Abstract
Cannabinoids are part of an endogenous signaling system consisting of cannabinoid receptors and endogenous cannabinoids as well as the enzymatic machinery for their synthesis and degradation. Depolarization-induced suppression of excitation (DSE) is a form of cannabinoid CB1 receptor–mediated inhibition of synaptic transmission that involves the production of the endogenous cannabinoid 2-arachidonoyl glycerol (2-AG). Both diacylglycerol lipase α (DAGLα) and DAGLβ can produce 2-AG in vitro, but evidence from knockout animals argues strongly for a predominant, even exclusive, role for DAGLα in regulation of 2-AG–mediated synaptic plasticity. What role, if any, might be played by DAGLβ remains largely unknown. Cultured autaptic hippocampal neurons exhibit robust DSE. With the ability to rapidly modulate expression of DAGLα and DAGLβ in these neurons with short hairpin RNA, they are well suited for a comparative study of the roles of each isoform in mediating DSE. We find that RNA interference knockdown of DAGLα substantially reduces autaptic DSE, shifting the “depolarization-response curve” from an ED50 value of 1.7 seconds to 3.0 seconds. Surprisingly, DAGLβ knockdown diminishes DSE as much or more (ED50 6.4 seconds), suggesting that DAGLβ is also responsible for a portion of 2-AG production in autaptic neurons. Similarly, the two DAGLs both contribute to the production of 2-AG via group I metabotropic glutamate receptors. Our results provide the first explicit evidence for a role of DAGLβ in modulating neurotransmission.
Introduction
Cannabinoids first gained notoriety as the psychoactive ingredients of marijuana and hashish. These agents, chief among them ∆9-tetradhydrocannabinol (Gaoni and Mechoulam, 1964), act on endogenous targets, designated the CB1 and CB2 cannabinoid receptors (Matsuda et al., 1990; Munro et al., 1993). These receptors are found throughout much of the brain and body and are implicated in a host of physiologic functions (Piomelli, 2003). The body also makes endogenous cannabinoids, with 2-arachidonoyl glycerol (2-AG) (Stella et al., 1997) deeply implicated in endogenous modulation of neurotransmission via CB1 (Kano et al., 2009). Understanding the production and breakdown of 2-AG is necessary to appreciate its physiologic role and develop therapeutics targeting 2-AG metabolism. As a lipid, 2-AG is unlikely to be packaged and released as a conventional neurotransmitter, but is instead produced enzymatically by cleavage from a precursor lipid (likely diacylglycerol) primarily by either of two diacylglycerol lipases [diacylglycerol lipase α (DAGLα) and DAGLβ] (Stella et al., 1997; Bisogno et al., 2003; Piomelli, 2003). Several studies support a DAGL role in endocannabinoid-mediated neuronal plasticity (e.g., Chevaleyre and Castillo, 2003; Jung et al., 2005; Straiker and Mackie, 2005), but these all relied on pharmacological tools that do not distinguish between the two DAGLs. Separately, expression studies offered differing pictures of the relative prominence of these enzymes (Bisogno et al., 2003; Jung et al., 2005). Consequently, the question of which DAGL mediated cannabinoid modulation remained unresolved for several years.
Three independent reports of DAGL knockout mice appeared in 2010 and 2011 that seemed to resolve the question of the relative roles of DAGLα versus DAGLβ in endocannabinoid-mediated synaptic plasticity (Gao et al., 2010; Tanimura et al., 2010; Yoshino et al., 2011). Tanimura et al. (2010) found that synaptic plasticity was absent in DAGLα knockout mice for each of eight forms of cannabinoid-mediated plasticity examined, whereas limited tests of DAGLβ knockout mice showed no changes. A separate study by Gao et al. (2010) found that hippocampal depolarization-induced suppression of inhibition was similarly dependent on DAGLα. Last, a third study appeared the following year that showed a dominant role for DAGLα in synaptic plasticity in the prefrontal cortex (Yoshino et al., 2011). These three studies all strongly suggested that DAGLα, rather than DAGLβ, was the predominant 2-AG synthesizing enzyme in adults for endocannabinoid-mediated modulation of neurotransmission. A recent study (Hsu et al., 2012) implicates DAGLβ in inflammatory responses in macrophages.
However, questions remain regarding DAGLα and DAGLβ in synaptic transmission, particularly with respect to the functional role of DAGLβ, as it is more abundantly expressed in the developing central nervous system (Bisogno et al., 2003; Wu et al., 2010). Also, constitutive knockout mice are subject to adaptive responses that may obscure the true role of the targeted gene. As a consequence, there is a need to probe the relative roles of these two enzymes with other tools. This is particularly compelling for studies using DAGLα knockouts because of the known role of this enzyme in neurodevelopment (Berghuis et al., 2007; Keimpema et al., 2011).
To explore this question in detail, we developed RNA interference (RNAi) tools for use in autaptic hippocampal neurons. These cultured neurons express a robust, well characterized CB1-based cannabinoid signaling system with multiple forms of endocannabinoid-mediated synaptic plasticity, including depolarization-induced suppression of excitation (DSE) and metabotropic suppression of excitation (MSE) (Straiker and Mackie, 2005, 2007; Straiker et al., 2009). This system has recently allowed a detailed dissection of the enzymes capable of participating in the breakdown of 2-AG (Straiker et al., 2009, 2011). In the current experiments, we examined the consequences of knocking down DAGLα or DAGLβ on autaptic DSE and MSE.
Materials and Methods
Culture Preparation.
All procedures used in this study were approved by the Animal Care Committee of the Indiana University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Animals. Mouse hippocampal neurons isolated from the CA1–CA3 region were cultured on microislands as described previously (Furshpan et al., 1976; Bekkers and Stevens, 1991). Neurons were obtained from animals (age postnatal day 0–2, of either sex) and plated onto a feeder layer of mouse hippocampal astrocytes that had been laid down previously (Levison and McCarthy, 1991). Cultures were grown in high-glucose (20 mM) medium containing 10% horse serum, without mitotic inhibitors, and were used for recordings after 8 days in culture and for no more than 3 hours after removal from culture medium.
Electrophysiology.
When a single neuron is grown on a small island of permissive substrate, it forms synapses—or “autapses”—onto itself. All experiments were performed on isolated autaptic neurons. Whole-cell voltage-clamp recordings from autaptic neurons were carried out at room temperature using an Axopatch 200A amplifier (Axon Instruments, Burlingame, CA). The extracellular solution contained (in mM) 119 NaCl, 5 KCl, 2.5 CaCl2, 1.5 MgCl2, 30 glucose, and 20 HEPES. Continuous flow of solution through the bath chamber (∼2 ml/min) ensured rapid drug application and clearance. Drugs were typically prepared as stocks, then diluted into extracellular solution at their final concentration and used on the same day.
Recording pipettes of 1.8–3 MΩ were filled with (in mM) 121.5 K gluconate, 17.5 KCl, 9 NaCl, 1 MgCl2, 10 HEPES, 0.2 EGTA, 2 MgATP, and 0.5 LiGTP. Access resistance and holding current were monitored, and only cells with both stable access resistance and holding current were included for data analysis. The membrane potential was held at –70 mV and excitatory postsynaptic currents (EPSCs) were evoked by triggering an unclamped action current with a 1.0-millisecond depolarizing step. EPSC size was calculated by integrating the evoked current to yield a charge value [in picocoulombs (pC)]. Calculating the charge value in this manner yields an indirect measure of the amount of neurotransmitter released while minimizing the effects of cable distortion on currents generated far from the site of the recording electrode (i.e., the soma). Data were acquired at a sampling rate of 5 kHz and filtered at 2 kHz.
To evoke DSE, after establishing a 10–20-second 0.5-Hz baseline, DSE was evoked by depolarizing to 0 mV for 1–10 seconds, followed by resumption of a 0.5-Hz stimulus protocol for 10–80+ seconds, until EPSCs recovered to baseline values. In experiments investigating 2-AG, the likely endogenous mediator of DSE in this preparation was applied at 5 μM since this concentration was found to correspond to maximal DSE in autaptic cultures (Straiker and Mackie, 2005). Depolarization-response curves derived from a depolarization series were used to derive an ED50. Curves were compared using 95% confidence intervals (95% CIs). We did not observe an effect of knockdown on average baseline EPSC size (EPSC size with DAGLα RNAi: 1.6 ± 0.3 nA, n = 10; with DAGLβ RNAi: 2.3 ± 0.24 nA, n = 5; same-day untransfected controls: 2.3 ± 1.2 nA, n = 6). Responses to exogenously applied 2-AG were intact in RNAi-transfected neurons (relative EPSC charge for DAGLα RNAi: 0.60 ± 0.03, n = 8; for DAGLβ RNAi: 0.52 ± 0.07, n = 3).
Neuronal Transfection.
We transfected neurons using a calcium phosphate–based method adapted from Jiang et al. (2004). Briefly, plasmids for the protein of interest and enhanced yellow fluorescent protein (EYFP) or mCherry (2 μg/well) were combined with 2 M CaCl2 in water and gradually added to 2× HEPES buffered saline; the mixture was then added to serum-free neuronal medium. Coverslips were incubated with this mixture for 2.5 hours while extra serum-free medium was acidified in a 10% CO2 incubator. At the end of 2.5 hours, the reaction mixture was replaced with acidified serum-free medium for 20 minutes. After this, cells were returned to their home wells. Each data set was taken from at least two different neuronal platings.
Western Blot.
Human embryonic kidney 293 cells were grown to approximately 60% confluency in 6-well dishes. V5-tagged murine DAGLα or β (gifts from Danieli Piomelli, UC Irvine, Irvine, CA) with or without DAGL RNAi expression plasmids were transfected into these cells using Lipofectamine 2000 per the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Following a 72-hour incubation, cells were removed from the incubator, chilled on ice, and washed with ice-cold 1× phosphate-buffered saline (PBS). They were then covered with 200 μl of lysis buffer containing (in mM) 100 Tris (pH 7.4), 150 NaCl, 8 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 1 EDTA, 6 MgCl2, and 0.1 phenylmethanesulfonylfluoride and incubated on ice for 5 minutes. Cells were then scraped and suspensions were sonicated and centrifuged at 10,000g and 4°C. The supernatant was collected and protein concentration was determined using the Bradford assay. Samples (25 μg of protein) were run on a 10% discontinuous SDS-PAGE gel. The separated proteins were transferred to nitrocellulose, and Western blots were performed using a mouse anti-V5 antibody (Cat# R960-25; Invitrogen). Primary antibody was diluted 1:1,000 in a 1:1 mixture of Odyssey blocking buffer (Li-Cor Biosciences, Lincoln, NE) and 1× PBS. A goat anti-mouse conjugated with an IR680 dye (Cat# 926-32220; Li-Cor Biosciences) was used as the secondary antibody (diluted 1:5000 as described earlier). Western blots were scanned on an Odyssey scanner, and the signal intensities determined using ImageJ (NIH, Bethesda, MD). Background was subtracted from plots, and the area under the curve was determined for each DAGL-expressing sample. Four separate experiments were performed and knockdown analyzed via two-tailed Student’s t tests using Prism 4 (GraphPad Software, San Diego, CA).
Immunocytochemistry.
Cultured neurons were fixed in 4% paraformaldehyde for 30–60 minutes, washed, and treated with a detergent (Triton X-100, 0.3% or saponin, 0.1%) and milk (5%) in PBS. Neurons were incubated with antibodies against DAGLα or DAGLβ alone or in combination with monoclonal (mouse) antibodies microtubule associated protein 2 (MAP2) (to identify dendrites; No. MAB3418, 1:500; Millipore, Temecula, CA), or synaptic vesicle 2 (SV2) (to identify axon terminals; 1:500; Developmental Studies Hybridoma Bank, Iowa City, IA) or postsynaptic density 95 (PSD95) (to identify dendritic spines; GTX80682, 1:500; GeneTex, Irvine, CA) overnight at 4°C. For the staining of DAGLα and DAGLβ in combination, a DAGLα antibody raised in guinea pig was used. Antibodies against DAGLα or DAGLβ were developed in house and have been previously characterized (Hu et al., 2010). After washing, secondary antibodies (Alexa 488, Alexa 594, or Alexa 633, 1:500; Invitrogen) were applied the next day at room temperature for 1.5 hours. Images were acquired with a TCS SP5 confocal microscope (Leica, Wetzlar, Germany) using a 63× oil immersion objective (1.4 numerical aperture). Images were processed using ImageJ and/or Photoshop (Adobe Inc., San Jose, CA). Images were modified only in terms of brightness and contrast.
Knockdown of DAGLα or DAGLβ expression in neurons was determined as follows. For DAGLβ, neurons were transfected with DAGLβ RNAi and then stained for DAGLβ and the dendritic marker MAP2, since DAGLβ showed relatively strong colocalization with MAP2. Transfected neurons expressed EYFP to allow identification relative to untransfected neurons. Regions of interest (ROIs) were identified in EYFP+/MAP2+ (knockdown condition) and EYFP−/MAP2+ (no knockdown) from the same coverslip. DAGLβ staining intensity in the ROI was then measured using ImageJ. At least three ROIs were identified in a given image and the DAGLβ intensity values averaged. Staining intensities from multiple images were used to obtain an overall average for each condition. For DAGLα, we followed the same procedure except that we used PSD95 as the marker since this had somewhat better colocalization than MAP2.
RNAi Constructs.
DAGLα and DAGLβ small-interfering RNA sequences were identified using Primer-Blast from the National Center for Biotechnology Information (Bethesda, MD). Short hairpin sequences were designed, commercially synthesized, and inserted downstream of the RNA polymerase III H1 promoter in a pSuper EGFP plasmid (Oligoengine, Seattle, WA) using BglII and HindIII restriction sites.
DAGLα sense: GATCCCGggtgctggagaattacaacTTCAAGAGAgttgtaattctccagcaccTTTTTA.
DAGLα antisense: AGCTTAAAAAggtgctggagaattacaacTCTCTTGAAgttgtaattctccagcaccGGG.
DAGLβ sense: GATCCCGgctttcacgacaaggtgtaTTCAAGAGAtacaccttgtcgtgaaagcTTTTTA.
DAGLβ antisense: AGCTTaaaaagctttcacgacaaggtgtaTCTCTTGAAtacaccttgtcgtgaaagcCGG.
Results
Design and Characterization of RNAi Constructs.
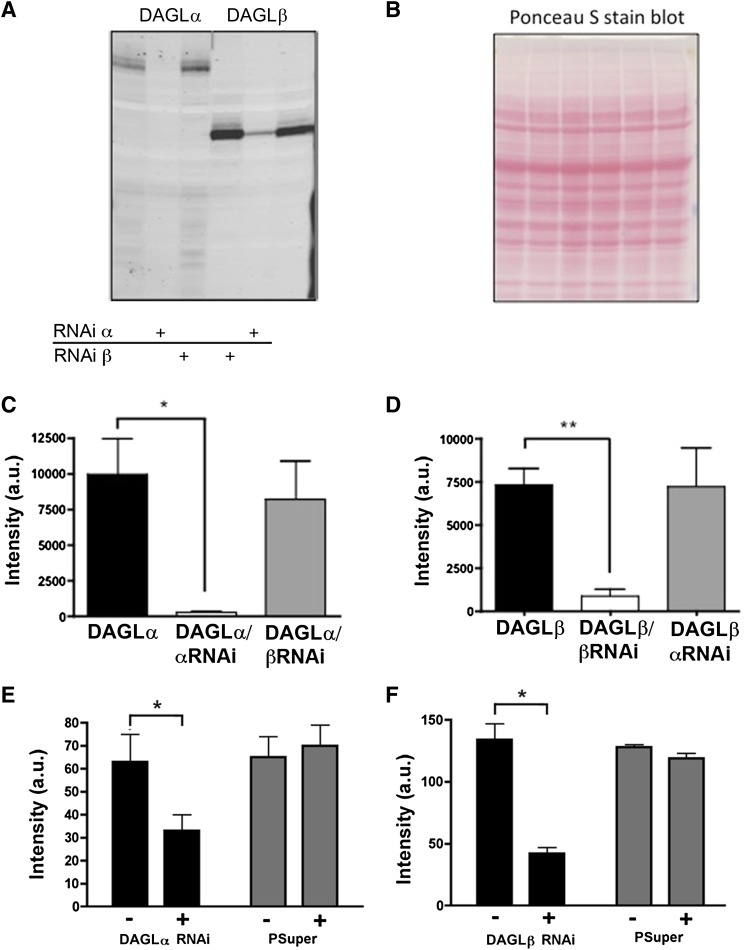
RNAi is a cellular process used to control gene expression. Using the pSuper EGFP expression plasmid, we specifically targeted mouse DAGLα and DAGLβ proteins for knockdown. RNAi-containing plasmids were transfected into human embryonic kidney 293 cells along with a plasmid containing either mouse V5-DAGLα or V5-DAGLβ (gifts from Danieli Piomelli, UC Irvine, Irvine, CA). αRNAi reduced DAGLα but not DAGLβ expression (Fig. 1A, lane 1 versus lanes 2 and 6). Conversely, RNAi specific for DAGLβ decreased DAGLβ expression, but not DAGLα expression (Fig. 1A, lane 4 versus lanes 5 and 3). RNAi for DAGLα and DAGLβ resulted in a decline of their respective detectable DAGL proteins of 97 and 88%, respectively (Fig. 1, A and C; n = 4; significance determined via two-tailed Student’s t test). To assess knockdown in neurons, we examined expression of DAGLα or DAGLβ protein in cells transfected with the appropriate RNAi or a pSuper transfection control. We found that, in each case, the DAGL signal declined in RNAi-transfected cells relative to untransfected cells, but not in pSuper-transfected cells relative to untransfected cells (Fig. 1, E and F; n = 6; significance determined via two-tailed Student’s t test).
Fig. 1.
Characterization of DAGL RNAi constructs. (A) V5-DAGLα or β was expressed in human embryonic kidney 293 (HEK293) cells with or without RNAi constructs. Expression of the enzyme was significantly reduced in cells coexpressing the targeted DAGL RNAi but not by RNAi against the other DAGL. (B) Ponceau S stain showing protein levels from blot in (A). (C) Summary of results from experiments such as (A) showing substantial knockdown of DAGLα. (D) Summary of results showing knockdown of DAGLβ. *P < 0.05; **P < 0.01 [two-tailed Student’s t test versus DAGLα (C) or DAGLβ (D) control]. Thus, RNAi for DAGLα specifically suppressed expression of DAGLα, but not DAGLβ and vice versa (C and D). (E) Summary of results showing knockdown of DAGLα in neurons. Left bars show DAGLα staining intensity in neurons untransfected (−) versus transfected with RNAi for DAGLα (+). Right bars show DAGLα staining intensity in neurons untransfected (−) versus transfected with pSuper (+). (F) Same as in (E) but for DAGLβ. *P < 0.05; **P < 0.01 (two-tailed Student’s t test). a.u., arbitrary units.
DAGLα and DAGLβ Both Regulate 2-AG Production in Autaptic Hippocampal Neurons.
To test whether DAGLα plays a role in DSE, we transfected autaptic hippocampal neurons with RNAi constructs for DAGLα. These neurons express presynaptic CB1 receptors as well as the machinery to produce and degrade endocannabinoids, likely 2-AG (Straiker and Mackie, 2005, 2009).
A simple way to quantify DSE and thereby assess CB1 signaling is to assemble a “depolarization-response curve.” Cells are depolarized for increasing durations (50, 100, 300, and 500 milliseconds, and 1, 3, and 10 seconds), resulting in increasing synthesis of endocannabinoids (likely 2-AG) and progressive EPSC inhibition (Straiker and Mackie, 2009). The resulting inhibition can be measured and analyzed in a manner very similar to a classic dose-response curve. The duration of depolarization required to reach 50% of maximal inhibition is referred to here as the ED50.
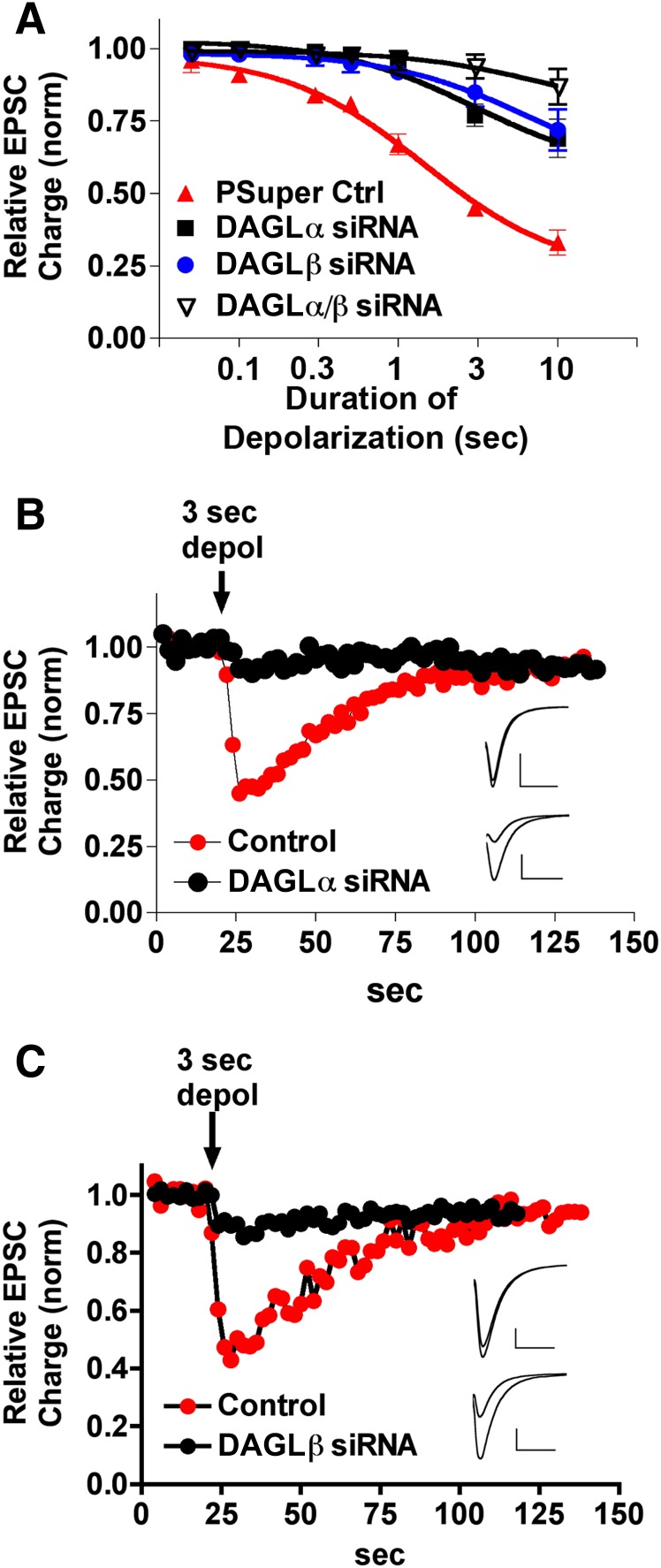
We have previously shown that the nonselective DAGL blocker O,O′-[1,6-hexanediyl bis(iminocarbony-l)]dioxime cyclohexanone (RHC80267) diminishes DSE (Straiker and Mackie, 2005). But because RHC80267 does not distinguish between DAGLα and DAGLβ, the possibility remains that either (or both) plays a role in DSE. If DAGLα is required for 2-AG production, then a knockdown of DAGLα should result in diminished DSE. We found that DAGLα RNAi did indeed substantially diminish DSE, shifting the depolarization-response curve to the right [Fig. 2, A and B; pSuper ED50: 1.5 seconds (95% CI: 1.2–1.8); DAGLα ED50: 3.0 seconds (95% CI: 2.4–3.8, n = 5; nonoverlapping 95% CI)]. This suggests that DAGLα is responsible for a substantial proportion of the DSE-related 2-AG release.
Fig. 2.
Both DAGLs regulate 2-AG release in DSE. (A) DSE depolarization-response curves, representing progressive inhibition in response to increasing durations of depolarization (50, 100, 300, and 500 milliseconds, and 1, 3, and 10 seconds). Depolarization-response curves for DSE in control pSuper-transfected (filled triangles), transfected DAGLα RNAi (squares), transfected DAGLβ RNAi (circles), and transfected DAGLα/DAGLβ (inverted open triangles). (B) Sample DSE time course of a DAGLα/RNAi-transfected neuron (black circles) and a nontransfected wild-type neuron (red circles) in response to a 3-second depolarization. (Inset) Corresponding traces for baseline and DSE-inhibited conditions in RNAi (upper) and control conditions (lower). Scale bars 1 nA, 10 milliseconds. (C) Sample DSE time course of a DAGLβ/RNAi-transfected neuron (black circles) and a nontransfected wild-type neuron (red circles) in response to a 3-second depolarization. Inset as in (B). Scale bars 500 pA, 10 milliseconds. Ctrl, control; dpol, depolarization; siRNA, small interfering RNA.
We next turned to DAGLβ. Using the previously characterized RNAi construct specific for DAGLβ, we transfected neurons and tested their DSE response profiles. Surprisingly, we found that knockdown of DAGLβ also diminished DSE [Fig. 2, A and C; DAGLβ ED50: 6.4 seconds (95% CI 5.7–7.1, n = 5; nonoverlapping 95% CI relative to pSuper)]. The ED50 of 6.4 seconds (relative to 3.0 after DAGLα knockdown) suggests that, in these neurons, DAGLβ plays a major role in 2-AG production.
This unexpected finding suggests that both enzymes synthesize the 2-AG that mediates DSE in autaptic hippocampal neurons. If both DAGLα and DAGLβ independently produce 2-AG in response to depolarization, one would expect knockdown of both enzymes to further diminish DSE. We found this to be the case [Fig. 3A; DAGLα/DAGLβ ED50: 8.7 seconds (95% CI: 6.1–12.4, n = 6; nonoverlapping 95% CI relative to control and DAGLα knockdown)].
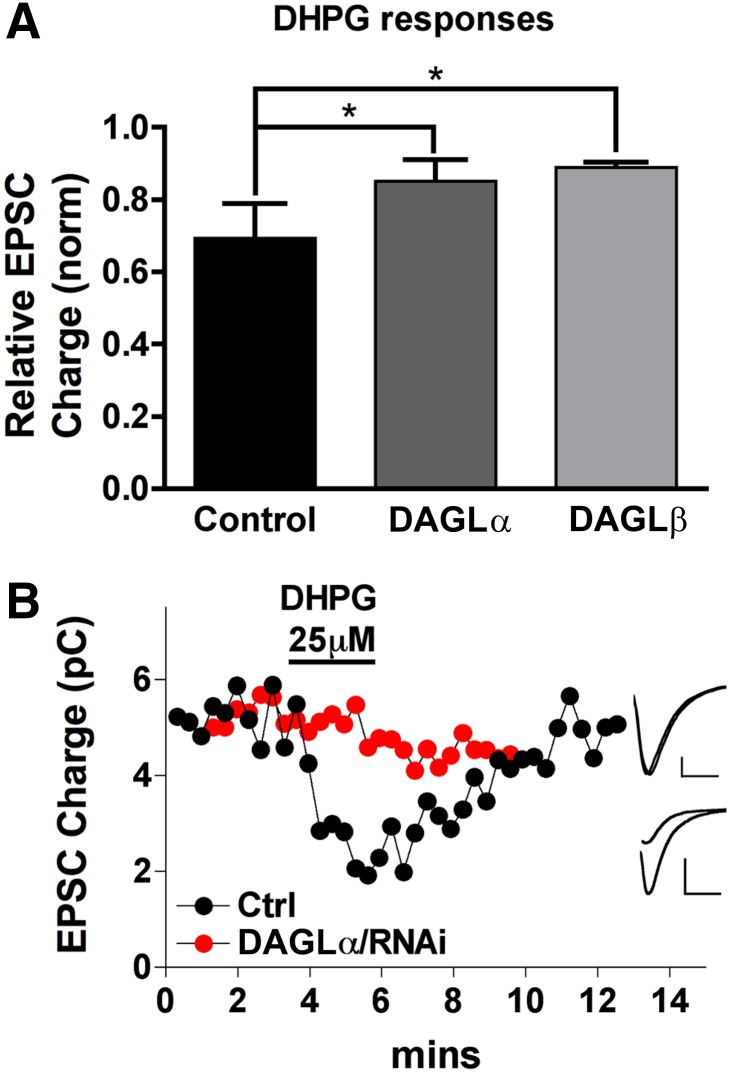
Fig. 3.
Both DAGLs regulate MSE. (A) Bar graph shows relative EPSC charge (1.0 = no inhibition) after treatment with the group I metabotropic glutamate receptor agonist DHPG (50 μM). Conditions: untransfected neurons (control) and DAGLα/RNAi- or DAGLβ/RNAi-transfected neurons. (B) Sample time courses for DHPG response in control (black circles) and DAGLα/RNAi-transfected neuron (red circles). *P < 0.05, one-way analysis of variance with Dunnett’s post-hoc test. (Inset) Corresponding traces for baseline and DHPG-treated conditions in RNAi (upper) and control conditions (lower). Scale bars 300 pA, 10 milliseconds.
DAGLα and DAGLβ Share in the Production of 2-AG by Group I Metabotropic Glutamate Receptors.
We have previously shown that autaptic hippocampal neurons not only express depolarization-dependent DSE but also MSE, particularly in response to activation of group I metabotropic glutamate receptors (Straiker and Mackie, 2007). In autaptic hippocampal cultures, MSE is mediated by 2-AG acting at presynaptic CB1 receptors (Straiker and Mackie, 2007). We investigated the impact of RNAi knockdown of DAGLα or DAGLβ on 50 μM dihydroxyphenyl glycine (DHPG)–induced MSE. Similar to DSE, DHPG inhibition was substantially diminished after knockdown of either DAGL (Fig. 3; DHPG results: DAGLα: 0.85 ± 0.06; DAGLβ: 0.89 ± 0.01; control: 0.63 ± 0.08; n = 5; P < 0.05 for DAGLα and DAGLβ versus control, 1-way analysis of variance with Dunnett’s post-hoc test).
DAGLα and DAGLβ Are Expressed Postsynaptically in Mature Autaptic Hippocampal Neurons.
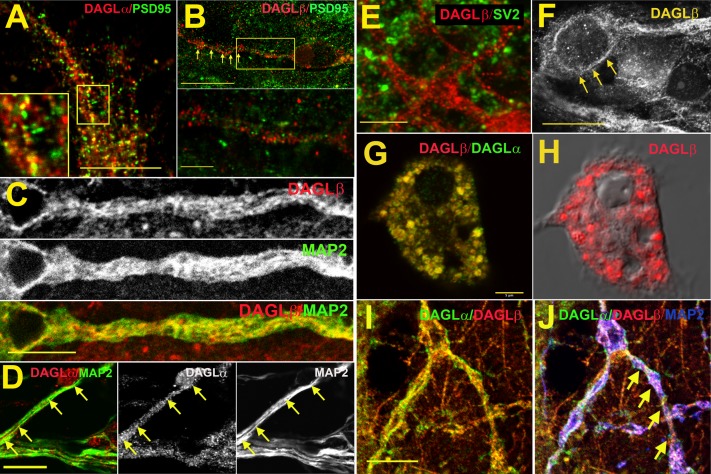
As noted earlier, the localization of DAGLα and DAGLβ has been previously described, with a generally axonal expression for both in embryonic neurons and dendritic expression in adult neurons (Bisogno et al., 2003). However, their expression has not previously been detailed in autaptic hippocampal neurons. Using antibodies developed against DAGLα and DAGLβ, we examined the localization of these proteins relative to markers for dendrites (MAP2), postsynaptic terminals (PSD95), and axon terminals (SV2). We found that both DAGLα and DAGLβ frequently colocalize with the dendritic marker MAP2, consistent with a postsynaptic localization (Fig. 4, C and D). DAGLβ was not generally seen colocalized with PSD95, in contrast to DAGLα (Fig. 4, A and B), suggesting DAGLβ is not enriched in dendritic spines. DAGLβ does not reliably colocalize with the presynaptic marker SV2 (Fig. 4E) but is often associated with membranes of neuronal somata (Fig. 4F). Therefore, DAGLβ and DAGLα exhibit a similar but not identical postsynaptic expression profile consistent with a role in retrograde neuronal signaling (Fig. 4, G and H).
Fig. 4.
DAGLα and DAGLβ are postsynaptically expressed in autaptic hippocampal neurons. Micrographs examining expression of DAGLα and DAGLβ in autaptic cultured hippocampal neurons. (A) DAGLα (red) strongly colocalizes with the dendritic spine marker PSD95 (green, overlap in yellow), although some PSD95-labeled spines lack detectable DAGLα (green). Inset (within frame) shows 2× magnification with points of overlap and nonoverlap. (B) DAGLβ (red) does not colocalize with PSD95 (green). Inset (bottom) shows detail from (B). (C) DAGLβ (red) colocalizes in part with MAP2 (green, overlap in yellow). (D) DAGLα (red) colocalizes with MAP2 (green). Middle and right panels show the corresponding DAGLα and DAGLβ staining, respectively. (E) DAGLβ (red) does not colocalize with the synaptic terminal marker, SV2 (green). (F) DAGLβ is frequently found in the membrane at or near the neuronal soma (arrows). (G) Double staining for DAGLα and DAGLβ shows a similar although not identical expression profile in the cell soma. (H) DAGLβ in the same neuron as (G), with differential interference contrast image for context; scale same as (G). (I) Another double staining for DAGLα and DAGLβ in neurites shows substantial overlap between DAGLα and DAGLβ. (J) Same staining as (I), but with MAP2 (blue, arrows) showing the predominantly dendritic staining. Scale bars: (A) 10 μm; (B) 15 μm; (B) (inset) 3 μm; (C) 5 μm; (D) 5 μm; (E) 5 μm; (F) 10 μm; (G and H) 5 μm; (I and J) 8 μm.
Discussion
Our chief finding using an RNAi approach is that both DAGLα and DAGLβ cooperate to produce 2-AG in a cultured neuron model system for endocannabinoid signaling. Furthermore, each of these isozymes participate in both depolarization-dependent and group I metabotropic glutamate receptor–mediated production of 2-AG. Our results offer the first evidence that DAGLβ is capable of playing an active role in synaptic plasticity, coupling electrical and metabotropic stimulation to the production of 2-AG, the consequent activation of presynaptic CB1 receptors, and inhibition of neurotransmitter release. Our results also indicate that DAGLα and DAGLβ are comparably coupled to both electrical and metabotropic stimulation in cultured neurons and that they act cooperatively.
Although DAGLα and DAGLβ were identified in 2003, it remains challenging to distinguish them pharmacologically. Preliminary investigations of DAGL expression did not strongly favor one over the other as a candidate to play a role in mediating cannabinoid plasticity. For instance, in immunohistochemical studies, expression of DAGLβ appeared to be lower than that for DAGLα in the cerebellum, but mRNA expression was 150-fold higher for DAGLβ than for DAGLα in the striatum (Bisogno et al., 2003; Jung et al., 2005). However, the recent publication of results from three independent lines of DAGL knockout mice seemed to largely settle the matter in favor of DAGLα being the predominant synthetic enzyme for 2-AG involved in synaptic plasticity (Gao et al., 2010; Tanimura et al., 2010; Yoshino et al., 2011), thus making our results surprising.
One possible explanation for the discrepancy in our results versus those of Gao et al. (2010), Tanimura et al. (2010), and Yoshino et al. (2011) has to do with the use of constitutive knockout animals. As noted earlier, knockout mice are valuable tools but come with the risk that, over the course of development, the animal has compensated in some manner for the absence of the gene, or that secondary effects of gene deletion have an indirect impact on the final phenotype. For instance, Tanimura et al. (2010) measured the levels of 2-AG in the cerebellum, striatum, and hippocampus, finding that the levels of 2-AG were markedly decreased in DAGLα but not DAGLβ mice. This is consistent with expectations regarding the role of DAGLs in 2-AG production. However, they also observed changes in anandamide and arachidonic acid levels in DAGLα−/− mice. Gao et al. (2010) reported that both anandamide and arachidonic acid decreased in DAGLα−/− mice, although Yoshino et al. (2011) did not report such a change. Anandamide is not synthesized by DAGL, suggesting that knockout of DAGLα has profound effects on the profiles of arachidonic acid–containing lipids. Conversely, it is possible that, in the absence of DAGLβ, DAGLα is upregulated or has its trafficking altered, accounting for the lack of apparent effect on cannabinoid plasticity. Arguing against the former possibility, Gao et al. (2010) reported that whole-brain DAGLα mRNA levels were unchanged in DAGLβ knockout animals. Because only limited experiments were conducted in DAGLβ knockouts, it is more difficult to assess whether DAGLβ might play at least a supporting role in synaptic modulation. The risk of compensatory effects is inherent to the use of knockout mice; such risks are lessened by the use of RNAi with its transient diminishment of gene function at specific developmental time points.
The distribution of DAGLα and DAGLβ has previously been shown to shift over the course of development from a predominantly axonal distribution, consistent with a role in axonal pathfinding, to dendritic enrichment (Bisogno et al., 2003; Berghuis et al., 2007). The latter profile is consistent with the generally accepted model of the majority of endocannabinoid signaling whereby 2-AG is synthesized postsynaptically but then travels across the synapse to act on presynaptically expressed CB1 receptors. Our evidence for functional postsynaptic DAGLβ is consistent with this model.
Our results demonstrate that DAGLβ is capable of playing a role in neurotransmission via 2-AG production, but whether/when/where this capability is realized remains an open question. The prominent role of DAGLβ may be restricted to autaptic cultures or the immature nervous system. Also, DSE in autaptic neurons is quite robust, and it is possible that the DAGLβ system is selectively engaged when DSE is especially strong. The enrichment of DAGLα in spines points to a more localized production, whereas the diffuse dendritic/somatic expression pattern of DAGLβ suggests that it may be more involved in “volume” production of 2-AG, or during periods of more pronounced activation. However, the question of whether DAGLβ acts in DSE and/or MSE “in the wild” must await more sophisticated tools (such as inducible knockouts or more selective pharmacological blockers) capable of dissecting the roles of DAGLβ and DAGLα in these preparations.
In summary, we have found that, in contrast to expectations, DAGLα and DAGLβ share roles in production of 2-AG for two forms of cannabinoid-mediated synaptic plasticity in cultured autaptic hippocampal neurons. This represents the first explicit evidence supporting a role for DAGLβ in the regulation of synaptic transmission. Our results invite further investigation of DAGLβ in the context of synaptic modulation and offer a novel therapeutic target for cannabinoid-related studies.
Acknowledgments
The authors thank Dr. Daniele Piomelli for providing the DAGL constructs.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- 95% CI
95% confidence interval
- DAGLα
diacylglycerol lipase α
- DAGLβ
diacylglycerol lipase β
- DHPG
dihydroxyphenyl glycine
- DSE
depolarization-induced suppression of excitation
- EPSC
excitatory postsynaptic current
- EYFP
enhanced yellow fluorescent protein
- MAP2
microtubule associated protein 2
- MSE
metabotropic suppression of excitation
- PBS
phosphate-buffered saline
- PSD95
postsynaptic density 95
- RHC80267
O,O′-[1,6-hexanediyl bis(iminocarbony-l)]dioxime cyclohexanone
- RNAi
RNA interference
- SV2
synaptic vesicle 2
Authorship Contributions
Participated in research design: Straiker, Mackie.
Conducted experiments: Jain, Straiker, Wager-Miller.
Contributed new reagents or analytic tools: Jain, Wager-Miller.
Performed data analysis: Straiker.
Wrote or contributed to the writing of the manuscript: Straiker, Mackie.
Footnotes
This work was supported by the National Institutes of Health [Grants DA011322, DA021696, and EY021831]; the Indiana Metabolomics and Cytomics Initiative (METACyt) of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc.; and the Indiana University Light Microscopy Imaging Center.
References
- Bekkers JM, Stevens CF. (1991) Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA 88:7834–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. (2007) Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316:1212–1216 [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, et al. (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163:463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. (2003) Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38:461–472 [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O’Lague PH, Potter DD. (1976) Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci USA 73:4225–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, et al. (2010) Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci 30:2017–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647 [Google Scholar]
- Hsu KL, Tsuboi K, Adibekian A, Pugh H, Masuda K, Cravatt BF. (2012) DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat Chem Biol 8:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SS, Arnold A, Hutchens JM, Radicke J, Cravatt BF, Wager-Miller J, Mackie K, Straiker A. (2010) Architecture of cannabinoid signaling in mouse retina. J Comp Neurol 518:3848–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Deng L, Chen G. (2004) High Ca(2+)-phosphate transfection efficiency enables single neuron gene analysis. Gene Ther 11:1303–1311 [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. (2005) Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol 68:1196–1202 [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. (2009) Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89:309–380 [DOI] [PubMed] [Google Scholar]
- Keimpema E, Mackie K, Harkany T. (2011) Molecular model of cannabis sensitivity in developing neuronal circuits. Trends Pharmacol Sci 32:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, McCarthy KD. (1991) Characterization and partial purification of AIM: a plasma protein that induces rat cerebral type 2 astroglia from bipotential glial progenitors. J Neurochem 57:782–794 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65 [DOI] [PubMed] [Google Scholar]
- Piomelli D. (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884 [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388:773–778 [DOI] [PubMed] [Google Scholar]
- Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, Mackie K. (2009) Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol 76:1220–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. (2005) Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol 569:501–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. (2007) Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol 578:773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. (2009) Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience 163:190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Wager-Miller J, Hu SS, Blankman JL, Cravatt BF, Mackie K. (2011) COX-2 and fatty acid amide hydrolase can regulate the time course of depolarization-induced suppression of excitation. Br J Pharmacol 164:1672–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, Abe M, Kita Y, Hashimoto K, Shimizu T, Watanabe M, et al. (2010) The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65:320–327 [DOI] [PubMed] [Google Scholar]
- Wu CS, Zhu J, Wager-Miller J, Wang S, O’Leary D, Monory K, Lutz B, Mackie K, Lu HC. (2010) Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci 32:693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, Blat Y, Westphal RS, Zaczek R, Lewis DA, et al. (2011) Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol 589:4857–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]