selective (sulpiride) antagonists were generated for cells transfected with the D1R and D2R (Fig. 2, A and B). Cells were simultaneously stimulated with 1 μM DA and examined for calcium mobilization. We observed complete inhibition of the calcium signal with either SCH23390 or sulpiride treatment. The potencies of the antagonists (SCH23390 IC50 ∼8.0 nM, sulpiride IC50 ∼0.7 nM) are consistent with their known affinities for their selective subtypes as determined in our laboratory (unpublished data) as well as by other groups (Seeman and Van Tol, 1993; Millan et al., 2001). More importantly, complete inhibition of the calcium response is seen at antagonist concentrations that have no effect on the opposite receptor subtype. Thus, selectively blocking DA activation of either receptor subtype is sufficient to prevent calcium mobilization, further suggesting that both receptor protomers must be activated for this signaling to occur.
Fig. 2.
Inhibition of D1R + D2LR–mediated calcium mobilization by either D1R- or D2R-selective antagonists. HEK293T cells were transfected with D1R + D2LR as described and 24 hours later were plated in 384-well plates. Cells were incubated with the indicated concentrations of the D1R-selective antagonist SCH23390 (A) or the D2R-selective antagonist sulpiride (B) and then stimulated with an ∼EC80 of DA (1 μM; SCH23390 IC50 = 8.0 nM, sulpiride, IC50 = 0.7 nM). Data are expressed as a percentage of the control (10 μM) DA response and are representative of two independent experiments performed with the same assay conditions on different days. Error bars indicate S.E.M. from multiple wells within the representative experiment.
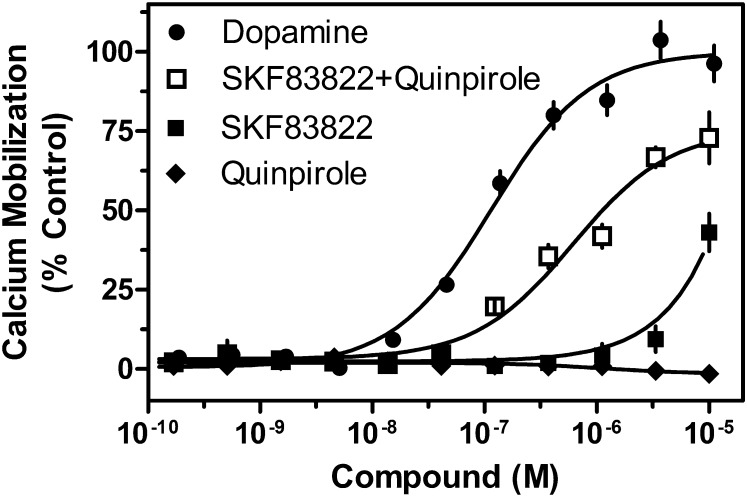
Whereas the studies using subtype-selective antagonists suggested that both D1R and D2R are required for calcium signaling, it might be possible that stabilizing one subtype into an inactive state within a heteromer might alter the conformation of the corresponding partner. Thus, to elucidate further the coupling mechanism, subtype-selective agonists were used to determine whether indeed activation of both protomers is required for calcium mobilization. As seen in Fig. 3, concurrent administration of a D1R-selective (SKF83822) and a D2R-selective (quinpirole) agonist to cells cotransfected with D1R and D2R resulted in a calcium mobilization response that nearly matched that of DA. In contrast, when D1R plus D2R–cotransfected cells were stimulated with quinpirole alone, no calcium mobilization was observed. Furthermore, when the cotransfected cells were stimulated with SKF83822, no calcium mobilization was seen at concentrations selective for D1R. A small response was observed at 10 μM, but this was at a concentration where SKF83822 loses receptor subtype selectivity and can begin to stimulate the D2R as well. Previous studies showed that SKF83822 has an affinity for D1R in the ∼2 nM range and D2R in the ∼200 nM range (O’Sullivan et al., 2004). Experiments done in our laboratory have demonstrated a D2R affinity that is greater than 10 μM (unpublished data), supporting the idea that the SKF83822-mediated calcium response seen at high concentrations is due to nonselective receptor activation. In addition, when cells were transfected with any of the subtypes individually, no signal was seen from any of the agonists (unpublished data). Taken together, these data indicate that stimulation of both receptor subtypes is necessary for calcium mobilization.
Fig. 3.
Stimulation of D1R + D2LR–mediated calcium mobilization by either D1R- or D2R-selective agonists. HEK293T cells were transfected with D1 R+ D2LR as described, plated 24 hours later in 384-well plates, and assayed for calcium accumulation the following day. Cells were stimulated with one of the following agonists as indicated: DA, the D1R-selective agonist SKF83822, the D2R-selective agonist quinpirole, or both SKF83822 and quinpirole (D1R + D2LR EC50 = 610.8 nM) combined. Control cells expressing the D1R, D2SR, or D2LR individually did not show a significant calcium response to concurrent agonist administration. Data are expressed as a percentage of control maximum DA-stimulated response and are representative of two independent experiments performed with the same assay conditions on different days. Error bars indicate S.E.M. from multiple wells within the representative experiment.
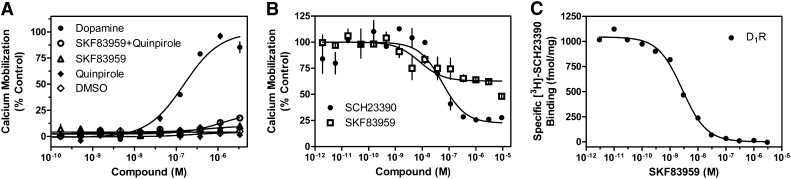
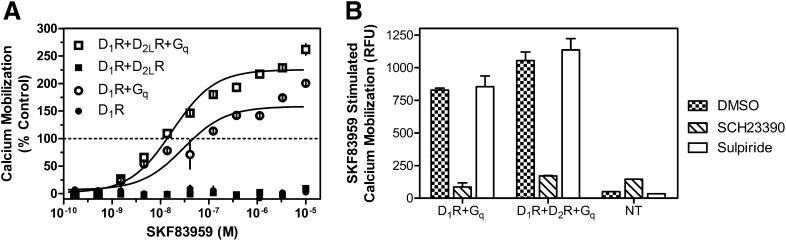
Previous studies suggested that SKF83959 may be a D1-D2 heteromer-selective compound, and a significant calcium response to this ligand has been reported in cells coexpressing the D1R and D2R (Lee et al., 2004; Rashid et al., 2007a,b; Beaulieu and Gainetdinov, 2011). This compound has also been reported to have seemingly paradoxical effects on the D1R, exhibiting both antagonist and agonist properties, depending on the system (Panchalingam and Undie, 2001; Cools et al., 2002; Zhang et al., 2005). In our current studies, we treated D1R and D2R cotransfected cells with SKF83959 and, surprisingly, were unable to elicit a calcium response (Fig. 4A). Furthermore, when SKF83959 was added in concert with the D2R selective agonist quinpirole, we were still unable to observe a significant calcium response. It should be noted that SKF83959 consistently failed to stimulate calcium mobilization even when this experiment was performed using different lots of compound from different vendors on separate days, as well as with different drug solvents (unpublished data). We also had one lot of compound chemically analyzed to verify its purity (unpublished data). To demonstrate that the SKF83959 compound was pharmacologically active in our hands, we performed two separate experiments. As shown in Fig. 4B, we stimulated calcium mobilization with DA and then dose dependently added either the D1R-selective antagonist SCH23390 as a control (see Fig. 2A) or SKF83959 to see whether it might function as an antagonist in this system. In fact, it did, exhibiting even higher potency than SCH23390, although its efficacy of antagonism was less, exhibiting a maximum inhibition of ∼50%. Finally, we performed a radioligand binding competition assay with SKF83959 and cells transfected with the D1R (Fig. 4C). SKF83959 was able to compete potently and fully for radioligand binding to the D1R. These experiments (Fig. 4, B and C) demonstrate that SKF83959 is active in binding to the monomeric D1R, as well as active as a partial antagonist of the calcium response observed in D1R and D2R cotransfected cells. In contrast, it does not appear to function as an agonist with respect to stimulating calcium mobilization in the D1R and D2R cotransfected cells.
Fig. 4.
Pharmacological characterization of SKF83959 on D1R + D2LR–mediated calcium mobilization. HEK293T cells were transfected with D1R + D2LR as described, plated 24 hours later in 384-well plates, and assayed for calcium accumulation the following day. (A) Cells were stimulated with one of the following conditions as indicated: DA, SKF83959, the D2R-selective agonist quinpirole, or both SKF83959 and quinpirole combined. (B) Cells were incubated with SKF83959 or the D1R-selective antagonist SCH23390, then stimulated with an ∼EC80 of DA (1 μM). Data are expressed as a percentage of control maximum DA-stimulated response and are representative of two or three independent experiments performed with the same assay conditions on different days. Error bars indicate S.E.M. from multiple wells within the representative experiment. (C) HEK293 cells stably transfected with D1R (Codex Biosolutions, Inc., Gaithersburg, MD) were grown and membranes harvested as described in Materials and Methods. Membranes were incubated with various concentrations of SKF83959 and 0.5 nM [3H]SCH23390 as indicated. Graph is representative of two independent experiments done on different days. Data are expressed as specific binding in units of fmol/mg. Ki value was calculated using the Cheng-Prushoff equation and a radioligand Kd value of 0.5 nM as determined via saturation binding isotherms (unpublished data). Average Ki for SKF83959 on D1R was 2.6 nM ± 0.7.
Given the apparent discrepancies of our findings with some previous studies (Lee et al., 2004; Rashid et al., 2007b; Hasbi et al., 2011) and the possibility that SKF83959 may not be as selective as previously thought, we sought to screen its selectivity against various GPCRs. This was accomplished through collaboration with the NIMH Psychoactive Drug-Screening Program (http://pdsp.med.unc.edu). For the primary screen, a single-point radioligand binding competition experiment was performed with 10 μM SKF83959 as a competitor against an appropriate receptor-specific radioligand of known properties. Forty-three GPCRs and signaling proteins were screened this way, and 20 of them resulted in >50% inhibition at 10 μM SKF83959 (Table 1). In contrast, 23 GPCR targets were found to have <50% inhibition at 10 μM SKF83959 and were therefore considered relatively “inactive/low affinity” for SKF83959 (Supplemental Table 1). The 20 “active” receptors/proteins underwent secondary radioligand competition binding experiments to generate full competition curves for SKF83959 and Ki values for these receptors were determined and are shown in Table 1. Of note is that the serotonin 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5A, and 5-HT6 receptors; the adrenergic α2A, α2B, and α2C receptors; the D1 D2, and D5 DARs; and the serotonin transporter all have nanomolar Ki values. SKF83959 demonstrated very high (sub-100 nM) affinity for four of these GPCRs: the serotonergic receptor subtypes 5-HT2C, the adrenergic receptor subtype α2C, the D1, and D5 DAR subtypes, and the serotonin transporter. Notably, SKF83959 has also recently been shown to be a potent allosteric modulator of the σ-1 receptor (Guo et al., 2013). Taken together, these data indicate that SKF83959 has significantly high affinities for a wide number of receptors and thus caution should be taken when interpreting in vivo experimentation and the selectivity of this agent.
TABLE 1.
SKF93959 competition binding experiments against various G protein–coupled receptors
Ki values were derived from radioligand binding competition curves generated against each of the above targets (n = 2) as described in Materials and Methods.
| Target | SKF83959 Ki | S.E.M. |
|---|---|---|
| nM | ||
| 5-HT1A | 1648.0 | 352.3 |
| 5-HT2A | 246.6 | 32.1 |
| 5-HT2B | 405.0 | 145.1 |
| 5-HT2C | 32.8 | 13.3 |
| 5-HT5A | 277.8 | 141.8 |
| 5-HT6 | 546.0 | 56.0 |
| α1A | 1290.5 | 154.5 |
| α1D | 1115.5 | 232.4 |
| α2A | 323.7 | 120.6 |
| α2B | 163.1 | 17.8 |
| α2C | 31.1 | 7.6 |
| D1R | 1.7 | 0.8 |
| D2R | 567.0 | 150.0 |
| D3R | 1018.3 | 109.8 |
| D4R | 1975.7 | 756.4 |
| D5R | 4.0 | 0.1 |
| H2 | 1699.3 | 640.3 |
| M4 | 5238.5 | 1985.5 |
| M5 | 3484.0 | 114.0 |
| SERT | 365.6 | 79.2 |
α1A, α-adrenergic receptor subtype 1A; α1D, α-adrenergic receptor subtype 1D; α2A, α-adrenergic receptor subtype 2A; α2B, α-adrenergic receptor subtype 2B; α2C, α-adrenergic receptor subtype 2C; 5-HT1A, serotonergic receptor subtype 1A; 5-HT2A, serotonergic receptor subtype 2A; 5-HT2B, serotonergic receptor subtype 2B; 5-HT2C, serotonergic receptor subtype 2C; 5-HT5A, serotonergic receptor subtype 5A; 5-HT6, serotonergic receptor subtype 6; M4, muscarinic receptor subtype 4; M5, muscarinic receptor subtype 5; SERT, serotonin transporter.
Whereas D1 and D2 receptors appear capable of signaling through calcium mobilization when both receptors are stimulated, the mechanism of transduction remains unclear. To understand more clearly the mechanisms involved, we tested the hypothesis that the receptors, perhaps within the context of a heteromer, may switch G protein-coupling selectivity and gain the ability to activate Gq. We first examined this possibility by overexpressing Gqα in cells expressing the D1R + D2R. Interestingly, the resulting DA-stimulated calcium signal was increased by 200% compared with cells transfected with the D1R + D2R alone (Fig. 5A). Expression of only the Gqα protein in the absence of either receptor did not enable the ability of DA to stimulate calcium mobilization (Fig. 5A). In parallel studies, we examined how overexpression of Gqα with the D1R or D2R alone could couple to intracellular calcium mobilization. Consistent with Fig. 1, cells transfected with D1R or D2R alone did not give a calcium response. However, when Gqα was overexpressed, the D1R was able to elicit a DA-stimulated calcium signal in the absence of the D2R (Fig. 5B), although the calcium response was not as large as that seen with the D1R + D2R + Gqα transfection (cf. Fig. 5, A and B). No such phenomenon was observed with the D2R. Taken together, these data suggest that the Gq protein may be involved in calcium mobilization mediated by a D1-D2 heteromer, but this interpretation is complicated by the fact that overexpression of Gqα can also lead to monomeric D1R coupling.
Fig. 5.
Influence of Gqα protein overexpression on D1R + D2LR–mediated calcium mobilization. (A) HEK293T cells were transfected with D1R + D2LR with and without Gqα or with Gqα alone (D1R + D2R EC50 = 168.3 nM, ECmax = 100%; D1R + D2R + Gq EC50 = 16.8 nM, ECmax = 300.1%). (B) HEK293T cells were transfected with D1R + D2LR, D1R, or D2R with and without Gqα (D1R + Gq EC50 = 10.3 nM, ECmax = 152.2%). Twenty-four hours later, cells were plated in 384-well plates and assayed the following day for calcium mobilization after stimulation by the indicated concentrations of DA. Data are expressed as a percentage of control maximum DA stimulation for D1R + D2LR alone and are representative of two or three independent experiments performed with the same assay conditions on different days. Error bars indicate S.E.M. from multiple wells within the representative experiment.
Given our results with Gqα overexpression, we re-evaluated SKF83959 stimulation of calcium mobilization under these conditions in the D1R and D2R coexpressed cells. We found that with Gqα overexpression, SKF83959 is able to stimulate calcium mobilization in a manner similar to that of DA (Fig. 6A), whereas it is unable to stimulate such a response in cells lacking Gqα overexpression (Figs. 4 and 6A). Interestingly, SKF83959 was also able to stimulate calcium mobilization in cells expressing the D1R and overexpressing Gqα, but not D1R alone (Fig. 6A). These results led us to test the antagonist sensitivity of the SKF83959 responses, as shown in Fig. 6B. We found that the D1R-selective antagonist SCH23390 could completely ablate SKF83959 stimulation of calcium mobilization in both D1R + Gqα transfected and D1R + D2R + Gqα transfected cells. However, in contrast to what we observed for DA stimulation of D1R + D2R cotransfected cells, the D2R-selective antagonist sulpiride was unable to block SKF83959 stimulation of calcium mobilization. These results suggest that overexpression of Gqα enables SKF83959 to stimulate monomeric D1R present in the D1R and D2R cotransfected cells, rather than enabling it to gain function as a D1-D2 heteromeric-selective agonist.
Fig. 6.
SKF83959 stimulates D1R-dependent calcium mobilization in the presence of Gqα. HEK293T cells were transfected with D1R + D2LR, Gqα or with Gqα alone as described, plated 24 hours later in 384-well plates, and assayed for calcium accumulation the following day. (A) Cells were stimulated with SKF83959. The line at 100% denotes the maximal DA response of D1R + D2LR cells. (B) Cells were incubated with the D1R-selective antagonist SCH23390 (1 μM) or the D2R-selective antagonist sulpiride (1 μM) and then stimulated with an ∼EC80 of SKF83959 (100 nM). Error bars indicate S.E.M. from multiple wells within the representative experiment, which was replicated twice with similar results. DMSO, dimethylsulfoxide.
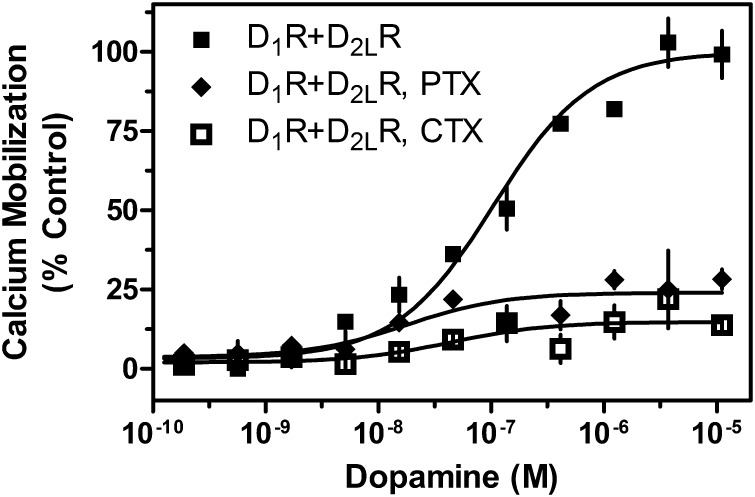
Although the extant hypothesis, which our overexpression data support, is that Gq is central to the stimulation of calcium mobilization, the central question is whether direct coupling with a D1-D2 heteromer may be involved. An alternative hypothesis is that the D1R and D2R signal through downstream pathways that converge on the Gq protein or other components of the calcium mobilization process. To test whether D1-D2 synergistic signaling is independent of Gi or Gs protein function, we interfered with the activity of Gi and Gs by treatment with toxins. D1R and D2R cotransfected cells were incubated overnight in media containing pertussis toxin (PTX) to inhibit Gi protein function (Namkung et al., 2009) or cholera toxin (CTX) to interfere with Gs protein function (Mannoury la Cour et al., 2011). Cells were then assayed for calcium mobilization in response to DA stimulation. We found that treatment with CTX or PTX drastically, but not entirely, reduced the calcium response (Fig. 7). These data support the involvement of D1R-Gs– and D2R-Gi–mediated mechanisms that majorly contribute to the calcium response in the D1R and D2R cotransfected cells.
Fig. 7.
G protein dependency of D1R + D2LR–mediated calcium mobilization. HEK293T cells were transfected with D1R + D2LR. Cells were incubated overnight in 1 μg/ml PTX or 1 μg/ml CTX; 48 hours post transfection, cells were assayed for calcium mobilization by stimulation with the indicated concentrations of DA (CTX ECmax = 14%, inhibition = 86% control, PTX ECmax = 24%, inhibition = 76% control). Data are expressed as a percentage of control maximum DA stimulation seen in untreated D1R + D2LR cells and are representative of two or three independent experiments performed with the same assay conditions on different days. Error bars indicate S.E.M. from multiple wells within the representative experiment.
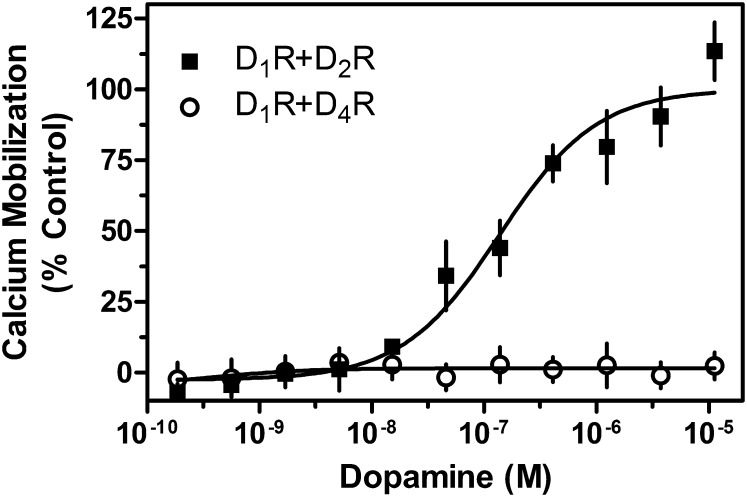
Another possibility, however, may be that general Gi-Gq “cross-talk” is occurring after receptor activation, which leads to PLC activation. Multiple cases of Gi-Gq cross-talk in other receptor systems and cell types have been documented (Okajima et al., 1989; Carroll et al., 1995; Toms and Roberts, 1999; Rebres et al., 2011), and Gi-Gq cross-talk in the D1-D2 receptor system could account for the PTX sensitivity of the calcium signal. In this model, any Gi-linked GPCR, not just the D2R, would be able to support a Gq-mediated calcium response. To test this possibility, we used the D4R, a Gi-linked DAR, which has not been found to form hetero-oligomers with the D1R (González et al., 2012). We cotransfected the D1R and D4R and compared the DA response with that in the D1R + D2R transfected cells (Fig. 8). In fact, the D4R did not support a calcium response in the presence of coexpressed D1R, indicating that nonspecific Gi-Gq cross-talk, at least as previously described (Okajima et al., 1989; Carroll et al., 1995; Toms and Roberts, 1999; Rebres et al., 2011). does not explain the D1-D2 heteromer-mediated calcium response.
Fig. 8.
Dopamine does not elicit a calcium response in cells co-expressing the D1R and D4R. HEK293T cells were transiently transfected with D1R + D2LR or D1R + D4R, as indicated and described in Materials and Methods. Twenty-four hours later, cells were plated in 384-well plates and assayed for calcium mobilization through stimulation by the indicated concentrations of DA. Data are expressed as a percentage of control maximum DA stimulation seen in cells transfected with D1R + D2LR only (EC50 = 162.0 nM) and are representative of two or three independent experiments done with the same assay conditions on different days. Expression of the D4R was confirmed using radioligand binding assays as described in Materials and Methods and was similar to that of the D2R. Error bars indicate S.E.M. from multiple wells within the representative experiment.
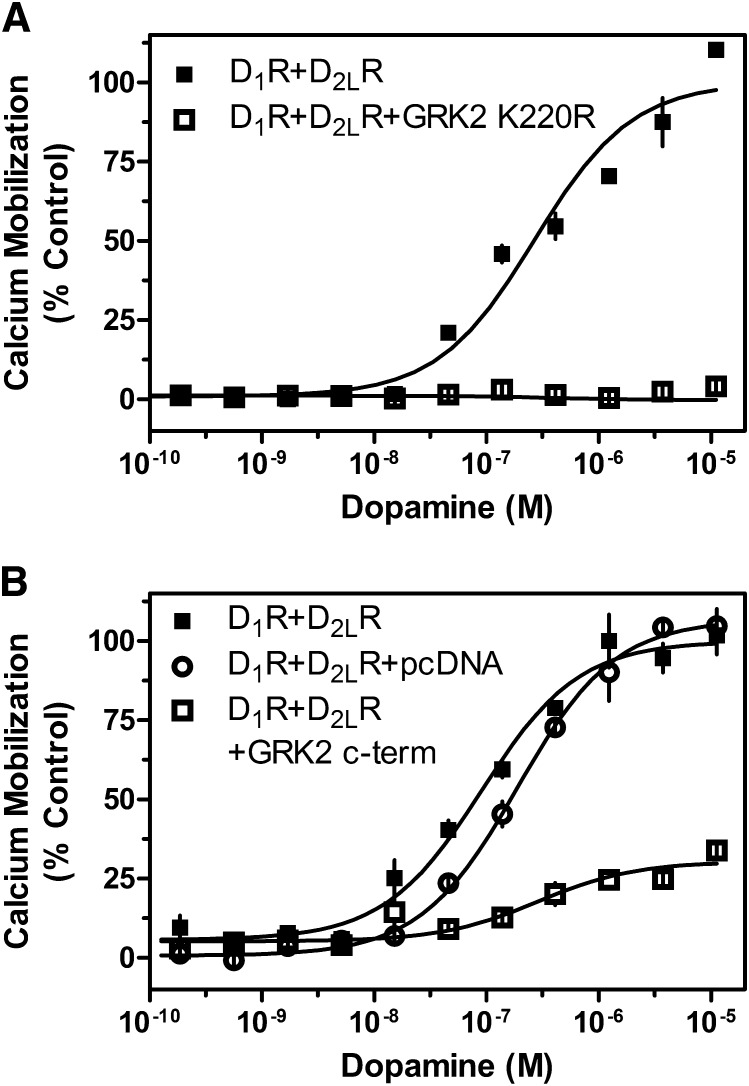
The potential involvement of multiple Gα-proteins led us to also investigate other mechanisms by which D1R and D2R activation could stimulate calcium mobilization. Notably, Gβγ subunits have been shown to increase cytoplasmic calcium concentrations by stimulating PLCβ (Beaulieu and Gainetdinov, 2011). A recent publication reported that the ghrelin receptor-D2R dimer-linked calcium response was PTX sensitive, required PLC activity, and could be ablated by sequestering the Gβγ subunits (Kern et al., 2012). To see whether Gβγ plays a role in the D1-D2 heteromer-mediated calcium release, we cotransfected the D1R and D2R with two different functionally dominant negative GRK2 mutants. The mutants we used were GRK2 K220R and the GRK2 C-terminal 495–689 peptide fragment (GRK2 c-term), both of which are unable to phosphorylate GPCRs but can bind to and sequester Gβγ subunits (Koch et al., 1994; Freedman et al., 1995). We found that overexpression of GRK2 K220R was able to ablate completely DA-stimulated calcium mobilization in the D1R and D2R cotransfected cells (Fig. 9A). Similarly, overexpression of GRK2 c-term drastically reduced, but did not completely ablate, the DA-stimulated calcium response (Fig. 7B). These data suggest that the observed calcium mobilization occurring in response to D1R and D2R activation is largely dependent on free Gβγ subunits.
Fig. 9.
GRK2 influence on DA-mediated D1R + D2LR calcium mobilization. HEK293T cells were transiently transfected with D1R + D2LR and either empty pcDNA vector the GRK2 catalytically inactive mutant GRK2 K220R (A) (D1R + D2R EC50 = 269.1 nM) or the GRK2 C-terminal 495–689 fragment (B; GRK2 c-term; D1R + D2R EC50 = 90.4 nM, ECmax = 100% control; D1R+D2R + pcDNA EC50 = 188.5 nM, ECmax = 106%; D1R + D2R +GRK2 c-term EC50 = 288.1 nM, ECmax = 30% control, 70% inhibition), as indicated and described in Materials and Methods. Twenty-four hours later, cells were plated in 384-well plates and assayed the following day for calcium mobilization after stimulation by the indicated concentrations of DA. Data are expressed as a percentage of control maximum DA stimulation seen in cells transfected with D1R + D2LR only and are representative of two or three independent experiments done with the same assay conditions on different days. Error bars indicate S.E.M. from multiple wells within the representative experiment.
Discussion
Receptor oligomers of many different GPCR types have been proposed to form homo- or hetero-oligomers with biochemical and functional characteristics that are unique to their oligomeric conformations (Ferre et al., 2009). These GPCR oligomers have been found not only to occur within a type of GPCR but also across different classes, families, types, and subtypes (Prinster et al., 2005). In addition to signaling, internalization and degradation of GPCRs in homo- and hetero-oligomers have been found to differ from their monomeric activities (Milligan, 2004; Terrillon and Bouvier, 2004; Prinster et al., 2005; Ferre et al., 2009; Missale et al., 2010). Like previously described receptor oligomers, it has been shown that the D1R and D2R can coimmunoprecipitate with each other (Lee et al., 2004; Pei et al., 2010; Supplemental Fig. 1), and fluorescence imaging has shown that the two receptors cointernalize when one or the other receptor is stimulated (O’Dowd et al., 2005, 2012; So et al., 2005; Dziedzicka-Wasylewska et al., 2006; Łukasiewicz et al., 2009). We have demonstrated that the calcium response is unique to cells that coexpress both D1 and D2 DARs and that the DARs must be costimulated, as an antagonist to either receptor blocks the transduction. However, the mechanism of action and whether heteromers or homomers form the functional units for calcium signaling remain unclear.
It has been suggested that the coactivation of the D1-D2 complex causes a conformational change that results in the direct interaction between the C terminus of the D1R and the third intracellular loop (ICL3) of the D2R (O’Dowd et al., 2012). The ICL3 is the only region of difference between D2LR and D2SR, and there is evidence that it results in differences in the G protein coupling and signaling capabilities of each D2R isoform (Kendall and Senogles, 2011). Recently, it was proposed that the ICL3 of D2LR, but not the D2SR, could form a complex with the D1R (Pei et al., 2010), but the findings were based on the use of glutathione S-transferase and trans-activator of transcription–fused D2R ICL3 fragments, which may not accurately mimic native receptor conformations and interactions. Later, it was shown that both D2R splice isoforms were able to cointernalize with the D1R (O’Dowd et al., 2012). Our results show that both D2SR and D2LR can couple with the D1R to mobilize calcium (Fig. 1), and we have found that this is also true for both human (unpublished data) and rat DARs. We have also confirmed that both receptors must be expressed in the same cell and coactivated to induce a calcium response in HEK293T cells.
Our data also suggest that Gq protein signaling may play a role in the calcium response elicited by the D1-D2 complex. This was demonstrated by observing increased calcium mobilization in response to DA in cells transfected with the D1R and D2R plus Gqα. However, we also observed that the D1R alone may couple to Gqα when the α subunit is expressed in significantly high amounts. This is likely due to the D1R having a relatively low affinity for Gqα; however, it may activate Gq-mediated calcium mobilization under conditions where Gq expression is very high. This is also supported by the enhanced calcium response we observed when the D1R and D2R are coexpressed in the presence of high levels of Gq protein, where the D1R is the protomer within the heteromer that likely activates Gqα (Rashid et al., 2007b). In this model, it is hypothesized that the D2R allosterically modulates the D1R (Rashid et al., 2007b; Hasbi et al., 2011). We believe, however, that the enhanced calcium mobilization seen in the D1R + D2R + Gqα transfected cells is not due solely to D1R monomer activation of Gq, as the degree of calcium mobilization (300% of control, Fig. 5A) is twice that seen in the D1R-Gqα transfected cells (Fig. 5B). Interestingly, another study has also reported D1R-mediated calcium release from internal stores in mouse cells lacking thymidine kinase transfected with the human D1R (Liu et al., 1992), indicating that this is not an event particular to our experimental paradigm. Thus, although Gq may play a role in the apparent ability of the D1-D2 heteromer to couple to calcium signaling, this role may be dependent on the level of Gq protein expression, either on a total cellular basis, which would thus be cell-type dependent, or this signaling may be localized to specific membrane microdomains (see discussion to follow).
It has also been suggested that SKF83959 may act as a D1-D2 heteromer-selective agonist, and it has been used as a putative heteromer-selective probe in vivo. However, these studies are not without controversy, as SKF83959 has a history of unusual pharmacology. Panchalingam and Undie (2001) found that SKF83959 inhibited D1R-stimulated cAMP formation and also induced striatal intracellular calcium mobilization in rats and monkeys. It lacked the side effects typical to D1R agonists that stimulate cAMP production but paradoxically seemed to cause typical D1R agonist-like behaviors in rats (Perreault et al., 2010) and is an effective anti-Parkinsonian agent in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydrapyridine)-lesioned monkeys unresponsive to l-Dopa (l-3,4,-dihydroxyphenylaline) (Andringa et al., 1999). In our hands SKF83959 did not stimulate a calcium response in cells transfected with both the D1R and D2R, despite the fact that it was active in binding to the D1R. In fact, it appeared to act as an antagonist of the DA-stimulated calcium response in D1R and D2R cotransfected cells. In contrast, when Gqα was overexpressed, SKF83959 stimulated a calcium response in cells cotransfected with the D1R and Gqα, as well as cells cotransfected with D1R, D2R, and Gqα. However, we observed that whereas the D1R-selective antagonist SCH23390 completely blocked the SKF83959-stimulated calcium response in both transfection conditions, the D2R-selective antagonist sulpiride was ineffective in the D1R and D2R cotransfection condition. This contrasts with sulpiride’s ability to block completely DA-stimulated calcium mobilization in the D1R and D2R cotransfected cells (cf. Figs. 2B and 6B). This finding suggests that SKF83959 is not activating the D1-D2 heteromer but rather is activating only D1R monomers that exist in the D1R and D2R cotransfected cells. This could be explained by the functionally selective or biased agonist properties of SKF83959 in that it can selectively activate D1R-Gq signaling, provided there is sufficient Gqα present, but our current results do not support its ability to activate the D1-D2 heteromer.
It has also been proposed that D1-D2 heteromer activation via SKF83959 in vivo and in vitro results in increased Ca2+/calmodulin-dependent protein kinase IIα levels in the striatum and nucleus accumbens, further resulting in enhanced brain-derived neurotrophic factor expression and increased neuronal maturation and differentiation (Rashid et al., 2007a; Hasbi et al., 2009; Ng et al., 2010; Perreault et al., 2012b). Given that our experiments indicated that SKF83959 could not induce D1-D2 heteromer–selective calcium mobilization in a controlled cell environment, we conducted a single-point competition-binding screen against an array of 43 GPCRs and additional signaling proteins (Supplemental Table 1; Table 1). We observed that SKF83959 demonstrated considerably high affinity for multiple receptors and other signaling proteins, and we conducted secondary competition binding experiments on the ones for which it showed the highest affinity. Surprisingly, SKF83959 showed nanomolar affinities for many different GPCRs, including several serotonergic, adrenergic, dopaminergic, and muscarinic receptor subtypes (Table 1). This result, as well as our functional data, questions whether SKF83959 may be useful as a selective probe to study D1-D2 heteromer or even D1-like receptor signaling in vivo.
Our data also suggest that calcium signaling through the D1-D2 receptor complex is largely sensitive to Gi and Gs inhibition by PTX and CTX, respectively. This led us to investigate additional hypotheses for the mechanism of D1-D2 calcium signaling. Recently, Kern et al. (2012) showed that the ghrelin receptor could hetero-oligomerize with the D2R. This heteromer induced calcium release from internal cellular stores in a PLC-dependent and PTX-sensitive manner and seemed to require Gβγ subunit activation. Previous studies have shown that GRK2 can bind to and sequester Gβγ subunits (Koch et al., 1994), and catalytically inactive GRK2 mutants that retain Gβγ binding have been used as tools to block Gβγ signaling without the complication of added receptor desensitization (Koch et al., 1994; Freedman et al., 1995). Our data demonstrated that the catalytically inactive GRK2 K220R mutant completely ablated the DA-stimulated calcium response in the D1R and D2R transfected cells, whereas GRK2 c-term (a truncated GRK2 protein that includes only the Gβγ binding domain) largely decreased the calcium response. Since activated Gβγ subunits can stimulate PLCβ activity (Camps et al., 1992), our results are consistent with the hypothesis that the DA-stimulated calcium response significantly involves Gβγ activation of PLCβ. Additionally, the N-terminal RGS domain of GRK2 has been shown to facilitate weak GTPase-activating protein-like activity on Gq, inhibiting PLC activation. This may explain the difference in degree of calcium signal inhibition between the GRK2 K220R mutant and the truncated GRK2 c-term mutant (Carman et al., 1999). Therefore, the activation of PLCβ may be Gqα- as well as Gβγ-dependent and due largely to synergistic cross-talk between the D1R and D2R.
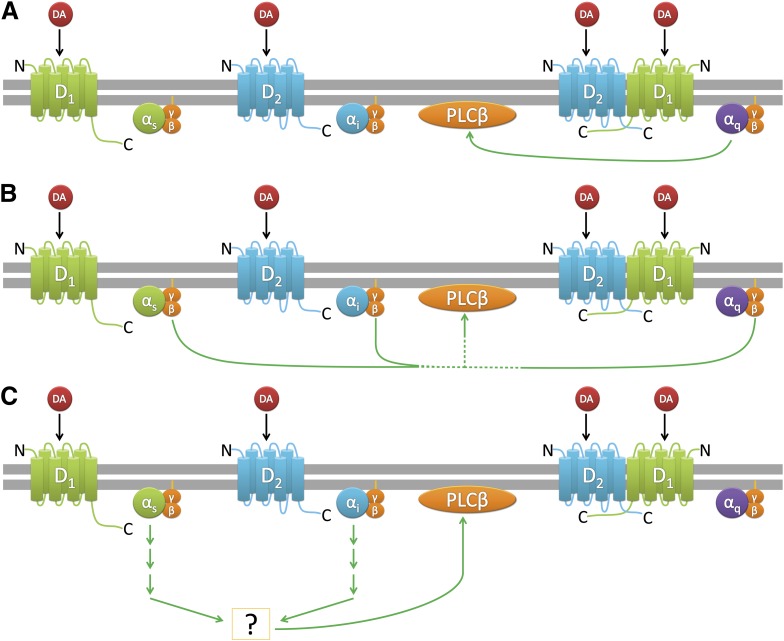
Figure 10 represents several hypothetical signaling pathways for D1-D2 receptor-calcium signaling in HEK293T cells. Pathway A represents D1-D2 heterodimer activation of Gq leading to Gqα activation of PLCβ, as has been hypothesized in the literature (Rashid et al., 2007b). Pathway B represents Gβγ activation of PLCβ, where free β/γ subunits could arise through activation of either Gs, Gi, or Gq. Pathway C represents coactivation of D1R and D2R monomers and cross-talk between Gs and Gi protein–mediated downstream signaling pathways, ultimately leading to PLCβ activation. Given that PTX and CTX can nearly eliminate the DA-stimulated calcium signaling, we believe that pathway A is largely inoperative in our system under basal conditions. Pathway C could readily account for the requirement for dual receptor activation, but the fact that Gβγ sequestration largely eliminates the DA calcium response suggests that pathway B is critically important. The PTX/CTX results further implicate Gs or Gi; however, the requirement for dual receptor activation in pathway B is not completely clear. Certainly, additional work is required to answer these questions, but it is clear from these studies that D1-D2 receptors can dually activate calcium signaling through more than a single mechanism.
Fig. 10.
Various mechanisms of PLCβ activation that may occur when the D1R and D2R are coexpressed and coactivated.
One additional consideration for D1-D2-calcium signaling, which does not necessarily exclude the possibility of heteromer formation, may involve the aggregation of the two DARs and their associated proteins in lipid rafts. Lipid rafts are a well-known but poorly understood platform for modulating certain protein-protein interactions in neurons as well as affecting GPCR ligand sensitivity, membrane trafficking, and signaling (Allen et al., 2007; Korade and Kenworthy, 2008; Björk and Svenningsson, 2011; Kong et al., 2011; Sebastião et al., 2011; Celver et al., 2012). Lipid rafts would readily enable cross-talk between the D1R and D2R and could assist in the multifaceted signaling profile of the D1-D2 receptor complex. In addition, differences in lipid raft composition, cell background, and assay detection may explain some of the differences observed between our data and the data generated by other groups. Despite the seeming complexity of the D1-D2 receptor signaling mechanisms, it may yet be useful to study how synergistic concurrent activation of the D1R and D2R may induce effects not seen when either receptor is expressed alone. This can be examined by coexpressing mutants of the the D1R and D2R, which have been reported to be unable to form dimers (O’Dowd et al., 2012), and studying the effect of coactivation on the generation of a calcium signal. Additionally, a compound that can selectively bias both receptors toward a conformation that promotes PLC activation may be useful in providing a clearer understanding of the DAR system in vivo.
Supplementary Material
Abbreviations
- CTX
cholera toxin
- D1R
D1 dopamine receptor subtype
- D2R
D2 dopamine receptor subtype
- D2LR
D2R long splice variant
- D2SR
D2R short splice variant
- DA
dopamine
- DAR
dopamine receptor
- FDSS
Functional Drug Screening System
- GRK2
G protein receptor kinase 2
- GPCR
G protein–coupled receptor
- HEK293T
human embryonic kidney cells 293-tsa201
- ICL3
third intercellular loop
- L-Dopa
l-3,4,-dihydroxyphenylaline
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydrapyridine
- NIMH
National Institute of Mental Health
- PDSP
Psychoactive Drug Screening Program
- PLC
phospholipase C
- PTX
pertussis toxin
- SCH23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- SKF83822
6-chloro-2,3,4,5-tetrahydro-1-(3-methylphenyl)-3-(2-propenyl)-1H-3-benzazepine-7,8-diol
- SKF83959
6-chloro-2,3,4,5-tetrahydro-3-methyl-1-(3-methylphenyl)-1H-3-benzazepine-7,8-diol
Authorship Contributions
Participated in research design: Chun, Free, Doyle, Sibley, Rankin, Huang.
Conducted experiments: Chun, Doyle, Rankin, Huang.
Contributed new reagents or analytic tools: Free.
Performed data analysis: Chun, Free, Doyle, Rankin, Huang.
Wrote or contributed to the writing of the manuscript: Chun, Free, Sibley, Rankin, Huang.
Footnotes
This work was supported in part by the Intramural Research Program of the National Institutes of Health [National Institute of Neurological Disorders and Stroke]; and the National Institutes of Health National Institute of Mental Health Psychoactive Drug Screening Program.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Aizman O, Brismar H, Uhlén P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. (2000) Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3:226–230 [DOI] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8:128–140 [DOI] [PubMed] [Google Scholar]
- Andringa G, Stoof JC, Cools AR. (1999) Sub-chronic administration of the dopamine D(1) antagonist SKF 83959 in bilaterally MPTP-treated rhesus monkeys: stable therapeutic effects and wearing-off dyskinesia. Psychopharmacology (Berl) 146:328–334 [DOI] [PubMed] [Google Scholar]
- Ariano MA, Larson ER, Noblett KL, Sibley DR, Levine MS. (1997) Coexpression of striatal dopamine receptor subtypes and excitatory amino acid subunits. Synapse 26:400–414 [DOI] [PubMed] [Google Scholar]
- Beaulieu J-M, Gainetdinov RR. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217 [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault J-A. (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28:5671–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk K, Svenningsson P. (2011) Modulation of monoamine receptors by adaptor proteins and lipid rafts: role in some effects of centrally acting drugs and therapeutic agents. Annu Rev Pharmacol Toxicol 51:211–242 [DOI] [PubMed] [Google Scholar]
- Camps M, Hou C, Sidiropoulos D, Stock JB, Jakobs KH, Gierschik P. (1992) Stimulation of phospholipase C by guanine-nucleotide-binding protein β γ subunits. Eur J Biochem 206:821–831 [DOI] [PubMed] [Google Scholar]
- Carman CV, Parent J-L, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T. (1999) Selective regulation of Galpha(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem 274:34483–34492 [DOI] [PubMed] [Google Scholar]
- Carroll RC, Morielli AD, Peralta EG. (1995) Coincidence detection at the level of phospholipase C activation mediated by the m4 muscarinic acetylcholine receptor. Curr Biol 5:536–544 [DOI] [PubMed] [Google Scholar]
- Celver J, Sharma M, Kovoor A. (2012) D(2)-Dopamine receptors target regulator of G protein signaling 9-2 to detergent-resistant membrane fractions. J Neurochem 120:56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools AR, Lubbers L, van Oosten RV, Andringa G. (2002) SKF 83959 is an antagonist of dopamine D1-like receptors in the prefrontal cortex and nucleus accumbens: a key to its antiparkinsonian effect in animals? Neuropharmacology 42:237–245 [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Górecka A, Andrecka J, Polit A, Kuśmider M, Wasylewski Z. (2006) Fluorescence studies reveal heterodimerization of dopamine D1 and D2 receptors in the plasma membrane. Biochemistry 45:8751–8759 [DOI] [PubMed] [Google Scholar]
- Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, et al. (2009) Building a new conceptual framework for receptor heteromers. Nat Chem Biol 5:131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman NJ, Liggett SB, Drachman DE, Pei G, Caron MG, Lefkowitz RJ. (1995) Phosphorylation and desensitization of the human beta 1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J Biol Chem 270:17953–17961 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432 [DOI] [PubMed] [Google Scholar]
- González S, Moreno-Delgado D, Moreno E, Pérez-Capote K, Franco R, Mallol J, Cortés A, Casadó V, Lluís C, Ortiz J, et al. (2012) Circadian-related heteromerization of adrenergic and dopamine D₄ receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol 10:e1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhao J, Jin G, Zhao B, Wang G, Zhang A, Zhen X. (2013). SKF83959 is a potent allosteric modulator of sigma-1 receptor. Mol Pharmacol 83:577–586 [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, George SR. (2009) Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci USA 106:21377–21382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR. (2011) Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol Brain 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. (1995) Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci 15:5222–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall RT, Senogles SE. (2011) Isoform-specific uncoupling of the D2 dopamine receptors subtypes. Neuropharmacology 60:336–342 [DOI] [PubMed] [Google Scholar]
- Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. (2012) Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron 73:317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. (1994) Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem 269:6193–6197 [PubMed] [Google Scholar]
- Kong MMC, Verma V, O’Dowd BF, George SR. (2011) The role of palmitoylation in directing dopamine D1 receptor internalization through selective endocytic routes. Biochem Biophys Res Commun 405:445–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korade Z, Kenworthy AK. (2008) Lipid rafts, cholesterol, and the brain. Neuropharmacology 55:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lança AJ, O’Dowd BF, George SR. (2004) Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem 279:35671–35678 [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. (1995) D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol 355:418–426 [DOI] [PubMed] [Google Scholar]
- Le Moine C, Normand E, Bloch B. (1991) Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci USA 88:4205–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester J, Fink S, Aronin N, DiFiglia M. (1993) Colocalization of D1 and D2 dopamine receptor mRNAs in striatal neurons. Brain Res 621:106–110 [DOI] [PubMed] [Google Scholar]
- Liu YF, Civelli O, Zhou QY, Albert PR. (1992) Cholera toxin-sensitive 3′,5′-cyclic adenosine monophosphate and calcium signals of the human dopamine-D1 receptor: selective potentiation by protein kinase A. Mol Endocrinol 6:1815–1824 [DOI] [PubMed] [Google Scholar]
- Łukasiewicz S, Faron-Górecka A, Dobrucki J, Polit A, Dziedzicka-Wasylewska M. (2009) Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the dopamine D1 and D2 receptor oligomerization. FEBS J 276:760–775 [DOI] [PubMed] [Google Scholar]
- Mahan LC, Burch RM, Monsma FJ, Jr, Sibley DR. (1990) Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc Natl Acad Sci USA 87:2196–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoury la Cour C, Salles M-J, Pasteau V, Millan MJ. (2011) Signaling pathways leading to phosphorylation of Akt and GSK-3β by activation of cloned human and rat cerebral D₂and D₃ receptors. Mol Pharmacol 79:91–105 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. (2001) The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology (Berl) 156:58–62 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2004) G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol 66:1–7 [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225 [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Collo G, Spano P. (2010) The neurobiology of dopamine receptors: evolution from the dual concept to heterodimer complexes. J Recept Signal Transduct Res 30:347–354 [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Mahan LC, McVittie LD, Gerfen CR, Sibley DR. (1990) Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci USA 87:6723–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Javitch JA, Sibley DR. (2009) G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem 284:15038–15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Rashid AJ, So CH, O’Dowd BF, George SR. (2010) Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1-D2 dopamine receptor complex. Neuroscience 165:535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd BF, Ji X, Alijaniaram M, Rajaram RD, Kong MMC, Rashid A, Nguyen T, George SR. (2005) Dopamine receptor oligomerization visualized in living cells. J Biol Chem 280:37225–37235 [DOI] [PubMed] [Google Scholar]
- O’Dowd BF, Ji X, Nguyen T, George SR. (2012) Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1-D2 heteromer formation. Biochem Biophys Res Commun 417:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F, Sato K, Sho K, Kondo Y. (1989) Stimulation of adenosine receptor enhances α 1-adrenergic receptor-mediated activation of phospholipase C and Ca2+ mobilization in a pertussis toxin-sensitive manner in FRTL-5 thyroid cells. FEBS Lett 248:145–149 [DOI] [PubMed] [Google Scholar]
- O’Sullivan GJ, Roth BL, Kinsella A, Waddington JL. (2004) SK&F 83822 distinguishes adenylyl cyclase from phospholipase C-coupled dopamine D1-like receptors: behavioural topography. Eur J Pharmacol 486:273–280 [DOI] [PubMed] [Google Scholar]
- Pacheco MA, Jope RS. (1997) Comparison of [3H]phosphatidylinositol and [3H]phosphatidylinositol 4,5-bisphosphate hydrolysis in postmortem human brain membranes and characterization of stimulation by dopamine D1 receptors. J Neurochem 69:639–644 [DOI] [PubMed] [Google Scholar]
- Panchalingam S, Undie AS. (2001) SKF83959 exhibits biochemical agonism by stimulating [(35)S]GTP gamma S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacology 40:826–837 [DOI] [PubMed] [Google Scholar]
- Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. (2010) Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med 16:1393–1395 [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Alijaniaram M, O’Dowd BF, George SR. (2012b) Dopamine D1-D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: regulation of BDNF, GAD67 and VGLUT1/2. PLoS ONE 7:e33348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O’Dowd BF, George SR. (2010) The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem 285:36625–36634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, O’Dowd BF, George SR. (2012a) Reduced striatal dopamine D1-D2 receptor heteromer expression and behavioural subsensitivity in juvenile rats. Neuroscience 225:130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR. (2011). The dopamine D1–D2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinster SC, Hague C, Hall RA. (2005) Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev 57:289–298 [DOI] [PubMed] [Google Scholar]
- Rashid AJ, O’Dowd BF, Verma V, George SR. (2007b) Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci 28:551–555 [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MMC, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. (2007a) D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA 104:654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebres RA, Roach TIA, Fraser IDC, Philip F, Moon C, Lin K-M, Liu J, Santat L, Cheadle L, Ross EM, et al. (2011) Synergistic Ca2+ responses by Gαi- and Gαq-coupled G-protein-coupled receptors require a single PLCβ isoform that is sensitive to both Gβγ and Gαq. J Biol Chem 286:942–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetz JA, Sibley DR. (2001) The binding-site crevice of the D4 dopamine receptor is coupled to three distinct sites of allosteric modulation. J Pharmacol Exp Ther 296:359–363 [PubMed] [Google Scholar]
- Sebastião AM, Assaife-Lopes N, Diógenes MJ, Vaz SH, Ribeiro JA. (2011) Modulation of brain-derived neurotrophic factor (BDNF) actions in the nervous system by adenosine A(2A) receptors and the role of lipid rafts. Biochim Biophys Acta 1808:1340–1349 [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HHM. (1993) Dopamine D4 receptors bind inactive (+)-aporphines, suggesting neuroleptic role: sulpiride not stereoselective. Eur J Pharmacol 233:173–174 [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr (1992) Molecular biology of dopamine receptors. Trends Pharmacol Sci 13:61–69 [DOI] [PubMed] [Google Scholar]
- So CH, Varghese G, Curley KJ, Kong MMC, Alijaniaram M, Ji X, Nguyen T, O’dowd BF, George SR. (2005) D1 and D2 dopamine receptors form heterooligomers and cointernalize after selective activation of either receptor. Mol Pharmacol 68:568–578 [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. (1992) Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA 89:10178–10182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song W-J, Yan Z. (1996) Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16:6579–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. (1988) SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol 8:466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. (2004) Roles of G-protein-coupled receptor dimerization. EMBO Rep 5:30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms NJ, Roberts PJ. (1999) Group 1 mGlu receptors elevate [Ca2+]i in rat cultured cortical type 2 astrocytes: [Ca2+]i synergy with adenosine A1 receptors. Neuropharmacology 38:1511–1517 [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. (1990) Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther 253:987–992 [PubMed] [Google Scholar]
- Wang HY, Undie AS, Friedman E. (1995) Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol Pharmacol 48:988–994 [PubMed] [Google Scholar]
- Zhang LJ, Lachowicz JE, Sibley DR. (1994) The D2S and D2L dopamine receptor isoforms are differentially regulated in Chinese hamster ovary cells. Mol Pharmacol 45:878–889 [PubMed] [Google Scholar]
- Zhang Z-J, Jiang X-L, Zhang SE, Hough CJ, Li H, Chen J-G, Zhen X-C. (2005) The paradoxical effects of SKF83959, a novel dopamine D1-like receptor agonist, in the rat acoustic startle reflex paradigm. Neurosci Lett 382:134–138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.