Abstract
Introduction:
Waterpipe tobacco smoking has in recent years become a popular international phenomenon, particularly among youth. While it has been shown to deliver significant quantities of several carcinogenic and toxic substances, phenols, an important class of chemical compounds thought to promote DNA mutation and cardiovascular diseases, however, has not been studied. Due to the relatively low temperature characteristic of waterpipe tobacco during smoking (i.e., <450 °C), it was hypothesized that phenolic compounds, which form at approximately 300 °C, will be found in abundance in waterpipe smoke.
Methods:
In this study, phenolic compounds in the particle phase of waterpipe mainstream smoke were quantified. Waterpipe and cigarette mainstream smoke generated using standard methods were collected on glass fiber pads and analyzed using gas chromatography/mass spectroscopy selected ion current profile chromatogram method for quantification.
Results:
We found that relative to a single cigarette, a waterpipe delivers at least 3 times greater quantities of the 7 analyzed phenols (phenol, o-cresol, m-cresol, p-cresol, catechol, resorcinol, and hydroquinone). Moreover, phenol derivatives such as methylcatechol, and flavorings such as vanillin, ethyl vanillin, and benzyl alcohol were found in quantities up to 1,000 times greater than the amount measured in the smoke of a single cigarette.
Conclusion:
The large quantities of phenols and phenol derivatives in waterpipe smoke add to the growing evidence that habitual waterpipe use may increase the risk of cancer and cardiovascular diseases.
INTRODUCTION
Waterpipe tobacco smoking has in recent years become prevalent across the globe. A waterpipe consists of a head, body, water bowl, and corrugated hose. It is often smoked with a moist, sweetened, and flavored tobacco mixture known as ma’assel, with burning charcoal placed atop as a heat source. Thus, in addition to smoke constituents originating from the tobacco, waterpipe smoke also contains charcoal combustion emissions. While a common perception among users is that waterpipe is relatively safe, it has been shown to deliver to the user large quantities of polycyclic aromatic hydrocarbons, aldehydes, tar, nicotine, and carbon monoxide (Al Rashidi, Shihadeh, & Saliba, 2008; Sepetdjian, Shihadeh, & Saliba, 2008; Shihadeh & Saleh, 2005). Also, waterpipe smoke can be a major source of furans specially 5-(hydroxymethyl)-2-furaldehyde (Schubert, Bewersdorff, Luch, & Schulz, 2012).
Phenols are listed as priority pollutants by the U.S. Environmental Protection Agency due to their high toxicity (Yan & Quan, 2009). In particular, polyphenolic compounds such as catechols and hydroquinones and their methyl derivatives that are present in tobacco smoke are important tumor promoters and provoke an increase in metastasis of lung cancer (Gopalakrishna, Chen, & Gundimeda, 1994; Hoffmann, Djordjevic, & Hoffmann, 1997). Other health impacts caused by phenolic compounds have been linked to genotoxic activities and cardiovascular effects (Fowles & Dybing, 2003; Harvey, Howe, Lynch, & Rees, 2005; Leanderson & Tagesson, 1990).
The generation of phenols in cigarette mainstream smoke is attributed to the distillation, depolymerization, and decomposition of tobacco components during pyrolysis. The precursors of catechol formation are the polyphenols such as quinic acid and quinic acid derivatives present in tobacco. Also, chlorogenic acid and lignin are considered precursors of hydroquinone formation at lower temperatures (<600 °C) (Czégény et al., 2009; Torikaiu, Uwano, Nakamori, Tarora, & Takahashi, 2005). McGrath, Brown, Meruva, and Chan (2009) showed that hydroquinone, catechol, and methyl derivatives of catechol are produced in highest amounts at tobacco pyrolysis temperatures ≤350°C due to their limited thermal stability at higher temperatures. Formation of other phenols such as cresols, phenol, and resorcinol is favored between 350 and 600 °C. Hence, temperature is considered an essential factor for the formation and the decomposition of many toxins like phenolic compounds in the smoke. The temperature at which the tobacco is heated inside the head of the waterpipe reaches up to 450 °C (Shihadeh 2003; Shihadeh & Saleh, 2005), whereas the burning temperatures of cigarette tobacco can reach up to 950 °C (Czégény et al., 2009). Therefore, high levels of phenols and their derivatives are expected in the waterpipe smoke because the smoke is generated at optimal temperatures for phenols production.
Many analytic techniques were developed to determine phenols in cigarette smoke (Moldoveanu & Kiser, 2007). The most common techniques are high-performance liquid chromatography (HPLC) with ultra violet or fluorescence detection and capillary gas chromatography with mass spectrometry (GC-MS) (Moldoveanu & Kiser, 2007). In this study, the identification and quantification of phenolic compounds and their derivatives in the particle phase of the mainstream waterpipe smoke was realized using GC-MS selected ion current profile (GC-MS-SICP) chromatogram method after sample derivatization.
EXPERIMENTAL
Chemicals
A standard mixture of seven phenols (phenol, o-cresol, m-cresol, p-cresol, catechol, resorcinol, and hydroquinone) and a mixture of two deuterated phenols (phenol-d6 and 4-methylphenol- d8) at 1000 µg/ml each were purchased from Absolute Standards. Polystyrene divinylbenzene (PS-DVB) SPE cartridges (CHROMABOND®; EASY 200mg, 3ml) were purchased from Sorbent Technology. The derivatizing reagent, bis (trimethylsilyl)-trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) and HPLC-grade solvents of methanol, dichlomethane, and ethylacetate were purchased from Sigma-Aldrich.
Smoke Generation and Collection
Mainstream waterpipe smoke was generated and collected in accordance with the Beirut method (Katurji, Daher, Sheheitli, Saleh, & Shihadeh, 2010; Shihadeh & Saleh, 2005) using Nakhla Double Apple (Egypt) waterpipe tobacco preparation and Three Kings (Holland) charcoal. During each machine smoking session, the smoke exiting the waterpipe mouthpiece was split into four parallel branches and each stream drawn through a 47-mm glass fiber filter pad (47mm, Pall Gelman Type A/E). As in Shihadeh et al. (2012), filters were changed during a smoking session whenever the filter assembly flow friction coefficient exceeded a predetermined threshold; the threshold indicated a mass loading approaching 150mg, the maximum allowed by smoking machine testing standard ISO 4387:1991. Three to four filter sets (i.e., 12–16 filters in total) were required during a smoking session. Additional details of the waterpipe smoking session and smoke sampling procedures can be found in Shihadeh et al. (2012). For each smoking session, all of the filters from one of the four parallel branches were analyzed for phenol content. The mass of consumed tobacco and charcoal was determined gravimetrically by comparing the weight of each prior to and at the end of the smoking session. The waterpipe hoses used in this study were of leather construction and exhibited infiltration rates of 0.93–1.8 standard liters per minute (slpm) at a nominal waterpipe flow rate of 12.2 slpm as determined using the method described by Saleh and Shihadeh (2008).
Cigarette smoke was generated using the Federal Trade Commission protocol and trapped on glass fiber filters (47mm, Pall Gelman Type A/E). Smoke from three cigarettes (Marlboro-KG) was drawn through each analyzed filter. The puff regimens and resulting tobacco, charcoal consumption, total particulate matter, and carbon monoxide amounts generated for waterpipe and cigarette smoke are provided in Table 1.
Table 1.
Puff Parameters for Cigarette and Waterpipe Smoke Generation
| Topography | Cigarette | Waterpipe |
|---|---|---|
| Tobacco product | Marlboro-KG Lebanon | Nakhla Double Apple |
| Number of puffs | 8.86±0.5 | 171 |
| Total volume (L) | 0.31±0.01 | 90.30±0.15 |
| Flow rate (L/min) | 1.01 | 12.19±0.02 |
| Puff duration (s) | 2 | 2.6 |
| Interpuff interval (s) | 58 | 17 |
| Tobacco consumed (g/session) | – | 4.9±0.3 |
| Charcoal consumed (g/session) | – | 7.4±0.2 |
| TPM (mg) | 11±2 | 1586.2±202 |
| Carbon monoxide (mg) | 4.85±0.8 | 186±20.8 |
Sample Preparation
Glass fiber filter pads of cigarette and waterpipe smoke were spiked with 100 µg/ml deuterated internal standards and extracted through mechanical shaking (IKA Vortex Genius 3) for 2hr at room temperature with 20ml of acidified water (0.1M HCl with 0.1% ascorbic acid). Ascorbic acid was added to prevent any potential oxidation of phenols. Two and five milliliters of the respective cigarette and waterpipe resulting solutions were loaded on separate PS-DVB cartridges preconditioned with 10ml of each: dichloromethane, methanol, and HCl (0.05M). PS-DVB cartridges with polymeric materials are mainly used to concentrate phenols from water samples, have been shown to be more stable when compared to silica base sorbents in high acidic media (Rodríguez, Lompart, & Cela, 2000). After loading the samples, the cartridges were washed 3 times with 3ml 1% acetic acid in order to eliminate interferences while retaining the phenolic compounds. Next, the cartridges were left to dry for 2hr under vacuum. Phenol samples were eluted with 12ml ethylacetate and the collected volume was reduced to approximately 150 µl under a flow of nitrogen. The 100 µl of the derivatizing agent BSTFA with 1% TMCS was added to the samples (Moldoveanu & Kiser, 2007). The vials were capped and heated at 80 °C for 30min to obtain the trimethylsilyl derivatives of phenols analyzed by GC-MS-SICP. The derivatization step was recommended by Mußmann, Levsen, and Radeck (1994) to avoid the broadening and tailing of chromatographic peaks otherwise characteristic of phenols due to their high polarity. The GC-MS-SICP method ensures high selectivity even though standards of phenolic derivatives are not available.
Detection and Calibration
Detection and quantification of the samples were done using derivatized standard mixtures injected on GC-MS in the full scan mode. Calibration curves were prepared using seven concentration ratios of phenol standards/deuterated phenol standards. Phenol standard concentrations ranging from 0.5 to 20 µg/ml were mixed with 2 µg/ml of deuterated phenol standard. The R 2 ranged between 0.998 and 0.999. Blank filters were analyzed in parallel to assess the background level of phenols during sample preparation.
Recovery and Limit of Detection
Percent recovery of phenols and deuterated standards were calculated by spiking glass fiber filters with 100 µl of phenols standard mixture at concentrations ranging from 0.5 to 20 µg/ml and 2 µg/ml for deuterated phenol standards. Standards were extracted and analyzed using the same procedure used for analyzing the cigarette and waterpipe samples. High recoveries (81%–89%) for all tested concentrations were obtained for phenol, o-cresol, m-cresol, and p-cresol. Due to the unavailability of their respective deuterated compounds, lower recoveries (17%–43%) were found for catechol, resorcinol, and hydroquinone (Vaughan, Stanfill, Polzin, Ashley, & Watson, 2008). This could be attributed to the loss of these compounds during drying and evaporation steps. The limits of detection ranged between 0.23 and 0.30 µg and were calculated as 3 times the standard deviations of their lowest detected concentrations by GC-MS.
RESULTS
As shown in Table 2, the yields of the seven phenols determined for cigarette mainstream smoke fall within the reported values (Baker, Massey, & Smith, 2004; Moldoveanu & Kiser, 2007; Vaughan et al., 2008), indicating the basic reliability of the method used in this study.
Table 2.
Mass (µg) of Phenols in Cigarette and Waterpipe Mainstream Smoke
| Compound | Cigarette (current study) | Cigarette (previous studies) | Waterpipe (current study) | ||||
|---|---|---|---|---|---|---|---|
| Average (n = 6), µg/cigarette | % RSD | Vaughan et al. (2008) a, µg/cigarette | Moldovean and Kiser (2007)b, µg/cigarette | Baker et al. (2004)c, µg/cigarette | Average (n = 12), µg/waterpipe | % RSD | |
| Phenol | 22.3 | 22 | 16.3 | 4.86–16.07 | 10.0–19.6 | 58.03 | 44 |
| o-Cresol | 5.79 | 21 | 4.9 | 1.23–3.70 | 2.81–4.75 | 4.409 | 61 |
| m-Cresol | 4.33 | 20 | 3.5 | 0.93–2.95 | 8.48–13.7d | 4.655 | 56 |
| p-Cresol | 10.1 | 21 | 9.1 | 2.12–6.97 | 5.375 | 55 | |
| Catechol | 40.7 | 18 | 49.5 | 20–62.35 | 44.2–54.5 | 316.1 | 42 |
| Resorcinol | 0.79 | 15 | 1.7 | 0.72–1.81 | <1.1 | 1.689 | 28 |
| Hydroquinone | 34.6 | 19 | 44.0 | 19.78–56.44 | 44.7–51.2 | 110.7 | 39 |
Note. RSD = Relative Standard Deviation.
aMicrogram per cigarette phenols Marlboro brand, 35-ml puff volume, 2-s puff duration, 60-s puff interval.
bMicrogram per cigarette phenols range in three common commercial cigarettes.
cMicrogram per cigarette phenols range in cigarettes after adding a range of ingredients to the tobacco (n = 5).
dValues of m- + p-cresols.
Derivatives of phenol and cresols such as dimethylphenols and ethylphenols (m/z = 194) showed similar amounts in cigarette and waterpipe samples (Table 3; Figure 1a). However, three methylated-dihydroxybenzene compounds (m/z = 268) were identified in waterpipe smoke compared to four such compounds in cigarette smoke (Figure 1b). The quantity of the methylated-dihydroxybenzene compounds identified in the waterpipe smoke was at least 8 times greater than that determined for cigarette smoke (Table 3). Dihydroxybenzene derivatives were quantified using the catechol calibration curve in absence of methylated-dihydroxybenzene standards.
Table 3.
List of Phenols Derivatives Identified in the Cigarette and Waterpipe Samples
| Compound | Total amount of possible isomers, µg/cigarette (n = 6) | Total amount of possible isomers, µg/waterpipe (n = 12) | ||||
|---|---|---|---|---|---|---|
| Average | SD | % RSD | Average | SD | % RSD | |
| Derivatives of phenol and cresola (m/z = 194) | 7.6 | 1.4 | 18 | 6.000 | 3.686 | 61 |
| Derivatives of catechol, resorcinol, and hydroquinoneb (m/z = 268) | 30 | 5.3 | 18 | 259.8 | 92.62 | 36 |
| Vanillin (m/z = 194) | 2.2 | 1.2 | 57 | 3192 | 1242 | 39 |
| Ethyl vanillin (m/z = 194) | 0.5 | 0.5 | 102 | 616.0 | 271.6 | 44 |
| Benzyl alcohol (m/z = 165) | 0.9 | 0.2 | 20 | 232.4 | 74.54 | 32 |
Note. RSD = Relative Standard Deviation.
aPossible isomers: 2-ethylphenol, 4-ethylphenol, 2,5-dimethylphenol, 3,5-dimethylphenol, 2,6-dimethylphenol, 2,3-dimethylphenol, 3,4-dimethylphenol.
bPossible isomers: 4-methylcatechol, 3-methylcatechol, 3-methylresorcinol, 2-methylresorcinol, methylhydroquinon.
Figure 1.
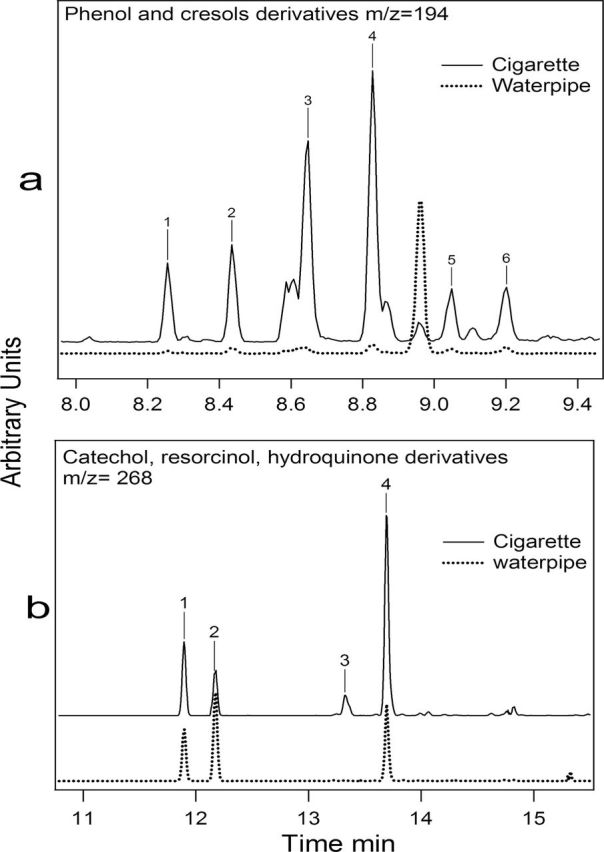
Selected ion current profile chromatograms of phenol derivatives in cigarette and waterpipe smoke. According to the National Institute of Standards and Technology library, the numbers are assigned to (a) phenol and cresol derivatives, namely, 1: 2-ethylphenol, 2: 4-ethylphenol, 3: 2,5-dimethylphenol, 4: 3,5-dimethylphenol, 5: 2,6-dimethylphenol, and 6: 2,3-dimethylphenol and (b) catechol, resorcinol, and hydroquinone derivatives, namely, 1: 4-methylcatechol, 2: 3-methylcatechol, 3: 2-methylresorcinol, and 4: 2-methylhydroquinone.
In addition to phenols’ derivatives, additives such as vanillin, ethyl vanillin, and benzyl alcohol show mass exceedance in waterpipe mainstream smoke that reached up to 1,000 times the amount calculated for the cigarette mainstream smoke (Table 3).
DISCUSSION
In comparison to cigarette smoke, the total yield of phenolic compounds quantified for the first time in the waterpipe smoke was at least 3 times higher than that in mainstream cigarette smoke. Variations in phenols amount between different waterpipe smoking sessions (28 < % Relative Standard Deviation < 61; Table 2) are attributed to differences in smoke production that result from natural variations in charcoal briquette burning rates, and hand-packing of the tobacco mixture in the waterpipe head. These variations are also apparent in the amounts of total particulate matter produced per session (Table 1). Furthermore, knowing that more additives and flavors are added to the ma’assel tobacco in waterpipe than cigarettes, it is expected that additives such as sugars, cellulose, and polysaccharides present at such high quantities can lead to an increase in the toxicity of the waterpipe smoke. Toxicity could be ascribed to increase in the total particulate matter concentrations as well as the formation of more additive-related pyrolysis or combustion harmful products (i.e., formaldehyde) (Baker et al., 2004; Wertz, Kyriss, Paranjape, & Glantz, 2011; Xiaomin et al., 2012).
It is also noteworthy that though phenols are highly polar and soluble in water (high Henry’s constants) (Feigenbrugel, Le Calvé, Mirabel, & Louis, 2004), and susceptible to be trapped in the water bubbler, they are apparently present in high enough quantities that there is still a lot left in the smoke reaching the mouthpiece. Therefore, the reported yields of phenols and their derivatives represent the lower limits of their actual values in the waterpipe smoke.
If reported phenol emissions are normalized by the volume of smoke inhaled or Total Particulate Matter (TPM) produced, cigarette smoke appears more toxic than waterpipe smoke. In terms of physiological/clinical relevance vis-à-vis the human body’s toxicant clearance mechanisms, however, an equally important measure could be the intake of phenols per hour of smoking, in which case the waterpipe smoking involves higher hourly phenol intake than cigarette smoking. After considering the various parameters that could be reported, phenol emissions per unit smoked would be the most relevant to the community. Those in need of toxicant concentrations per milliliter smoke or per milligram TPM can derive such values from the presented data.
CONCLUSION
When heated or burned, tobacco ingredients such as cellulose, polyphenols, chlorogenic acid, and quercetin dehydrate generate phenols and their derivatives. In waterpipe smoking, the relatively low temperature of the burning tobacco mixture favors production and survival of phenol compounds; in this study we observed high yields of hydroquinone, catechol, and methyl derivatives of catechol. In particular, we found that the yields of the seven standard phenol compounds in mainstream waterpipe smoke were at least 3 times higher than in mainstream cigarette smoke, and that derivatives and flavorings such as methylated-dihydroxybenzenes, vanillin, ethyl vanillin, and benzyl alcohol were up to 1,000 times more abundant in waterpipe mainstream smoke than they were in cigarette mainstream smoke. This study adds to the growing evidence that waterpipe smoking presents significant health risks.
FUNDING
This work was supported by U.S. Public Health Service Grants R01CA120142 and R01DA025659.
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Al Rashidi M., Shihadeh A., Saliba N. A. (2008). Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food and Chemical Toxicology, 46, 3546–3549. 10.1016/ j.fct.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. R., Massey E. D., Smith G. (2004). An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food and Chemical Toxicology, 42,(Suppl.), S53–S83. 10.1016/j.fct.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Czégény Z., Blazsó M., Várhegyi G., Jakab E., Liu C., Nappi L. (2009). Formation of selected toxicants from tobacco under different pyrolysis conditions. Journal of Analytical and Applied Pyrolysis, 85, 47–53. 10.1016/j.jaap.2008.10.001 [Google Scholar]
- Feigenbrugel V., Le Calvé S., Mirabel P., Louis F. (2004). Henry’s law constant measurements for phenol, o-, m-, and p-cresol as a function of temperature. Atmospheric Environment, 38, 5577–5588. 10.1016/j.atmosenv.2004.06.025 [Google Scholar]
- Fowles J., Dybing E. (2003). Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tobacco Control, 12, 424–430. 10.1136/tc.12.4.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Chen Z. H., Gundimeda U. (1994). Tobacco smoke tumor promoters, catechol and hydroquinone, induce oxidative regulation of protein kinase C and influence invasion and metastasis of lung carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America, 91, 12233–12237 Retrieved from http://www.pnas.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. S., Howe J. R., Lynch A. M., Rees R. W. (2005). The results of five coded compounds: Genistein, metaproterenol, rotenone, p-anisidine and resorcinol tested in the pH 6.7 Syrian hamster embryo cell morphological transformation assay. Mutagenesis, 20, 51–56. 10.1093/mutage/gei009 [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Djordjevic M. V., Hoffmann I. (1997). The changing cigarette. Preventive Medicine, 26, 427–434 Retrieved from http://www.journals.elsevier.com/preventive-medicine/ [DOI] [PubMed] [Google Scholar]
- Katurji M., Daher N., Sheheitli H., Saleh R., Shihadeh A. (2010). Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhalation Toxicology, 22, 1101–1109. 10.3109/08958378.2010.524265 [DOI] [PubMed] [Google Scholar]
- Leanderson P., Tagesson C. (1990). Cigarette smoke-induced DNA-damage: Role of hydroquinone and catechol in the formation of the oxidative DNA-adduct, 8-hydroxydeoxyguanosine. Chemico-Biological Interactions, 75, 71–81. 10.1016/0009-2797(90)90023-G [DOI] [PubMed] [Google Scholar]
- McGrath T. E., Brown A. P., Meruva N. K., Chan W. G. (2009). Phenolic compound formation from the low temperature pyrolysis of tobacco. Journal of Analytical and Applied Pyrolysis, 84, 170–178. 10.1016/j.jaap.2009.01.008 [Google Scholar]
- Moldoveanu S. C., Kiser M. (2007). Gas chromatography/mass spectrometry versus liquid chromatography/fluorescence detection in the analysis of phenols in mainstream cigarette smoke. Journal of Chromatography A, 1141, 90–97. 10.1016/j.chroma.2006.11.100 [DOI] [PubMed] [Google Scholar]
- Mußmann P., Levsen K., Radeck W. (1994). Gas-chromatographic determination of phenols in aqueous samples after solid phase extraction. Fresenius’ Journal of Analytical Chemistry, 348, 654–659. 10.1007/BF00325568 [Google Scholar]
- Rodríguez I., Lompart M. P., Cela R. (2000). Solid-phase extraction of phenols. Journal of Chromatography A, 885, 291–304. 10.1016/S0021-9673(00)00116-3 [DOI] [PubMed] [Google Scholar]
- Saleh R., Shihadeh A. (2008). Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food and Chemical Toxicology, 46, 1461–1466. 10.1016/j.fct.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Schubert J., Bewersdorff J., Luch A., Schulz T. G. (2012). Waterpipe smoke: A considerable source of human exposure against furanic compounds. Analytica Chimica Acta, 709, 105–112. 10.1016/j.aca.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Sepetdjian E., Shihadeh A., Saliba N. A. (2008). Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food and Chemical Toxicology, 46, 1582–1590. 10.1016/j.fct.2007.12.028 [DOI] [PubMed] [Google Scholar]
- Shihadeh A. (2003). Investigation of mainstream smoke aerosol of the argileh water pipe. Food and Chemical Toxicology, 41, 143–152. 10.1016/S0278-6915(02)00220-X [DOI] [PubMed] [Google Scholar]
- Shihadeh A., Saleh R. (2005). Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food and Chemical Toxicology, 43, 655–661. 10.1016/j.fct.2004.12.013 [DOI] [PubMed] [Google Scholar]
- Shihadeh A., Salman R., Jaroudi E., Saliba N., Sepetdjian E., Blank M. D., et al. (2012). Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, “ tar” and nicotine yields. Food and Chemical Toxicology, 50, 1494–1498. 10.1016/j.fct.2012.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torikaiu K., Uwano Y., Nakamori T., Tarora W., Takahashi H. (2005). Study on tobacco components involved in the pyrolytic generation of selected smoke constituents. Food and Chemical Toxicology, 43, 559–568. 10.1016/j.fct.2004.12.011 [DOI] [PubMed] [Google Scholar]
- Vaughan C., Stanfill S. B., Polzin G. M., Ashley D. L., Watson C. H. (2008). Automated determination of seven phenolic compounds in mainstream tobacco smoke. Nicotine & Tobacco Research, 10, 1261–1268. 10.1080/14622200802123146 [DOI] [PubMed] [Google Scholar]
- Wertz M. S., Kyriss T., Paranjape S., Glantz S. A. (2011). The toxic effects of cigarette additives. Philip Morris’ project mix reconsidered: An analysis of documents released through litigation. PLoS Medicine, 8. 10.1371/journal.pmed.1001145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaomin C., Weia M., Zhangb H., Luoa C., Chena Y., Chen Y. (2012). Effect of vanillin and ethyl vanillin on cytochrome P450 activity in vitro and in vivo. Food and Chemical Toxicology, 50, 1897–1901. 10.1016/j.fct.2012.03.060 [DOI] [PubMed] [Google Scholar]
- Yan J., Quan G. (2009). Equilibrium and kinetic studies of phenol sorption by chitosan coated montmorillonite. Journal of the Chilean Chemical Society, 54, 73–76 Retrieved from http://www.scielo.cl/scielo.php?script=sci_serial&pid= 0717-9707&lng=es&nrm=iso [Google Scholar]


